Abstract
Male genital schistosomiasis (MGS) is hypothesized to increase seminal shedding of HIV-1. This prospective pilot study assessed seminal HIV-1 RNA shedding in men on long-term ART with and without a diagnosis of MGS. Study visits occurred at 0, 1, 3, 6 and 12 months. MGS was diagnosed by egg positivity on semen microscopy or PCR of seminal sediment. After optimization of the HIV-RNA assay, we examined 72 paired plasma and semen samples collected from 31 men (15 with and 16 without MGS) over 12 months. HIV-1 RNA was detected in 7/72 (9.7%) seminal samples and 25/72 (34.7%) plasma samples. When comparing sample pairs, 5/72 (6.9%) showed HIV-1 RNA detection only in the seminal sample. Overall, 3/31 (9.7%) participants, all with MGS, had detectable HIV-1 RNA in semen while plasma HIV-1 RNA was undetectable (< 22 copies/mL), with seminal levels ranging up to 400 copies/mL. Two participants showing HIV-1 RNA in seminal fluid from the MGS-negative group also had concomitant HIV-1 RNA detection in plasma. The findings suggest that MGS can be associated with low-level HIV-1 RNA shedding despite virologically suppressive ART. Further studies are warranted to confirm these observations and assess its implications.
Similar content being viewed by others
Introduction
Three-quarters of the global burden of HIV-1 infection resides within sub-Saharan Africa1 which also bears a disproportionately high burden of neglected tropical diseases. Here, schistosomiasis, a parasitic infection caused by water-borne blood flukes2, afflicts some 180 million people whereas an estimated 6 million are living with HIV-13,4. Although levels of co-infection of schistosomiasis and HIV are not accurately reported, an increased risk of HIV-1 acquisition may be assumed in those with underlying schistosomiasis5.
Indeed, HIV-1 prevalence is often raised in fishing communities where schistosomiasis is prevalent6,7. In endemic areas, there is a higher prevalence of HIV-1 infection in women with female genital schistosomiasis (FGS), and schistosomiasis has been shown to increase the risk of HIV-1 acquisition by a factor of three8,9,10. This association is due to genital mucosal breach, neovascularisation, and increased density of HIV receptive CD4 + cells in women with FGS. Genital schistosomiasis has also been associated with increased seminal levels of interleukin (IL)-4, IL-6, IL-10, and tumour necrosis factor-alpha in men with seminal egg excretion11. However, there are limited data on the relationship between male genital schistosomiasis (MGS) and HIV-1 infection among men. A study in Zimbabwe examined four antiretroviral therapy (ART)-naïve men living with HIV-1 and demonstrated a decline in seminal HIV-1 RNA levels (by a median of 0.6 log10 copies/mL) 10 weeks after treatment with praziquantel (PZQ), the only available medicine against schistosomiasis12.
ART is highly effective in suppressing HIV-1 replication in both plasma and genital tract, reducing the risk of HIV-1 transmission through both heterosexual and homosexual intercourse. Prospective studies of HIV-discordant couples where the partner living with HIV was established on virologically suppressive ART (defined as plasma HIV-1 < 200 copies/mL) showed no HIV-1 transmission despite condomless sex13,14,15,16. Nonetheless, detection of HIV-1 RNA in seminal fluid has been previously reported in men receiving virologically suppressive ART17,18. One explanation is that HIV-1 RNA suppression occurs more slowly in seminal fluid than in plasma and thus seminal shedding may be relatively common in the early phase after ART initiation19. Furthermore, it has been hypothesised that like sexually transmitted and other genital infections, MGS may promote genital shedding of HIV-1 RNA despite effective ART due to chronic egg-induced inflammation of the genital tract20.
In Malawi, within a population of 19.9 million, 990 000 (5%) were estimated to be living with HIV-1 in 202121. Whilst epidemiological data is generally heterogenous across sub-Saharan Africa, in 2015, one study in Malawi reported schistosomiasis prevalence of 47.4% in communities around its lakeshores and other environmental water bodies22. The aim of this pilot study was to assess the extent of any putative relationship between MGS and seminal HIV-1 RNA shedding among men living with HIV-1 and receiving long-term ART in Malawi.
Methods
Population and sampling
Study participants were heterosexual men ≥ 18 years of age living with HIV-1 who attended HIV outpatient services along Lake Malawi between October 2017 and December 2018 (Fig. 1). Mid-morning urine, semen and whole blood in EDTA were to be collected at each planned study visit (0, 1, 3, 6 and 12 months). The sampling methodology was reported previously23 and is described in Supplementary Information. Seminal samples were collected in a clear plastic bag following two days of abstinence from sexual activity. In total, 74 participants enrolled, of which 23 did not submit blood or urine samples for schistosomiasis testing. Of the remaining 51, 33 tested negative for schistosomiasis, of whom 16 provided blood, urine, and semen. This cohort of 16 was deemed the ‘MGS-negative group'. Of the 18 participants that tested positive for schistosomiasis, 15 provided blood, urine, and semen. This cohort of 15 was deemed the ‘MGS-positive group'. The disposition of the whole study population is shown in Supplementary Fig. 1. Given the high prevalence of schistosomiasis in the region22, participants received PZQ at all study visits regardless of a diagnosis of MGS.
Schematic map of study area showing health facilities along the shores of Lake Malawi (https://www.cia.gov/library/publications/the-world-factbook/attachments/locator-maps/MI-locator-map.gif and https://www.cia.gov/library/publications/the-world-factbook/attachments/maps/MI-map.gif)24.
Laboratory procedures
Samples were processed within 3 hours of collection. Whole blood samples were centrifuged at 3000 xg for 5 minutes to separate plasma for storage at −80°C. Seminal samples were allowed to liquefy at ambient temperature and examined under microscopy for the presence of Schistosoma eggs, followed by centrifugation at 3000 xg for 5 minutes to separate supernatant seminal fluid and sediments. Seminal fluid was stored at −80°C. Seminal sediment samples were re-dissolved in saline and 2–3 drops were placed on a glass slide for microscopy to detect Schistosoma eggs. Leftover seminal sediments were preserved in ethanol and shipped to the Elisabeth-TweeSteden Ziekenhuis (ETZ) Hospital in Tilburg, the Netherlands, for Schistosoma DNA detection by in-house real-time PCR as previously described23. The parasitological procedure was previously detailed23 and is described in Supplementary Information. Urine samples were tested for Schistosoma circulating cathodic antigen (CCA) using the point-of care parasite CCA test (POC-CCA) (Rapid Medical Diagnostics, South Africa), followed by urine filtration and microscopy for Schistosoma eggs. Cryopreserved seminal fluid and plasma samples were shipped on dry ice to the United Kingdom for HIV-1 RNA testing.
Schistosomiasis diagnosis and definition of MGS
Schistosoma positivity was defined by visual evidence of eggs by microscopy of filtrated urine, semen, or seminal sediment; PCR detection in seminal sediment, urine or stool; or positive POC-CCA test in urine. Male genital schistosomiasis (MGS) was defined as Schistosoma positivity in semen.
HIV-1 RNA testing
HIV-1 RNA was detected in plasma and seminal fluid using the Cepheid Xpert assays25. The assays perform qualitative (HIV-1 Qual assay) and quantitative (HIV-1 Viral Load assay) detection of HIV-1 RNA in plasma26. With 1 mL input, the HIV-1 Qual assay reports qualitative HIV-1 RNA detection with a lower limit of detection (LLOD) of 278 copies/mL; the manufacturer describes 25% detection rate at 60 copies/mL27,28. With 1mL input, the HIV-1 Viral Load assay has a lower limit of quantification (LLOQ) of 40 copies/mL and a LLOD of 22 copies/mL26. To detect HIV-1 RNA in seminal fluid, the assays were first validated using seminal fluid samples collected from two HIV negative donors and spiked with known amounts of HIV-1 RNA, as detailed in Supplementary Information. The assay LLOD for seminal fluid ranged from 55 to 220 copies/mL according to the dilution applied. HIV-1 RNA results were reported as either a quantified level or as qualitative targeted detected.
Statistical analyses
Baseline characteristics were described as categorical and continuous variables and compared using Mann–Whitney–Wilcoxon test (continuous variables) or Fisher’s exact test (categorical variables). Analyses was performed using IBM SPSS Statistics (version 27) and GraphPad Prism version 8.0.0.
Ethical considerations
Ethical approval was provided by the National Health Sciences Research Committee (NHSRC) of Malawi (Approval No.: 1805) and the Liverpool School of Tropical Medicine Research Ethics Committee (Approval No.: 17-018). Participants provided written informed consent to be recruited and participate in the study in accordance with the Declaration of Helsinki. All research methods were performed in accordance with relevant guidelines and regulations. All participants reserved the right to opt-out at any stage of the study.
Results
Study population and sampling
A total of 31 participants established on ART provided at least one paired semen and blood sample over the 12 months of the study. Their baseline characteristics are summarised in Table 1. At study entry, participants had received ART for a median of 7.5 years (IQR 1.9–13.1). Most were receiving coformulated tenofovir disoproxil fumarate/lamivudine/efavirenz (TDF/3TC/EFV). A total of 72 paired samples were collected, with a median of 2 paired samples per participant (range 1–5), including 30 samples at study entry (baseline), and 10, 12, 9 and 11 samples at 1, 3, 6 and 12 months. Eleven participants donated only one set of paired samples. Overall, based on the date of the last paired samples, the median duration of follow-up was 10.2 months (IQR 4.0–14.2). All except 6 participants (one with MGS and 5 without) had received PZQ in the 12 months prior to recruitment. None showed symptoms suggestive of sexually transmitted infections (STIs) and no samples were collected to investigate asymptomatic STIs.
MGS status
Details of parasitology testing are summarised in Table 2. At baseline, 8 participants tested MGS positive by semen microscopy, 4 tested positive only by real-time PCR of seminal sediment, and 20 tested negative by all tests. Among the 20 participants with a negative baseline test, 3 had a positive test during follow-up (1 by real-time PCR only; 1 by PCR and POC-CCA test and 1 by urine filtration, PCR and POC-CCA test) yielding a total of 15 participants who were classed as MGS positive (A01–A15), whereas 16 were classed as MGS negative (A16–A31). There were no significant differences when comparing the baseline characteristics of the two groups (Table 1). During the study period, 42 paired samples were provided by the MGS positive participants while 30 samples were provided by MGS negative participants.
HIV-1 RNA testing
Plasma
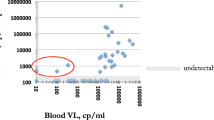
At baseline, 5/30 (16.7%) participants showed quantifiable plasma HIV-1 RNA (\(\ge\) 40 copies/mL) including 3/14 (21.4%) in the MGS-positive group (A09, A11, A15) and 2/16 (12.5%) in the MGS-negative group (A21, A30) (Table 3). HIV-1 RNA levels ranged from 41 to 56,000 copies/mL During follow-up, 1/5 participants (A09) achieved suppression < 40 copies/mL at 6 months, while continuing to show detectable HIV-1 RNA below the assay LLOQ of 40 copies/mL (estimated 22–39 copies/mL); 1/5 (A11) showed persistent viraemia between 816 and 277 copies/mL at 6 and 12 months; and 3/5 had no follow-up samples collected. A further 4 participants showed detectable plasma HIV-1 RNA below the assay LLOQ of 40 copies/mL (estimated 22–39 copies/mL) at baseline, including 2/14 (14.3%) in the MGS-positive group (A07, A13) and 2/16 (12.5%) in the MGS-negative group (A16, A23). Among 21 participants with undetectable HIV-1 RNA at baseline, two in the MGS-negative group showed plasma viral load rebound > 40 copies/mL at 1 month (A17; 64 copies/mL) and 3 months (A20; 107,000 copies/mL) respectively, followed by resuppression < 40 copies/mL) but detectable HIV-1 RNA at 12 months (Table 3).
Seminal fluid
Among participants with baseline samples, 3/30 (10.0%) showed detectable HIV-1 RNA in seminal fluid (Table 3). In the MGS-positive group, 2/14 (14.3%) participants (A03, A04) showed detectable HIV-1 RNA (estimated levels between 55 and 400 copies/mL) in seminal fluid, while plasma HIV-1 RNA was undetectable (< 22 copies/mL). One of the two had no follow-up samples. The other showed undetectable HIV-1 RNA in both plasma and seminal fluid at months 1, 3 and 12. In the MGS-negative group, 1/16 (6.3%) participants (A21) showed seminal HIV-1 RNA levels of 4840 copies/mL, but in this case plasma HIV-1 RNA levels were also high at 26,900 copies/mL. A total of 20 participants had at least one follow-up sample (Table 3). Among these, 2 participants (A05, A20) showed newly detectable HIV-1 RNA in seminal fluid. One participant (A05) in the MGS-positive group had persistent HIV-1 RNA in seminal fluid at months 1, 3 and 6, with levels ranging up to 123 copies/mL, while plasma HIV-1 RNA remained persistently undetectable (< 22 copies/mL). The second participant (A20) was in the MGS-negative group. At 3 months, HIV-1 RNA levels were 528 copies/mL in seminal fluid while plasma HIV-1 RNA had rebounded from undetectable to 107,000 copies/mL. Overall, 7/72 (9.7%) seminal fluid samples had detectable or quantifiable HIV-1 RNA, comprising 3 samples at baseline (2 in the MGS-positive group) and 1, 2 and 1 samples at 1, 3 and 6 months, respectively (3 in the MGS-positive group).
Patterns of HIV-1 RNA detection by MGS status
When comparing the 72 paired plasma and seminal samples, HIV-1 RNA detection (with or without quantification) was concordant in 44/72 (61.1%) samples (Table 4). The remaining 28/72 (38.9%) sample pairs were discordant; most had HIV-1 RNA detected in plasma only. In the MGS-negative group, 2/16 participants had detectable HIV-1 RNA in 2 seminal samples, and in both cases the paired plasma showed high HIV-1 RNA levels. In contrast, in the MGS-positive group, 3/15 participants had detectable HIV-1 RNA in 5 seminal samples, and in all cases the paired plasma showed undetectable HIV-1 RNA. The characteristics of the 3 participants are detailed in the Table 5. They had been established on ART long-term, ranging between 8 and 12 years at study entry. Overall, 4 of the 5 seminal samples with detectable HIV-1 RNA had concomitant Schistosoma test positivity.
Discussion
Across sub-Saharan Africa, evidence of the impact of MGS on HIV-1 shedding in semen is limited despite the significant epidemiological overlap between HIV-1 infection and endemic schistosomiasis29. We sought to assess prospectively over 12 months, the rates and kinetics of seminal HIV-1 RNA shedding in heterosexual men living with HIV-1 and established on first-line NNRTI-based ART in Malawi, stratifying the data according to a diagnosis of MGS. In our pilot study, we observed low-level HIV-1 RNA shedding in seminal fluid of men with MGS despite fully suppressed plasma HIV-1 RNA, whereas in those without an MGS diagnosis seminal HIV-1 RNA shedding was always concomitant with high-level viremia. Our numbers are limited. In total, we observed HIV-1 RNA detection in 5 seminal samples from 3 of 15 participants in the MGS group. Larger studies are needed to confirm these interesting observations.
Urogenital schistosomiasis is thought to be similar to STIs such as HSV-2 in facilitating HIV-1 transmission; this is due to presence of mucosal lesions and local recruitment of HIV-susceptible cells through egg-induced inflammation3. Collectively, this is expected to increase the risk of HIV acquisition and propagation by increasing viral replication. Whilst data reporting the impact of urogenital schistosomiasis on HIV-1 shedding in semen are scarce, one study showed a decrease in seminal HIV-1 RNA levels following treatment with PZQ during a follow-up period of 10 weeks12.
Virologically effective ART reduces the risk of transmitting HIV sexually via both heterosexual and homosexual routes14,15,30. One question remains as to the relevance of the reported delay in HIV-1 RNA suppression in seminal fluid relative to plasma in the early phase of ART. In our study, participants entered the analysis while already established on ART with a median ART duration of 7.5 years (IQR 1.9–13.0). In this population established on long-term ART, 5/31 (16.1%) participants had at least one episode of seminal HIV-1 RNA detection over 12 months of follow-up. Two of the participants were negative for Schistosoma and had concomitant high HIV-1 RNA detection in plasma. Among participants positive for Schistosoma, 3 were established on ART with TDF/3TC/EFV for 8–12 years and had fully suppressed, undetectable HIV-1 RNA in plasma, yet they showed detectable HIV-1 RNA in a total of 5 seminal samples. It is important to highlight that the levels of seminal HIV-1 RNA in these 5 samples were low and never exceeded an estimated 400 copies/mL, despite 4 of the 5 samples coinciding with a positive Schistosoma test. The implications in terms of risk of HIV-1 transmission are uncertain.
As a pilot study, we acknowledge several limitations. The study population was small and participants did not provide samples at all scheduled time points. We had small seminal sample volumes which constrained our ability to use a high input volume in the HIV-1 RNA tests. Due to lack of samples, we did not attempt to perform HIV drug resistance testing when HIV-1 RNA was detected and we did not investigate the concomitant occurrence of clinically unrecognised STIs.
Conclusion
Taken together, our study found that 3/15 (20%) men with HIV-1 and MGS who were established on NNRTI-based first-line ART for at least 8 years had detectable HIV-1 RNA in 5 seminal samples while plasma HIV-1 RNA was fully suppressed. Seminal HIV-1 RNA shedding coincided with Schistosoma detection in 4 of the 5 samples. However, HIV-1 RNA was detected in seminal fluid at low levels, raising doubts about significance in terms of risk of transmission, particularly if shedding is sporadic or intermittent. We recommend that future studies should aim to (1) evaluate the definitive role of MGS in enhancing HIV-1 RNA shedding in semen during ART; (2) investigate the infectiousness and drug resistance profile of seminal HIV-1 RNA; and (3) determine how the introduction of dolutegravir-based ART across Africa may impact on the findings especially as reports have started to observe treatment failure across populations31,32.
Data availability
All data generated or analysed during this study are included in this manuscript and its supplementary information files. Any additional information can be reasonably requested from the corresponding author.
References
(UNAIDS). JUNPoHA. Global HIV Statistics—2020 Fact sheet. (Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland, 2020).
Colley, D. G., Bustinduy, A. L., Secor, W. E. & King, C. H. Human schistosomiasis. Lancet 383(9936), 2253–2264 (2014).
Downs, J. A. et al. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: A nested case-control study. PLoS Negl. Trop. Dis. 11(9), e0005968 (2017).
WHO. Schistosomiasis Geneva, Switzerland: World Health Organization; 2018 [updated 20th February 2018. Available from: http://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis.
Patel, P. et al. Association of schistosomiasis and HIV infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 102, 544–553 (2020).
Kissling, E. et al. Fisherfolk are among groups most at risk of HIV: Cross-country analysis of prevalence and numbers infected. AIDS 19(17), 1939–1946 (2005).
Seeley, J., Tumwekwase, G. & Grosskurth, H. Fishing for a living but catching HIV: AIDS and changing patterns of the organization of work in fisheries in Uganda. Anthropol. Work. Rev. 30(2), 66–76 (2009).
Jourdan, P. M., Holmen, S. D., Gundersen, S. G., Roald, B. & Kjetland, E. F. HIV target cells in schistosoma haematobium-infected female genital Mucosa. Am. J. Trop. Med. Hyg. 85(6), 1060–1064 (2011).
Kjetland, E. F., Leutscher, P. D. C. & Ndhlovu, P. D. A review of female genital schistosomiasis. Trends Parasitol. 28(2), 58–65 (2012).
Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20.
Leutscher, P. D. C. et al. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium-infected individuals. J. Infect. Dis. 191(10), 1639–1647 (2005).
Midzi, N., Mduluza, T., Mudenge, B., Foldager, L. & Leutscher, P. D. C. Decrease in seminal HIV-1 RNA load after praziquantel treatment of urogenital schistosomiasis coinfection in HIV-positive men—An observational study. Open Forum Infect. Dis. 4(4), ofx199 (2017).
Rodger, A. J. et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. The Lancet 393, 2428–2438 (2019).
Rodger, A. J. et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 316(2), 171–181 (2016).
Cohen, M. S. et al. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: Where do we go from here?. The Lancet. 382(9903), 1515–1524 (2013).
Nelson, J. A. et al. Female genital tract shedding of HIV-1 is rare in women with suppressed HIV-1 in plasma. AIDS 34(1), 39–46 (2020).
Marcelin, A.-G. et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS 22(13), 1677–1679 (2008).
Sheth, P. M. et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS 23(15), 2050–2054 (2009).
Mujugira, A. et al. HIV transmission risk persists during the first 6 months of Antiretroviral therapy. JAMA 72(5), 579 (2016).
Leutscher, P. et al. Community-based study of genital schistosomiasis in men from Madagascar. The Lancet 355(9198), 117–118 (2000).
WHO. Malawi: HIV Country Profile 2022. HIV Country Intelligence. (WHO, Geneva, Switzerland, 2022).
Mtethiwa, A. H., Nkwengulila, G., Bakuza, J., Sikawa, D. & Kazembe, A. Extent of morbidity associated with schistosomiasis infection in Malawi: A review paper. Infect. Dis. Poverty 4, 25 (2015).
Kayuni, S. A. et al. How can schistosome circulating antigen assays be best applied for diagnosing male genital schistosomiasis (MGS): An appraisal using exemplar MGS cases from a longitudinal cohort study among fishermen on the south shoreline of Lake Malawi. Parasitology 146, 1785–1795 (2019).
Kayuni, S. A. et al. Male genital schistosomiasis along the Shoreline of Lake Malawi: Baseline prevalence and associated knowledge, attitudes and practices among local fishermen in Mangochi District Malawi. Front. Public Health 9, 590695 (2021).
Villa, G. et al. Determining virological suppression and resuppression by point-of-care viral load testing in a HIV care setting in sub-Saharan Africa. EClinicalMedicine. 18, 100231 (2020).
Cepheid. Xpert HIV-1 Viral Load United States of America2018 [22/11/2019]. Available from: https://www.cepheid.com/en/cepheid-solutions/clinical-ivd-tests/virology/xpert-hiv-1-viral-load.
Xpert HIV-1 QUAL datasheet. [Internet]. 2018. Available from: https://p.cdn.net/sde8xc/Cepheid-Xpert_hiv_1_qual_Brochure_CE-IVD-3053_English.
WHO. WHO Prequalification of in Vitro Diagnostics. Public report product: Xpert HIV Qual assay WHO reference number: PQDx 0259-070-00. (World Health Organization, Geneva, Switzerland, 2017)
Bustinduy, A. et al. HIV and schistosomiasis co-infection in African children. Lancet. Infect. Dis 14(7), 640–649 (2014).
Cohen, M. S. et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N. Engl. J. Med. 375(9), 830–839 (2016).
van Oosterhout, J. J. et al. Dolutegravir resistance in Malawi’s National HIV treatment program. Open Forum Infect. Dis. 9, ofac148 (2022).
Abdullahi, A. et al. Limited emergence of resistance to integrase strand transfer inhibitors (INSTIs) in ART-experienced participants failing dolutegravir-based antiretroviral therapy: A cross-sectional analysis of a Northeast Nigerian cohort. J. Antimicrob. Chemother. 78, 2000–2007 (2023).
Acknowledgements
Many thanks to the Director of Health and Social Services, District Medical Officers and management team of Mangochi District Health Office for their overwhelming support toward the study; Messrs Bright Mainga, Pilirani Mkambeni, Patrick Hussein, Mkonazi Nkhoma, Matthews Elias, and Boniface Injesi for technical assistance; and all the fishermen who participated in the longitudinal cohort study in Mangochi district. Also grateful to the head and staff of the laboratory department at Mangochi District Hospital; district ART coordinators, in-charge, and staff of Tikondane clinic; in-charges and staff of Billy Riordan Memorial Clinic; Monkey-Bay Community Hospital; Nkope and Koche Health Centres; Lumbua Katenda, Anwar, and staff of Mangochi LMJ clinic; local community health workers: Ambali Makochera, Alfred Kachikowa, Rodgers Wengawenga, Michael Tsatawe, Chikondi Mtsindula, Mambo Amin, Elias Matemba, Austin Kaluwa, Justin Mndala, Promise Mwawa, Dickson Tabu, George Matiki, Alfred Mdoka, and Charles Katandi; Brother Henry Chagoma and staff of Montfort Mission Guest House; and all traditional leaders in the hosting fishing communities and beach committee members for their enthusiasm and support during the study. Much appreciation to the Commonwealth Scholarship Commission (CSC) UK for the Ph.D. scholarship, British Society for Parasitology (BSP) for the International Travel Fellowship and World Friendship Charity for Travel grant to S.A.K. to conduct the study field data collection; and the Ministry of Health, Kingdom of Saudi Arabia for M.H.A. Ph.D. scholarship.
Funding
This work for S.A.K. was funded by the African Research Network for Neglected Tropical Diseases (ARNTD) through United States Agency for International Development (USAID), UK aid from the British people (UK aid) and Coalition for Operational Research on Neglected Tropical Diseases (COR-NTD). The funders had no role in the study design, data collection or analysis; or in the decision to submit this for publication. The contents in this publication are solely the responsibility of the authors and do not necessarily represent the views of ARNTD, COR-NTD, UK aid or the USAID.
Author information
Authors and Affiliations
Contributions
S.A.K., A.A., J.J.K., E.J.L., A.M.G. and J.R.S. conceptualised the study; S.A.K., M.H.A., P.M., E.J.L. and J.R.S. conducted the field data collection; S.A.K. and A.A. performed the laboratory work; S.A.K, A.A, A.M.G and J.R.S. performed data analysis. S.A.K. and A.A. wrote the main manuscript text, all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
AMG has received personal fees from Abbott, Gilead, GSK, Roche, and ViiV and research funding (to the Institution) from Roche and ViiV, while A.A is supported by the Takemi Program in International Health at the Harvard T.H. Chan School of Public Health, outside of the work presented. All the other authors have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kayuni, S.A., Abdullahi, A., Alharbi, M.H. et al. Prospective pilot study on the relationship between seminal HIV-1 shedding and genital schistosomiasis in men receiving antiretroviral therapy along Lake Malawi. Sci Rep 13, 14154 (2023). https://doi.org/10.1038/s41598-023-40756-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40756-8
- Springer Nature Limited
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.