Abstract
We determined whether COVID-19 vaccination was associated with Quality of Life (QoL) changes among individuals previously infected with SARS-CoV-2 in Israel. Using a validated questionnaire, we collected information about socio-demographics, SARS-CoV-2 infection, COVID-19 vaccination and QoL (using the EQ-5D-5L tool) 3–18 months post-infection among adults tested for SARS-CoV-2 by polymerase chain reaction in Northern Israel between March 2020–June 2022. We compared post-COVID QoL between those vaccinated against COVID-19 at the time of infection and those not, using an adjusted linear regression model, stratified by time elapsed since infection. Of 951 participants, mean EQ-5D Utility Index (EQ-5D UI) was 0.82 (SD = 0.26) and 0.83 (SD = 0.25) among the 227 double and 250 triple vaccinated respectively, compared to 0.76 (SD = 0.33) among those who received 0 dose (n = 243). The size of the effect of vaccination was small (Cohen’s d = 0.2). In the adjusted model, previously infected individuals vaccinated with two or more doses reported a QoL score post- infection 0.05 points higher (CI = 0.01–0.10, p = 0.02) compared with those unvaccinated when infected. No association between vaccination and QoL was detected beyond 12 months post-infection. Vaccination with two or more doses of COVID19 vaccine, or at least the BNT162b2 vaccine, may modestly mitigate QoL losses associated with post-acute COVID-19 symptoms, at least in the first 12 months post-infection.
Similar content being viewed by others
Introduction
Post-COVID condition, also referred to as post-acute sequelae of COVID-19 (PASC) or Long COVID was defined by the World Health Organization (WHO) as “a condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection and occurs three months after the initial COVID-19 symptoms, whereby the symptoms reported by post-COVID patients cannot be explained by alternative diagnoses”1. PASC affects approximately 10–30% of individuals previously infected with SARS-CoV-22. Suggested pathophysiological mechanisms for PASC include long-term organ damage resulting from the initial infection, central nervous system damage, immune dysregulation, endothelial dysfunction, viral persistence, and coagulation activation3, 4. It has also been postulated that several pathological mechanisms may occur concurrently, manifesting as a wide range of seemingly unrelated post-COVID symptoms3 and that the patient experience of long COVID results from the interplay between biological, social, psychological and experiential factors5.
Post-SARS-CoV-2 infection persistence of symptoms is a widely reported phenomenon in the literature. A living systematic review (ongoing as of January 2023) suggested that severity of the acute episode was associated with post-COVID condition6. Chronic conditions such as diabetes, hypertension, Parkinson’s disease, chronic obstructive pulmonary disease and others were also associated with developing post-COVID condition7. Post-Covid condition has also been reported among asymptomatic COVID19 patients, albeit to a lesser extent than symptomatic individuals7. Post-viral syndrome is not unique to SARS-CoV-2 and has been described with other viral infections including Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS)8.
Mass COVID-19 vaccination has been one of the key measures to mitigate the impact of the pandemic. Despite decreased effectiveness against infection with Omicron sub-variants, COVID-19 vaccines remain effective against severe disease9. By December 2022, approximately 70% of the world population and 71% of the Israeli population had received at least one dose of a COVID-19 vaccine10, 11. Israel overwhelmingly used the BNT162b2 mRNA vaccine. As of February 2023, Israel offers up to 5 doses of COVID-19 vaccine, including an updated bivalent vaccine used as a booster that has shown efficacy against severe disease caused by the Omicron variants of SARS-CoV-212.
Beyond protecting against acute COVID-19, being vaccinated at the time of infection is also associated with a reduction in reported post-acute symptoms, with most studies published on the topic agreeing on the direction of the association, if not the strength of the effect13,14,15. The evidence of post-infection vaccination on long-term symptoms is less clear16. Available evidence suggests that post-acute COVID-19 symptoms impacts quality of life (QoL)17, 18, although the extent of the impact on QoL depends on factors such as acute disease severity19 and gender17, 20 The emerging consensus is that QoL impairments sustained during acute-COVID-19 persists for months as a result of ongoing physical and mental health issues17, 20. Despite the growing consensus around the mitigating effect of COVID19 vaccinations against post-acute symptoms and the impact of post-acute COVID symptoms on QoL, there is a gap in evidence on whether COVID-19 vaccination has the potential to mitigate any QoL losses among SARS-CoV-2 infected individuals suffering from long-term symptoms.
Understanding the impact of vaccination on long-term QoL resulting from post-acute COVID-19 symptoms will help estimate the global burden of disease likely to emerge as a result of the COVID-19 pandemic, and better define the role of vaccination in mitigating it. We therefore aimed to identify associations between COVID-19 vaccination and QoL among individuals previously infected with SARS-CoV-2, up to 18 months after infection by comparing QoL between vaccinated and unvaccinated individuals.
Results
Baseline characteristics
Between July 2021 and June 2022, 95,604 persons were invited to participate in the study. Of these, 6964 (7.3%) individuals responded and provided complete data on their COVID-19 vaccination and SARS-CoV-2 RT-PCR testing status and were thus included in the study. Of the 6,964 included participants, 2579 (37.0%) participants reported a positive test. Of those, 1,227 (47.6%) participants reported complete information about post-COVID symptoms and QoL. Of these, 276 (22.5%) individuals reported their symptoms less than 60 days following their positive PCR test and were excluded from the study so as to not include the impact of acute illness on QoL. The remaining 951 participants were included in the final analysis. The baseline socio-demographic characteristics of the study participants are shown in Table 1. Overall, mean (SD) age was 46 (± 14.74) years old, 65.7% of participants were female, and 76.9% were of Jewish ethnicity, comparable to the 74% in the general population21. Compared to infected participants not included in the study (because they did not have complete QoL data), participants were similar in terms of age (mean 46 vs 49 years old, p = 0.88), gender (34 vs 39% male, p = 0.06), vaccination status (50.1 vs 53.1% who received at least 2 doses, p = 0.26) and severity of disease (14.3% vs 12.2% hospitalized, p = 0.23). In terms of vaccination status, of the 951 participants, 243 participants (25.6%) were unvaccinated, and 231 (24.3%), 227 (23.9%) and 250 (26.2%) received 1, 2 and 3 doses of COVID-19 vaccine respectively. Vaccinated participants were comparable with unvaccinated participants with respect to gender and marital status. The unvaccinated were more likely to be hospitalized for COVID-19 and slightly younger than those vaccinated (44.3 vs. 47.9 years, p < 0.001), likely reflecting the fact that vaccination in Israel was first available to older individuals. In the unvaccinated group, the mean duration between reporting testing positive for SARS-CoV-2 and answering the survey was 251 days compared to 401, 267 and 137 days for 1-dose, 2-doses, and 3-doses vaccinated, respectively (Table 1). The longer follow-up time for those who received one dose reflects the fact that the vast majority (206/231, 89.2%) of those who received a single dose were infected prior to vaccination, as the policy in Israel was initially for those infected to received single dose of vaccine. Of the 951 participants, 572 (60.1%) reported at least one post-COVID symptom and 547 (57.5%) one of the ten most common symptoms (listed in supplementary Table s1). Of the 547 participants reporting symptoms, 298 had received 0 or 1 vaccine dose and 127 and 147 had received 2 and 3 doses respectively.
QoL and post-COVID symptoms
EQ-5D-5L dimensions
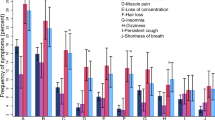
Compared with 2 and 3-dose vaccinated participants, a higher proportion of unvaccinated and one-dose vaccinated participants reported scores of 4 and 5 (indicating a lower QoL) in the mobility, pain, discomfort, and anxiety and depression dimensions of EQ-5D-5L, a standardized Quality of life questionnaire collecting information on 5 dimensions of quality of life, each with a score between 1 (lowest quality of life) and 5 (highest) (Fig. 1, supplementary Table s2). The proportion of individuals reporting no or only slight impairment in their usual activities was higher among those who received two or three doses compared with those who received 0 or 1 dose for the self-care, usual activities, and anxiety and depression dimensions (Fig. 1 and supplementary Table s2).
EQ-5D utility index (UI) scores
Regardless of their COVID-19 vaccination status, participants not reporting post-acute COVID-19 symptoms had mean EQ-5D UI of 0.92 (SD = 0.20), compared to 0.72 (SD = 0.32) among individuals reporting at least one symptom 3–18 months post COVID. There was no effect of vaccination on QoL among those not reporting post-acute symptoms (Cohen’s d = − 0.06). Overall, the mean EQ-5D UI was 0.82 (SD = 0.26) and 0.83 (SD = 0.25) among the double and triple vaccinated respectively, compared to 0.76 (SD = 0.33) and 0.78 (SD = 0.31) among those who had received 0 or 1 dose, respectively (Table 2). The overall size of the effect of being vaccinated at the time of infection was small (Cohen’s d = 0.2). Among participants reporting at least one post-COVID symptom, those unvaccinated had a mean EQ-5D UI of 0.68 (SD = 0.40) compared with 0.74 (SD = 0.27) and 0.77 (SD = 0.27) for those doubly and triply vaccinated, respectively, a small effect size (Cohen’s d of 0.22 and 0.32 respectively). In all age, gender, and ethnicity subgroups, the double and triple-vaccinated individuals reported higher mean UIs compared to the unvaccinated, with small effect sizes in all strata (Cohen’s d < 0.5, Table 2). The largest effect size of vaccination on QoL was seen among individuals aged 60 and over (Cohen’s d = 0.4). By time since infection those vaccinated reported higher unadjusted UIs compared to those unvaccinated (0-doses and 1-dose) at 3–6 months, (0.84 ± 0.24 vs. 0.76 ± 0.32, p = 0.019, Cohen’s d = 0.3, Fig. 2). No overall significant difference in UI was found according to vaccination status among those reporting 7–12 months or more than 12 months after their acute SARS-CoV-2 infection.
Association between COVID-19 vaccine and EQ‑5D‑5L UI and patient characteristics
After adjusting for age, ethnicity, hypertension, hospitalization (as a proxy for severity), and duration since testing positive for SARS-CoV-2), SARS-CoV-2-infected individuals vaccinated with 2 or 3 doses reported 0.05 points higher UI compared to those unvaccinated at the time of infection (95%CI = 0.01–0.10, p = 0.024, Table 3). Compared with those not vaccinated at the time of infection, the double-vaccinated reported an overall 0.06 points higher mean QoL score post-infection (95%CI = 0.004–0.11, p = 0.036, Table 3), but the mean UI in those triply-vaccinated was not significantly different (+ 0.05, 95%CI = -0.01–0.10, p = 0.096, Table 3). When hospitalization was removed from the model, we found that vaccination was associated with a 0.06 point (p = 0.011) higher UI among those vaccinated with two doses or more compared to those not (supplementary Table s3).
When restricting the analysis to those experiencing ongoing post-COVID symptoms and after adjustment for potential confounders, participants who received two or three doses reported 0.08 higher UI compared to those unvaccinated at the time of infection (CI = 0.02–0.14, p = 0.013). When stratifying by the number of doses received, the association was only statistically significant with three doses (+ 0.09, 95%CI = 0.02–0.16, p = 0.024, Table 3). When removing hospitalization out of the model, elevation in UIs overall among the vaccinated compared to the unvaccinated at the time of infection were 0.08 (p = 0.012), 0.07 (p = 0.059) and 0.08 (p = 0.016) overall and for two and three or more doses respectively (supplementary Table s3).
Association between COVID-19 vaccine and EQ‑5D‑5L UIs at different time points post-SARS-CoV-2 infection
Among participants who answered the survey between 3- and 6-months post-SARS-CoV-2 infection, before adjusting for confounders, those who received 2 or 3 doses of vaccine reported a 0.08-point higher QoL (95%CI 0.03–0.14, p < 0.003). The effect size was lower, and the association was no longer statistically significant after adjusting for confounders significant in the univariate analysis (+ 0.03, 95% CI = − 0.03–0.10, p = 0.303, Table 4). When adjusting for all confounders except hospitalization, vaccination was associated with a 0.07-point higher EQ-5D-UI (p = 0.036, supplementary Table s3). Conversely, when adjusting for hospitalization only, the association between vaccination and post-COVID QoL 3–6 months post-infection was not significant (+ 0.08 points, p = 0.303). Among those reporting 7–12 months post-infection, there was no overall association between COVID-19 vaccination and mean EQ‑5D‑5L UI, however among those reporting post-acute symptoms, double and triple vaccinated participants reported 0.15 higher mean UI compared to those unvaccinated at the time of infection (95% CI 0.02–0.29, p = 0.024, Table 4). We did not detect any association between COVID-19 vaccination and mean EQ‑5D‑5L UI among participants reporting beyond 12 months post-SARS-CoV-2 infection, whether taking acute disease severity into account or not (Table 4).
Discussion
To our knowledge, this is the first study that investigates the long-term impact of COVID-19 vaccination on QoL outcomes among individuals previously infected with SARS-CoV-2. After adjusting for potential confounders, we found that being vaccinated with 2 or more doses of COVID-19 vaccine at the time of infection was associated with higher QoL post-SARS-CoV-2 infection, more so among individuals experiencing post-COVID symptoms. The size of the effect identified was small and time limited. These results suggest that, overall, COVID-19 vaccination, or at least with the BNT162b2 vaccine widely used in Israel, may partly mitigate losses of QoL post-acute COVID-19, as measured by the EQ-5D-5L, at least in the first 12 months. The small effect size suggests that while vaccines should be considered as part of an array of tools and approaches to mitigate long COVID, it is not a silver bullet against the QoL life loss associated with post-acute COVID symptoms. We could not find a minimally clinically important difference (MCID) in EQ-5D for patients with post-viral symptoms. While evidence from other diseases suggest that a change in UI as small as 0.03 can be clinically important22,MCIDs are subjective and not easily transferrable from one clinical condition to another. MCIDs in EQ5D for post viral diseases are needed to interpret the clinical relevance of changes in QoL following interventions (vaccines or otherwise) intending to mitigate Long COVID. . In our cohort, the severity of the initial COVID-19 illness (measured by hospitalization) was the most important confounding factor associated with QoL in the post-COVID period. In the 3–6 months following acute infection, hospitalization explained most of the association between vaccination and QoL. This suggests that the well documented effectiveness of COVID-19 vaccination against severe acute disease,9 also impact of post-acute symptoms, since severity of acute disease is a strong predictor of post-acute COVID symptoms23. In other words, COVID-19 vaccination mitigates the loss of QoL associated with post-acute COVID symptoms by reducing the severity of the acute illness, which in turn reduces the likelihood and severity of ongoing, post-acute disease. Our results also suggest that this may not be the only mechanism of action: overall and among those reporting 7–12 months post-acute infection and reporting post-acute COVID symptoms, those triply vaccinated reported a higher QoL compared to those unvaccinated, even after adjusting for hospitalization. These findings suggest that even in instances where vaccinated patients report post-acute symptoms, and after taking disease severity into account, the impact of these symptoms on QoL is less than among those who are unvaccinated. There was no significant association between vaccination and QoL among those reporting 12 months or more post-infection. While we refrain from statistically analysing trends in UI over time because patients are different at each time point, this regression towards the UI of those unvaccinated suggests that the positive effect that vaccination may have on QoL may wane over time. This hypothesis should be tested more formally with longitudinal studies. Waning of COVID-19 vaccine effectiveness against reinfection and severity of symptoms of acute COVID-19 illness has been reported previously24, 25. Our findings suggest that booster doses may be required to offer continued mitigation against the post-acute effects of SARS-CoV-2 infection, although the data presented here cannot answer with any level of certainty whether this is the case.
To a large extent, the demographic characteristics of our study participants approximated that of the Israeli population in terms ethnicity and age distribution and reflected the national vaccine roll out strategy which targeted individuals older than 50 years first. The lower proportion of vaccinated patients reporting post-acute symptoms, and the lower proportion of vaccinated patients being hospitalized is also compatible with the existing literature9, 13,14,15. The most prevalent post-COVID symptoms in our cohort (supplementary Table s1) were similar to the symptoms of post-COVID condition frequently reported in the literature6, 16.
The study faced several limitations. Measured outcomes in the study were self-reported, therefore the possibility of reporting bias is a concern. In addition, our results are not generalisable to other COVID-19 vaccines as the population we reported on in this study were predominantly vaccinated with BNT162b2 vaccine and we did not determine which SARS-CoV-2 variant individuals were infected with. Furthermore, our study reports results from a cross-sectional study, therefore it was not possible to adequately compare the impact of COVID-19 vaccination on QoL over time. Consequently, caution should be taken while interpreting time trends results of COVID-19 vaccines presented in this study. The small numbers of individuals who received three doses and answered the survey more than 6 months post-infection was small, limiting the power of our dose-specific analysis. Finally, in the absence of a suggested minimally clinically important difference for this type of clinical presentation, it is difficult to extrapolate to what extent the changes in reported QoL scores translate clinically.
Conclusions
Results from our study suggest that among individuals previously infected with SARS-CoV-2 virus, QoL in those unvaccinated at the time of infection was significantly lower than that in those vaccinated at the time of infection. COVID-19 vaccination, or at least vaccination with BNT162b2, can therefore modestly mitigate the decrease in QoL associated with symptoms of post-COVID illness, at least in the first 12 months. This mitigation could be largely explained by the reduction in severe acute illness associated with vaccination, but also by reducing the impact of post-acute COVID-19 symptoms on QoL. We could only detect positive associations between vaccination and QoL in those reporting up to 12 months following SARS-CoV-2 infection, but not beyond. Longitudinal studies are required to understand with more precision and certainty how symptoms post COVID-19 can affect QoL over time, and the role of vaccines and boosters in mitigating the long-term post-acute effects of SARS-CoV-2 infection. With Long COVID looking to become a durable public health issue affecting the quality of life of millions around the globe, studies estimating the MCID for Long COVID will help better understand the impact of interventions aimed at mitigating the effects of the disease.
Methods
Study design and participants
We invited individuals aged 18 years and older whose COVID-19 reverse transcription polymerase chain reaction (RT-PCR) test was done between 15th March 2020 and 15th June 2022 in one of three government hospitals in Northern Israel (Ziv Medical Centre, Padeh-Poriya Medical Centre, Galilee Medical Centre) to participate in the study. We included hospitalized patients and community patients whose PCR test was processed at a hospital laboratory. Participants recruitment and data collection has been described previously14. Briefly, using patient telephone records, eligible participants were invited to participate between July 2021 and June 2022, through a Short Message Service (SMS) with a link to an online survey available in four commonly spoken languages in Israel: Hebrew, Arabic, Russian, and English. In the current study, analysis was restricted to participants who reported having tested positive for SARS-CoV-2 by RT-PCR. We categorised participants according to the number of vaccine doses they received and then compared the groups according to their vaccination status in terms of reported QoL outcomes 3–18 months following their infection, both overall and among those reporting post-acute symptoms.
Measurement tools
We used the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) COVID-19 follow-up tool26 and adapted it to the Israeli context. The questionnaire included the EQ-5D-5L tool, a widely used validated instrument for QoL measurement based on 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is measured on a score scale from 1-(high QoL) to 5-(low QoL)27. A composite utility index (UI) was then generated, using country-specific weighting28. UI can range from 1 (complete health) to less than 0, acknowledging that extremely poor health statuses can lead to a QoL worse than death29.
Data sources and variables
Baseline characteristics
Baseline characteristics recorded in the questionnaire included socio-demographics (marital status, age, sex, religion, ethnicity, and level of education), comorbidities (hypertension, diabetes, asthma, and COPD), and details about the acute COVID-19 episode including history of hospitalisation and intensive care admission.
Exposure groups
We categorised participants according to the number of COVID-19 vaccine doses received (0, 1, 2, or 3). In Israel, in the early phases of COVID-19 vaccination roll out, infected individuals were only eligible for a single dose. As a result, in our study, almost 90% of participants who received a single dose of vaccine were vaccinated after infection and were therefore not vaccinated at the time of infection. In the final analysis, we therefore grouped those who had received a single dose together with participants who reported not receiving any COVID-19 vaccine dose as a single group unvaccinated at the time of infection. Because Israel almost exclusively used the BNT162b2 vaccine in the study period, results apply to this vaccine only.
Assessment of symptoms of post-COVID disease
Participants were asked to select from a list of 39 symptoms which ones they were experiencing in the week prior to answering the survey. Participants who reported experiencing at least one of the ten most common symptoms were classified as experiencing post-acute COVID symptoms. To avoid misclassification between prolonged acute disease and post-acute symptoms, participants who reported symptoms in the first 60 days following their reported positive PCR test were excluded from the analysis.
Outcome: QoL
We measured participants’ QoL at the time of answering the survey using the EQ-5D-5L instrument27. The EQ-5D is a generic instrument for measuring quality of life. The instrument is based on a 5-level Likert scale descriptive system that measures health in 5 dimensions including: mobility, self-Care, usual activities, pain or discomfort, and anxiety/depression. Since no Israel-specific EQ-5D value set exists, following recommendations from the EuroQol Research Foundation, we computed the UI score using the USA EQ-5D value set.
Statistical analysis
We described participant characteristics at baseline using means and standard deviations and proportions for continuous and categorical variables respectively. Two-sided t-tests were used to test the differences between group means and chi-square tests to compare proportions between groups. We computed the proportions of patients reporting specific scores for each of the 5 dimensions of the EQ-5D according to the number of vaccine doses received and presented the findings graphically. The mean QoL UIs with corresponding standard deviations (SDs) were computed for the included participants according to age, sex, ethnicity, vaccination status, time periods, education level, marital status, chronic illnesses status, and presence of post-COVID symptoms. We estimated the size of the effect of vaccination on Quality of life by calculating the standardized mean difference (Cohen’s d) between those vaccinated at the time of infection ( with 2 + doses) and those not (received 0 or 1 dose) among each stratum in our group according to age, gender, ethnicity, presence of post-acute symptoms and time since infection. In line with consensus thresholds we classified effect sizes as small (d = 0.2), medium (d = 0.5), and large (d ≥ 0.8).30.
We determined associations between vaccination status and post-COVID QoL using ordinary least square (OLS) linear regression, using a model adjusting for potential confounders. Variables considered in the model were those significant in the univariate analysis or deemed important in the literature and included time since SARS-CoV-2 infection (3–6 months, 7–12 months, and more than 12 months up to 18 months), number of COVID-19 vaccine doses received at the time of infection, presence of hypertension (the only underlying condition significantly different among those vaccinated and those not in the univariate analysis), age, sex, ethnicity and hospitalization. Because vaccination is associated with a reduction in severe disease9 and because severity of the acute episode is associated with post-COVID condition25, we ran the model both including and excluding hospitalization during the acute COVID-19 episode (as a proxy for disease severity), to determine whether any changes in QoL resulted from a decrease in acute disease severity or otherwise. We compared individuals vaccinated with 2 and 3 doses with individuals unvaccinated at the time of infection (either having received 0 doses or 1 dose after their infection) in terms of changes in reported QoL UI, together with 95% confidence intervals (95% CI). The regression analysis was then repeated stratified by the duration of time elapsed between vaccination and QoL reported: 3–6 months, 7–12 months, and 13–18 months. It is important to note that each time point includes different participants and we therefore do not directly test for trends over time.
Ethical approval
The study was conducted in compliance with all relevant guidelines and regulations according to good clinical practice (GCP). All patients provided informed consent prior to participating in the study. The study was approved by the ethical committees of each of the three participating hospitals, namely Ziv Medical Centre, Padeh-Poriya Medical Centre, and Galilee Medical Centre ethical committees, reference numbers; 0007-21-ZIV, 009-21-POR, and 0018-21-NHR, respectively.
Data availability
The dataset will be made available upon reasonable request to the authors. To request the dataset for secondary use please contact michael.edelstein@biu.ac.il.
References
Soriano, J. B., Murthy, S., Marshall, J. C., Relan, P. & Diaz, J. V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet. Infect. Dis. 22(4), 102–107. https://doi.org/10.1016/S1473-3099(21)00703-9 (2022).
Ballering, A. V., van Zon, S. K., Olde Hartman, T. C. & Rosmalen, J. G. Lifelines corona research initiative. persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. The Lancet. 400(10350), 452–461 (2022).
Castanares-Zapatero, D. et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 54(1), 1473–1487. https://doi.org/10.1080/07853890.2022.2076901 (2022).
Yong, S. J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 53(10), 737–754. https://doi.org/10.1080/23744235.2021.1924397 (2021).
Saunders, C., Sperling, S. & Bendstrup, E. A new paradigm is needed to explain long COVID. Lancet. Respir. Med. https://doi.org/10.1016/S2213-2600(22)00501-X (2023).
Michelen, M. et al. Characterising long COVID: a living systematic review. BMJ Glob. Health 6(9), e005427 (2021).
Adler, L. et al. Long-COVID in patients with a history of mild or asymptomatic SARS-CoV-2 infection: a Nationwide Cohort Study. Scand. J. Prim. Health Care. 29, 1–8. https://doi.org/10.1080/02813432.2022.2139480 (2022).
Khaswal, A., Kumar, V. & Kumar, S. Long-term health consequences of SARS-CoV-2: Assumptions based on SARS-CoV-1 and MERS-CoV infections. Diagnostics. 12(8), 1852. https://doi.org/10.3390/diagnostics12081852 (2022).
Feikin, D. R. et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 399(10328), 924–944. https://doi.org/10.1016/S0140-6736(22)00152-0 (2022).
Edouard Mathieu, Hannah Ritchie, Lucas Rodés-Guirao, et al. "Coronavirus Pandemic (COVID-19)". Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/coronavirus' [Online Resource]. Accessed December 29th, (2022)
Hamzah, F. B. et al. CoronaTracker: worldwide COVID-19 outbreak data analysis and prediction. Bull World Health Organ. 1(32), 1–32. https://doi.org/10.2471/BLT.20.255695 (2020).
Lin, D. Y. et al. Effectiveness of bivalent boosters against severe omicron infection. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2215471 (2023).
Notarte, K. I. et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. E Clin. Med. 53, 101624. https://doi.org/10.1016/j.eclinm.2022.101624 (2022).
Kuodi, P. et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020–21. Israel. Npj Vaccines 7, 101. https://doi.org/10.1038/s41541-022-00526-5 (2022).
Gao, P., Liu, J. & Liu, M. Effect of COVID-19 vaccines on reducing the risk of long COVID in the real world: A systematic review and meta-analysis. Int. J. Environ. Health Res. 19(19), 12422. https://doi.org/10.3390/ijerph191912422 (2022).
Davis, H. E. et al. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146. https://doi.org/10.1038/s41579-022-00846-2 (2023).
Malik, P. et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (QoL)—A systematic review and meta-analysis. J. Med. Virol. 94(1), 253–262. https://doi.org/10.1002/jmv.27309 (2022).
Figueiredo, E. A. et al. The health-related quality of life in patients with post-COVID-19 after hospitalization: A systematic review. Rev. Soc. Bras. Med. Trop. 28, 55. https://doi.org/10.1590/0037-8682-0741-2021 (2022).
Halpin, S. J. et al. Post discharge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 93(2), 1013–1022. https://doi.org/10.1002/jmv.26368 (2021).
Poudel, A. N. et al. Impact of Covid-19 on health-related quality of life of patients: A structured review. PLoS ONE 16(10), e0259164. https://doi.org/10.1371/journal.pone.0259164 (2021).
CBS, ICBD report-Israel in Figures, 2021—Selected data from the statistical abstract of Israel, 2021. https://www.cbs.gov.il/en/Pages/search/searchResultsIsraelnFigures.aspx. Accessed December 29th, (2022)
Coretti, S., Ruggeri, M. & McNamee, P. The minimum clinically important difference for EQ-5D index: A critical review. Expert Rev. Pharmacoecon. Outcomes Res. 14(2), 221–233. https://doi.org/10.1586/14737167.2014.894462 (2014).
Righi, E. et al. Determinants of persistence of symptoms and impact on physical and mental wellbeing in Long COVID: A prospective cohort study. J. Infect. 84(4), 566–572. https://doi.org/10.1016/j.jinf.2022.02.003 (2022).
Menni, C. et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet. Infect. Dis. https://doi.org/10.1016/S1473-3099(22)00146-3 (2022).
Patalon, T. et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 13, 3203. https://doi.org/10.1038/s41467-022-30884-6 (2022).
ISARIC. ISARIC Covid-19 long term follow-up study Oxford, UK, 2020. Available: https:// isaric. org/ research/ covid- 19- clinical- research- resources/ covid- 19- long- term- follow- up- study/ 19 ISARIC. Accessed December 30th, 2022.
Herdman, M. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20, 1727–1736. https://doi.org/10.1007/s11136-011-9903-x (2011).
Devlin, N., Parkin, D. (2007). Guidance to users of EQ-5D value sets. In: SZENDE, A., OPPE, M., DEVLIN, N. (eds) EQ-5D Value Sets. EuroQol Group Monographs, Vol 2. Springer, Dordrecht. https://doi.org/10.1007/1-4020-5511-0_4
McClure, N. S., Al Sayah, F., Xie, F., Luo, N. & Johnson, J. A. Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. Value Health. 20(4), 644–650. https://doi.org/10.1016/j.jval.2016.11.015 (2017).
Sullivan, G. M. & Feinn, R. Using effect size-or why the P value is not enough. J. Grad. Med. Educ. 4(3), 279–282. https://doi.org/10.4300/JGME-D-12-00156.1 (2012).
Acknowledgements
We wish to thank Yehudit Hakmon, Eliran Levi, Zion Levi, Nissim Neeman (Ziv Medical Centre), Mark Lifishitz, and Shelly Shalem (Galilee Medical Centre) for their technical help and support in disseminating the questionnaire.
Funding
This study was partly funded from a donation from the Harvey Goodstein Charitable Foundation. The funder had no role in the writing of the manuscript or the decision to submit for publications. The funding enabled to fund software licenses for statistical software, SMS packages used for sending invitations to participants, and will fund the open access publication fees. The authors were not paid to write this article by a pharmaceutical company or any other agency. The authors were not precluded from accessing data in the study and accept responsibility to submit for publication.
Author information
Authors and Affiliations
Contributions
M.E. conceptualised the original research idea, acquired the funding, designed the methodology, supervised the analysis and interpretation of the results, and critically revised the manuscript. P.K. formally analysed the data, interpreted the findings, and wrote the first version of the manuscript. Y.G. coordinated data management and curation across the data collection sites. J.E. contributed to formal analysis. H.Z., O.W., K.B.W., K.A.J., A.D., S.N., J.E., and D.G. coordinated the data collection and curated the data from their respective hospitals and provided input on the methodology and clinical interpretation of the findings. All authors critically reviewed and edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuodi, P., Gorelik, Y., Zayyad, H. et al. Association between BNT162b2 vaccination and health-related quality of life up to 18 months post-SARS-CoV-2 infection in Israel. Sci Rep 13, 15801 (2023). https://doi.org/10.1038/s41598-023-43058-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43058-1
- Springer Nature Limited