Abstract
Coronary bifurcation lesions represent a challenging anatomical subset, and the understanding of their 3D anatomy and plaque composition appears to play a key role in devising the optimal stenting strategy. This study proposes a new approach for the 3D reconstruction of coronary bifurcations and plaque materials by combining intravascular ultrasound (IVUS) and angiography. Three patient-specific silicone bifurcation models were 3D reconstructed and compared to micro-computed tomography (µCT) as the gold standard to test the accuracy and reproducibility of the proposed methodology. The clinical feasibility of the method was investigated in three diseased patient-specific bifurcations of varying anatomical complexity. The IVUS-based 3D reconstructed bifurcation models showed high agreement with the µCT reference models, with r2 values ranging from 0.88 to 0.99. The methodology successfully 3D reconstructed all the patient bifurcations, including plaque materials, in less than 60 min. Our proposed method is a simple, time-efficient, and user-friendly tool for accurate 3D reconstruction of coronary artery bifurcations. It can provide valuable information about bifurcation anatomy and plaque burden in the clinical setting, assisting in bifurcation stent planning and education.
Similar content being viewed by others
Introduction
Interventional cardiology faces challenges in treating coronary bifurcation lesions because of low procedural success rates and increased rates of adverse cardiovascular events1,2. Because of these unique anatomical locations, different stenting strategies have been developed, which have been the subject of ongoing debate. To determine the best bifurcation stenting strategy and achieve favorable clinical outcomes, several factors, including bifurcation anatomy and disease extent, must be considered3,4. In recent years, interventional cardiologists have expressed the need for the three-dimensional (3D) representation of bifurcation anatomy and disease burden, as this can lead to a better understanding of the anatomical complexity of bifurcation disease and the optimization of stenting strategies.
Intravascular ultrasound (IVUS) is a powerful intravascular imaging technology that allows for the cross-sectional imaging of the coronary arteries. IVUS images have several anatomical features, including the lumen, external elastic membrane (EEM), and plaque materials. Several studies have proposed methods for combining IVUS and angiography images for 3D reconstruction of coronary arteries5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23; however, these approaches were focused on non-bifurcated vessels. Some studies have performed 3D reconstruction of bifurcations but relied on computed tomography (CT) images to correct the IVUS frame orientation, limiting their clinical applicability24,25. Furthermore, efforts to 3D reconstruct the plaque materials by IVUS have been limited to single vessels21,22,23,26,27.
In this study, we present a new method that combines IVUS imaging and angiography with advanced modeling techniques to perform 3D reconstruction of coronary bifurcations, including plaque materials. The study aimed to: (1) provide a detailed methodology for the 3D reconstruction of coronary bifurcations along with plaque materials, and (2) evaluate the methodology's accuracy, feasibility, and reproducibility in both patient-specific silicone bifurcation models and patient cases with varying degrees of disease.
Methodology
All methods were carried out in accordance with the relevant guidelines and regulations. The angiograms and IVUS data used in the study were obtained from the Kyushu Medical Center, Fukuoka, Japan. The study was reviewed and approved by ethics committee of the National Hospital Organization Kyushu Medical Center (protocol number 20C035). Informed consent was obtained from all participants.
Experimental studies
Experimental coronary artery models, flow chamber studies, and imaging procedures
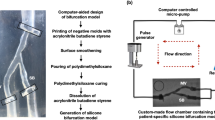
Figure 1 depicts a flowchart that explains the 3D reconstruction process. Three patient-specific silicone coronary bifurcation models were created using the previously described technique28. In brief, the 3D bifurcation geometries were created using two angiographic projections in 3D CAAS Workstation 8.2 (Pie medical imaging, Maastricht, The Netherlands). Negative moulds were created based on the geometries and 3D printed in acrylonitrile butadiene styrene. Polydimethylsiloxane was mixed with its curing agent before being poured into dry-clean moulds for curing. The silicone models were transferred to an acetone beaker to dissolve the acrylonitrile butadiene styrene material. The silicone models were placed in a custom-made flow chamber. A bioreactor circuit was connected to the flow chamber’s inlet and outlet, allowing steady blood-mimicking flow at a 100 ml/min rate at room temperature.
The models were first imaged with angiography in two projections with at least a 30-degree difference in viewing angles to provide the main vessel (MV) and side branch (SB) centerlines. The three silicone models were subjected to IVUS imaging with Opticross 6 HD, 60 MHz (Boston Scientific, Marlborough, MA, USA). The imaging catheter was advanced using a 6F guiding catheter. An automatic pullback was performed at a constant speed of 0.5 mm/s or 1.0 mm/s, with a distance of 0.017/0.033 mm between two consecutive frames. All the pullback frames were analyzed offline, and the lumen sections were segmented using EchoPlaque 4.0 (INDEC Medical Systems, Los Altos, CA, USA) (Fig. 2). Finally, after injecting an iodinated contrast media (37%), the bifurcation models were imaged with µCT (Skyscan 1172 version 1.5, Antwerp, Belgium) with the following parameters: image pixel size 26.94 µm, voltage 100 kV, current 100 µA, and slice thickness 27 µm. The bifurcations were 3D reconstructed from µCT images with 3D medical imaging software (Rhino3dmedical, Mirrakoi, Switzerland) and smoothed with modeling software Meshmixer (Autodesk Research, New York, NY, USA).
IVUS processing for bifurcation lumen reconstruction
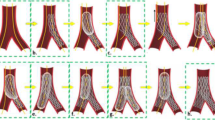
The detailed steps of the bifurcation lumen reconstruction are listed in Fig. 1. We applied Electrocardiogram (ECG)-gating to obtain the IVUS frames at the end-diastolic phase. The frames were uploaded into Grasshopper (GH) 3D, a Rhinoceros plug-in. These frames were then aligned and packaged in a straight line along the central path of the IVUS catheter. The packaged frames were oriented in 3D space by placing them perpendicular to the centerline and registering each frame centroid. The MV and SB frames were then rotated to align with the same bifurcation carina reference to match the relative position. The lofting function of GH created the original surfaces of MV and SB. This process’s detailed steps can be found in our previous work on 3D reconstruction using optical coherence tomography (OCT) images28.
To secure a smooth 3D reconstruction of the MV and SB surfaces, especially at the carina where the MV/SB surfaces exhibited irregular protrusions, in each IVUS frame, we identified the catheter point. All the catheter points of the entire sequence of IVUS frames created a virtual catheter path. We noticed that the catheter points at the carina were deviating. To correct the surface protrusion at the carina, we manually aligned the deviating catheter points (Fig. 3). To further refine the 3D reconstructed lumen surface, we mapped back the segmented IVUS frames to the 3D reconstructed surface (Fig. 4). Using a Rhinoceros tool, named subdivision surfaces (SubD)29, we were able to directly adapt the 3D geometry of the lumen to the segmented IVUS frames.
Mapping back technique. (a) Silicone model. (a1) Cross-section of the 3D reconstructed lumen. (a2) Mapping back of the IVUS image to the corresponding 3D reconstructed lumen cross-section. (a3) Cross-section view of the mapping-back. (b) Clinical case. (b1) Cross-section of the 3D reconstructed lumen, calcium, fibrolipid, and fibrosis. (b2) Mapping back of the IVUS image to the corresponding 3D reconstructed vessel cross-section. (b3) Cross-section view of the mapping-back. IVUS, intravascular ultrasound.
Validation of the 3D reconstruction method
The 3D IVUS reconstructed bifurcation models were compared to the corresponding 3D µCT reconstructed ones. The carina point was used to co-register the 3D IVUS and µCT reconstructed models. For comparisons, two metrics were used: lumen diameter and shape. To minimize biases, different operators performed the IVUS-based 3D reconstruction, µCT-based 3D reconstruction, and the comparisons of IVUS- and µCT-based models.
In the IVUS and µCT models, serial cross-sections were identified at every 0.1 mm along the lumen of the MV and SB. We observed a consistent difference in lumen diameter between IVUS and µCT (Supplementary Fig. S1), with IVUS measurements overestimating the actual lumen size30. For that reason, the lumen diameters were normalized using the z-score31 to account for the systemic discrepancy in lumen size between the IVUS and µCT.
In each cross-section, the ratio of the maximum distance between the two furthest points of the circumference (distance X) to the maximum length perpendicular to distance X (distance Y) was calculated to compare the lumen shape. The lumen was assumed to be oval-shaped, and the ratio of distance Y/distance X was used as a lumen shape indicator.
Reproducibility
To calculate the reproducibility of the IVUS-based 3D reconstruction method, two independent operators 3D reconstructed all the silicone models, respectively. The reconstructed models were compared in terms of the lumen diameter and shape.
Clinical studies
The clinical feasibility and processing times of our bifurcation reconstruction method were evaluated in n = 3 patient coronary artery bifurcations (Supplementary Table S1). The above-mentioned imaging protocols were used to collect IVUS and angiography data. Using a customized platform (based on Rhinoceros and GH), we manually segmented the vessel lumen and EEM, as well as the plaque materials (calcium, fibrosis, and fibrolipid) (Fig. 3). To correct the twisting of the IVUS catheter due to the pulsatile motion of the artery28, we rotated the successive IVUS frames (including lumen, EEM, and plaque materials) around the catheter center until the segmented lumens were aligned (Supplementary Fig. S2). Finally, the correctly oriented IVUS frames (lumen, EEM, and plaque materials) were lofted and smoothened to create the 3D bifurcation reconstruction. The mapping back technique was also used to further refine the quality of the 3D reconstructed lumen, EEM, and plaque materials. To evaluate the time efficiency of our 3D reconstruction method, we calculated the processing time for each step in all three clinical cases.
Statistical analyses
Statistical analyses were performed with the statistical package GraphPad Prism 9.5 (GraphPad Inc., San Diego, CA, USA). Continuous variables were expressed as median (Inter quartile range; IQR). The lumen diameters of IVUS and μCT models were normalized by calculating the Z score as (absolute diameter-µ)/s with µ representing the mean diameter and s the standard deviation of the mean. The comparisons and reproducibility were performed with linear regression and Bland–Altman analysis. p value < 0.01 was considered as the level of significance.
Results
Silicone model validation
Lumen diameter
All n = 3 silicone models were successfully 3D reconstructed (Fig. 5a). In the diameter/length graphs, the normalized lumen diameters (z-score) of the 3D reconstructed bifurcations from 3D IVUS vs. µCT show high agreement (Fig. 5b). Linear regression analysis revealed r2 values ranging from 0.88 to 0.99, with slopes close to one and intercepts near zero (Table 1).
Comparison between IVUS-based and μCT-based 3D reconstruction of silicone bifurcation models. (a) IVUS-reconstructed models and μCT-reconstructed models. (b) Normalized lumen diameter/length graphs. The lumen length is from distal to proximal. IVUS, intravascular ultrasound; MV, main vessel; SB, side branch.
Lumen shape
Table 1 shows the median, 25th, and 75th percentiles of the maximum distances perpendicular to each other (distance Y/distance X) of IVUS- and µCT-reconstructed bifurcation models. The median, 25th, and 75th percentile ratios of reconstructed IVUS and µCT models revealed mean differences of − 0.003 (0.046 to 0.039), 0.013 (− 0.067 to 0.093), and − 0.007 (− 0.045 to 0.032), indicating a high level of agreement (Supplementary Fig. S3).
Reproducibility
Table 2 displays the reproducibility test results for our method. The lumen diameters of the 3D IVUS reconstructed bifurcation models from two independent operators showed high agreement, with r2 ranging from 0.96 to 1.00 (p < 0.0001), indicating that our method was quite reproducible.
Clinical feasibility
The validated 3D reconstruction algorithm successfully reconstructed all n = 3 patient bifurcations. The reconstructed bifurcation models, including lumen, EEM, and plaque materials, are shown in Fig. 6. The lumen shape was in good agreement with the corresponding angiograms (Fig. 6b, f, j). The mapping back technique was used in all models to test the accuracy of the plaque materials reconstruction, showing that the reconstructed lumen and plaque materials matched their respective IVUS images in location and size (Fig. 6d, h, l).
3D reconstruction of coronary artery bifurcation geometries. (a, e, i) Reconstructed lumen of n = 3 patient-specific geometries using angiography and IVUS imaging. (b, f, j) Angiography of the reconstructed coronary bifurcation lumen showing the main vessel (MV) and side branch (SB). (c, g, k) Reconstructed lumen, calcium, fibrosis, and fibrolipid of n = 3 patient-specific geometries using angiography and IVUS imaging. (d, h, l) Mapping back of a cross-section of the reconstructed lumen, calcium, fibrosis, and fibrolipid to its respective IVUS image, showing the high accuracy of the reconstruction. IVUS, intravascular ultrasound; MV, main vessel; SB, side branch.
Table 3 summarizes the processing time for each step, from image processing to final 3D lumen and plaque reconstruction. The average time for image pre-processing was about 100 min, and for 3D reconstruction of a bifurcation about 60 min.
Discussion
In this study, we proposed a new method for 3D reconstruction of coronary bifurcations, including plaque materials, by combining IVUS imaging and coronary angiography. We performed in-vitro validation using silicone models and clinical feasibility tests, demonstrating that our reconstruction technique has high accuracy, reproducibility, and clinical feasibility. Our validated method has the potential to be used in the clinical setting to provide reliable information about the anatomy and plaque burden of coronary bifurcations, thereby facilitating clinical planning and decision-making during cardiac catheterization procedures.
The first studies on IVUS-based coronary vessel 3D reconstruction date back more than 20 years5,6,7. Thereafter, several studies have performed 3D reconstruction of non-bifurcated coronary arteries by combining IVUS with angiography5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23. To date, two studies have performed 3D reconstruction of coronary bifurcation arteries by combining IVUS and angiography, but both studies used CT to correct the IVUS frames’ orientation24,25. Our study is the first to perform 3D reconstruction of coronary artery bifurcation by combining IVUS and angiography only without the need for CT imaging. While some previous studies have performed 3D reconstruction of coronary plaque materials based on IVUS, they have certain limitations. Balocco et al.21 performed segmentation and reconstruction of fibrotic, calcified, and fibro-lipidic plaques considering thickness, but simplified the models by assigning plaques’ morphological values to vessel surface mesh. By collecting the IVUS-VH (Virtual Histology) cross-section image, Liang et al.26 reconstructed fibrous tissue, necrotic core, and calcification for single vessels. Following the 3D reconstruction methods proposed by Tang et al.32, a series of works applying IVUS or IVUS-VH were performed by Tang’s research group22,23,27. They reconstructed 3D models of fibrous tissue, lipid core, and calcification but the reconstructions were limited to non-bifurcated vessels. Compared to these prior works, our study provides a more comprehensive 3D bifurcation reconstruction incorporating all major coronary plaque materials: calcium, fibrosis, and fibro-lipid. Table 4 provides a comprehensive comparison of our methodology to that of other published studies.
We validated our work using 3D models reconstructed from µCT imaging, widely accepted as a gold standard33,34, after successfully reconstructing patient-specific silicone bifurcation models based on IVUS and angiography. The linear regression analysis on lumen diameter revealed that the r2 values ranged from 0.88 to 0.99, with a mean difference of -0.003 in the median lumen shape ratio. The linear regression analysis of the reproducibility test, performed by two independent operators, revealed high r2 values ranging from 0.96 to 1.00, suggestive of the high accuracy and reproducibility of the proposed method. During the clinical feasibility tests, the mapping back of the IVUS images onto the corresponding 3D reconstructed model demonstrated that our 3D reconstruction method could accurately locate and size the plaque materials.
Notably, in our work, we used two innovative techniques: (1) creation of the virtual catheter path to correct the irregular surface protrusion at the carina, and (2) mapping back technique combined with the SubD surfaces, which allowed us to directly modify and adapt the 3D reconstructed bifurcation model to the segmented IVUS frames (Supplementary Fig. S4).
This study’s findings have important clinical implications and future applications. Our 3D reconstruction method has the potential to improve procedural planning and clinical outcomes by providing interventional cardiologists with precise information about the anatomy and severity of coronary bifurcation disease. Our technique can also perform patient-specific computational simulations for bifurcation stenting. This can advance knowledge about bifurcation stenting mechanics and potentially lead to more personalized stenting techniques. This can be especially beneficial to the pharmaceutical industry when developing next-generation stents. Furthermore, if the imaging data is available to extract the required information, the innovative methodology of our technique has the potential to improve other invasive imaging modalities, such as OCT, or even non-invasive imaging, such as coronary computed tomography angiography. Finally, when combined with improved visualization techniques, the accurate 3D reconstructed bifurcation models can provide medical students with novel education, such as flying through the vessel using virtual reality.
Limitations
There were several limitations in our study. First, our study was performed on a small dataset (three silicone models and three clinical cases). However, the primary goal of our proof-of-concept study was to introduce a novel 3D reconstruction method by IVUS and angiography, validate this with silicone models, and show its clinical applicability. Second, the back-and-forth movement of the IVUS catheter (swinging effect) during pulsation could affect the accuracy of the 3D reconstruction35. However, the application of ECG-gating could minimize the swinging effect, as suggested by the previous works. Finally, manual segmentation of IVUS images and plaque material is a time-consuming process. To address this issue, we are currently working on machine learning-based codes that can be integrated into GH to fully automate the IVUS segmentation process, reducing processing time and allowing our algorithm to be applied in near real-time.
Conclusion
This study presents a new methodology for 3D reconstruction of coronary artery bifurcations using IVUS and angiography. The agreement between the IVUS-based 3D reconstructed bifurcation models and the µCT reference models was remarkably high, indicating the accuracy of our approach. Furthermore, our methodology was found to be clinically feasible and time-efficient for three patient-specific bifurcations, with a reconstruction time of about 60 min. Our technique provides a simple, easy-to-use, and accurate 3D reconstruction of coronary bifurcations, which can help with pre-procedural planning and clinical decision-making for bifurcation stenting procedures.
Data availability
Correspondence and requests for materials should be addressed to Y.S.C.
Change history
19 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-57034-w
References
Giannoglou, G. D., Antoniadis, A. P., Koskinas, K. C. & Chatzizisis, Y. S. Flow and atherosclerosis in coronary bifurcations. EuroIntervention 6(Suppl J), J16–23. https://doi.org/10.4244/eijv6supja4 (2010).
Antoniadis, A. P. et al. Biomechanical modeling to improve coronary artery bifurcation stenting: Expert review document on techniques and clinical implementation. JACC Cardiovasc. Intervent. 8, 1281–1296. https://doi.org/10.1016/j.jcin.2015.06.015 (2015).
Markl, M. et al. In vivo wall shear stress distribution in the carotid artery: Effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ. Cardiovasc. Imaging 3, 647–655. https://doi.org/10.1161/circimaging.110.958504 (2010).
Gwon, H.-C. Understanding the coronary bifurcation stenting. KCJ 48, 481–491. https://doi.org/10.4070/kcj.2018.0088 (2018).
Wahle, A., Prause, G. P. M., DeJong, S. C. & Sonka, M. Geometrically correct 3-D reconstruction of intravascular ultrasound images by fusion with biplane angiography-methods and validation. IEEE Trans. Med. Imaging 18, 686–699. https://doi.org/10.1109/42.796282 (1999).
Cothren, R. M. et al. Three-dimensional reconstruction of the coronary artery wall by image fusion of intravascular ultrasound and bi-plane angiography. Int. J. Cardiac Imaging 16, 69–85. https://doi.org/10.1023/A:1006304419505 (2000).
Slager, C. J. et al. True 3-dimensional reconstruction of coronary arteries in patients by fusion of angiography and IVUS (ANGUS) and its quantitative validation. Circulation 102, 511–516. https://doi.org/10.1161/01.cir.102.5.511 (2000).
Bourantas, C., Fotiadis, D. I., Kourtis, I. C., Michalis, L. K. & Plissiti, M. Three-dimensional coronary artery reconstruction using fusion of intravascular ultrasound and biplane angiography. Int. Congr. Ser. 1256, 1133–1138. https://doi.org/10.1016/S0531-5131(03)00334-0 (2003).
Bourantas, C. V. et al. A method for 3D reconstruction of coronary arteries using biplane angiography and intravascular ultrasound images. Comput. Med. Imaging Graph. 29, 597–606. https://doi.org/10.1016/j.compmedimag.2005.07.001 (2005).
Giannoglou, G. D. et al. In-vivo validation of spatially correct three-dimensional reconstruction of human coronary arteries by integrating intravascular ultrasound and biplane angiography. Coron. Artery Dis. 17, 85 (2006).
Bourantas, C. V. et al. ANGIOCARE: An automated system for fast three-dimensional coronary reconstruction by integrating angiographic and intracoronary ultrasound data. Catheter. Cardiovasc. Interv. 72, 166–175. https://doi.org/10.1002/ccd.21527 (2008).
Chatzizisis, Y. S. et al. In vivo comparative study of linear versus geometrically correct three-dimensional reconstruction of coronary arteries. Am. J. Cardiol. 101, 263–267. https://doi.org/10.1016/j.amjcard.2007.07.070 (2008).
Schuurbiers, J. C. H. et al. In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS). Catheter. Cardiovasc. Interv. 73, 620–626. https://doi.org/10.1002/ccd.21872 (2009).
Bourantas, C. et al. A new methodology for accurate 3-dimensional coronary artery reconstruction using routine intravascular ultrasound and angiographic data: Implications for widespread assessment of endothelial shear stress in humans. EuroIntervention 9, 582. https://doi.org/10.4244/EIJV9I5A94 (2013).
Doulaverakis, C. et al. IVUSAngio tool: A publicly available software for fast and accurate 3D reconstruction of coronary arteries. Comput. Biol. Med. 43, 1793–1803. https://doi.org/10.1016/j.compbiomed.2013.08.013 (2013).
Ma, H. T., Wang, H., Wang, C. & Hau, W. K. In 2013 IEEE International Conference of IEEE Region 10 (TENCON 2013) 1–4 (2013).
Zheng, S. & Mengchan, L. Reconstruction of coronary vessels from intravascular ultrasound image sequences based on compensation of the in-plane motion. Comput. Med. Imaging Graph. 37, 618–627. https://doi.org/10.1016/j.compmedimag.2013.09.003 (2013).
Bezerra, C. G. et al. Three-dimensional reconstruction of coronary arteries based on the integration of intravascular ultrasound and conventional angiography. Rev. Brasil. Cardiol. Invas. (Engl. Ed.) 23, 134–138. https://doi.org/10.1016/j.rbciev.2015.12.013 (2015).
Wang, X., Peng, C., Liu, X. & Pan, Z. Functional assessment of stenotic coronary artery in 3D geometric reconstruction from fusion of intravascular ultrasound and x-ray angiography. IEEE Access 6, 53330–53341. https://doi.org/10.1109/ACCESS.2018.2870950 (2018).
Jiang, J. et al. Fractional flow reserve for coronary stenosis assessment derived from fusion of intravascular ultrasound and X-ray angiography. Quant. Imaging Med. Surg. 11, 4543–4555. https://doi.org/10.21037/qims-20-1324 (2021).
Balocco, S. et al. Relation between plaque type, plaque thickness, blood shear stress, and plaque stress in coronary arteries assessed by X-ray Angiography and Intravascular Ultrasound. Med. Phys. 39, 7430–7445. https://doi.org/10.1118/1.4760993 (2012).
Wang, L. et al. IVUS-based FSI models for human coronary plaque progression study: Components, correlation and predictive analysis. Ann. Biomed. Eng. 43, 107–121. https://doi.org/10.1007/s10439-014-1118-1 (2015).
Yang, C. et al. In vivo IVUS-based 3-D fluid-structure interaction models with cyclic bending and anisotropic vessel properties for human atherosclerotic coronary plaque mechanical analysis. IEEE Trans. Biomed. Eng. 56, 2420–2428. https://doi.org/10.1109/TBME.2009.2025658 (2009).
Gijsen, F. J. H. et al. 3D reconstruction techniques of human coronary bifurcations for shear stress computations. J. Biomech. 47, 39–43. https://doi.org/10.1016/j.jbiomech.2013.10.021 (2014).
Son, J. & Choi, Y. Reconstruction of intima and adventitia models into a state undeformed by a catheter by using CT, IVUS, and biplane X-ray angiogram images. Comput. Math. Methods Med. 2017, 9807617. https://doi.org/10.1155/2017/9807617 (2017).
Liang, X. et al. Biomechanical factors in coronary vulnerable plaque risk of rupture: Intravascular ultrasound-based patient-specific fluid–structure interaction studies. Coron. Artery Dis. 24, 75–87. https://doi.org/10.1097/MCA.0b013e32835bbe99 (2013).
Fan, R. et al. Human coronary plaque wall thickness correlated positively with flow shear stress and negatively with plaque wall stress: An IVUS-based fluid-structure interaction multi-patient study. Biomed. Eng. Online 13, 32. https://doi.org/10.1186/1475-925X-13-32 (2014).
Wu, W. et al. 3D reconstruction of coronary artery bifurcations from coronary angiography and optical coherence tomography: Feasibility, validation, and reproducibility. Sci. Rep. 10, 18049. https://doi.org/10.1038/s41598-020-74264-w (2020).
Introduction to subdivision surfaces (SubD), a Rhinoceros 3D modeling tool. https://www.rhino3d.com/features/subd/ (2023).
Kubo, T. et al. OCT compared with IVUS in a coronary lesion assessment: The OPUS-CLASS study. JACC Cardiovasc. Imaging 6, 1095–1104. https://doi.org/10.1016/j.jcmg.2013.04.014 (2013).
Li, S. Z. & Jain, A. Encyclopedia of Biometrics 1134–1135 (Springer, 2009).
Tang, D. et al. Quantifying effects of plaque structure and material properties on stress distributions in human atherosclerotic plaques using 3D FSI models. J. Biomech. Eng. 127, 1185–1194. https://doi.org/10.1115/1.2073668 (2005).
Oliveira, L. B. et al. Validity of micro-CT for in vitro caries detection: A systematic review and meta-analysis. Dentomaxillofac. Radiol. 49, 20190347. https://doi.org/10.1259/dmfr.20190347 (2020).
Keklikoglou, K. et al. Micro-CT for biological and biomedical studies: A comparison of imaging techniques. J. Imaging 7, 172 (2021).
Hernàndez-Sabaté, A., Gil, D., Garcia-Barnés, J. & Martí, E. Image-based cardiac phase retrieval in intravascular ultrasound sequences. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 60–72. https://doi.org/10.1109/tuffc.2011.1774 (2011).
Acknowledgements
We would like to acknowledge the valuable contribution of Mallareddy Banala, MBBS.
Funding
Supported by the National Institute of Health (R01 HL144690) and Dr. Vincent Miscia Cardiovascular Research Fund.
Author information
Authors and Affiliations
Contributions
Y.S.C., W.W., conceived and designed the study, analyzed and interpreted the data, drafted the manuscript, revised critically for important intellectual content and approved the manuscript submitted. U.M.O., A.B., S.Z., A.K.T., V.K.G., C.H.V., K.M.H., A.N., and X.T., analyzed and interpreted the data, revised the manuscript critically for intellectual content and approved the manuscript submitted.
Corresponding author
Ethics declarations
Competing interests
Yiannis S. Chatzizisis: Speaker honoraria, advisory board fees, and research grant from Boston Scientific Inc.; Advisory board fees and research grant from Medtronic Inc.; Issued U.S. patent (No. 11,026,749) and international patent pending (application No. PCT/US2020/057304) for the invention entitled “Computational simulation platform for the planning of interventional procedures”; Co-founder of ComKardia Inc. All other authors have no relevant conflict of interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors. Modifications have been made to the Methodology section. Full information regarding the changes made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, W., Oguz, U.M., Banga, A. et al. 3D reconstruction of coronary artery bifurcations from intravascular ultrasound and angiography. Sci Rep 13, 13031 (2023). https://doi.org/10.1038/s41598-023-40257-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40257-8
- Springer Nature Limited