Abstract
Industrial wastewaters are different from sanitary wastewaters, and treatment complications due to their unique characteristics, so biological processes are typically disrupted. High chemical oxygen demand, dye, heavy metals, toxic organic and non-biodegradable compounds present in petroleum industry wastewater. This study intends to optimize the photocatalytic proxone process, utilizing a synthesized ZnO–Fe3O4 nanocatalyst, for petroleum wastewater treatment. The synthesis of ZnO–Fe3O4 was done by air oxidation and layer-by-layer self-assembly method and XRD, SEM, EDAX, FT-IR, BET, DRS, and VSM techniques were used to characterize the catalyst. Central composite design (CCD) method applied to investigated the effect of pH (4–8), reaction time (30–60 min), ozone gas concentration (1–2 mg/L-min), hydrogen peroxide concentration (2–3 mL/L) and the amount of catalyst (1–0.5 g/L) on the process. In the optimal conditions, biological oxygen demand (BOD5) and total petroleum hydrocarbon (TPH) removal, reaction kinetic, and synergistic effect mechanisms on the process were studied. Based on the ANOVA, a quadratic model with R2 = 0.99, P-Value = 0.0001, and F-Value = 906.87 was proposed to model the process. Based on the model pH = 5.7, ozone concentration = 1.8 mg/L-min, hydrogen peroxide concentration = 2.5 mL/L, reaction time = 56 min, and the catalyst dose = 0.7 g/L were proposed as the optimum condition. According to the model prediction, an efficiency of 85.3% was predicted for the removal of COD. To evaluate the accuracy of the prediction, an experiment was carried out in optimal conditions, and experimentally, a 52% removal efficiency was obtained. Also, at the optimum condition, BOD5 and TPH removal were 91.1% and 89.7% respectively. The reaction kinetic follows the pseudo-first-order kinetic model (R2 = 0.98). Also, the results showed that there is a synergistic effect in this process. As an advanced hybrid oxidation process, the photocatalytic proxone process has the capacity to treat petroleum wastewater to an acceptable standard.
Similar content being viewed by others
Introduction
The industry that has recently grown a lot and has caused environmental pollution is the oil-related industries. These include refineries, petrochemicals, oil reservoirs, and transmission lines. Wastewater produced in these industries is usually associated with water consumption and its subsequent contamination with oil1. Chemically stabilized water and oil emulsions pose many environmental hazards to water resources, including increased COD, odor, and turbidity, which all affect aquatic life2. Industrial wastewaters, as mentioned, have various properties that make its biodegradability difficult. The presence of non-biodegradable and hardly biodegradable organic matters, synthetic compounds, high oil, and grease, high concentrated minerals and heavy metals have led biological treatment processes to be less efficient3. So petroleum industry wastewater should be treated before discharging into the environment. To this aim, advanced oxidation processes (AOPs) that have been widely studied on a bench scale and have been scaled up to industrial-size applications recommend4. AOPs can generate strong oxidizing radicals, such as hydroxyls, superoxides, sulfate radicals, and so on. These radicals can destroy any types of organic pollutants that biological processes cannot remove because of their high oxidation and reduction potential (above 2 eV) and their non-selectivity5.
Novel AOPs used for advanced treatment of industrial wastewaters include catalytic ozonation6, Fenton process7, electrochemical and electro-oxidation processes8,9,10, etc. The ozonation is considered as a disinfection process because of its ability to remove natural and degradable organic compounds6. In contrast, ozone has a relatively low efficiency in removing resistant and non-biodegradable organic compounds because of its selective and direct oxidation properties. Conventional ozone used for water and wastewater treatment cannot completely oxidize and mineralize organic compounds. It produces intermediates such as bromates that can cause cancer in humans. Thus, in recent years, combined processes such as ozone/hydrogen peroxide, ozone/UV, Fenton/ozonation, and catalytic ozonation processes have been applied that these hybrid processes produce stronger oxidizing radicals11. The combination of ozone and hydrogen peroxide is known as the peroxone process, which speeds up the decomposition of the ozone molecules and leads to the production of more hydroxyl radicals. Their simultaneous application causes a tremendous synergistic increase in mineralization of organic compounds and produces hydroxyl radicals, which are much stronger oxidants than ozone molecules. In addition, hazardous intermediates, and byproducts are not produced during the process and the final products consist of water and oxygen12. Hence, it is considered as an environmentally friendly process. To further enhance this process, the use of other mechanisms has also been studied. In this context, the use of a popular method of ultraviolet recently been interested13. The ultraviolet (UV) can perform direct oxidation (photolysis) and indirect oxidation owing to high-energy photons which cause more synergistic effect in advanced oxidation processes. Furthermore, UV radiation along with ozonation give rise to the decomposition of hydrogen peroxide and finally cause to the release of hydroxyl radicals during the process. UV, has also the ability to convert the ozone molecules into stronger oxidizing compounds such as hydroxyl and peroxide radicals, which ultimately improves the process efficiency14.
Besides, semiconductors are known as catalysts in the photo-catalytic process. Therefore, researchers have focused on the synthesis and fabrication of these catalysts15. Numerous semiconductors oxides, such as ZnO, SnO2, TiO2, BiVO4, Bi2WO6 have been studied16. Among them, ZnO has been known as an excellent candidate for photocatalytic reaction due to its high photocatalytic activity, environmentally friendly properties, and relatively low cost. In addition, high specific surface area has led Nano ZnO to be commonly used in wastewater treatment. However, an essential problem to be solved in industrial photocatalytic applications is the efficient separation of ZnO from the reaction media. The magnetic carriers using external magnetic fields provide a very efficient and convenient way to separate and recycle catalysts17. Fe3O4 as an important member of the spinel type ferrite nanoparticles has been widely used in minerals separation, heat transfer, electrography, efficient hyperthermia for cancer therapy, and other fields. Thus, various studies on the synthesis, properties, and applications of magnetic Fe3O4 nanoparticles have been conducted18. Hence, the development of efficient reusable magnetic photocatalysis has become an important issue in oxidation processes. The utility of empirical strategies coupled with response surface methodology (RSM) has brought about to enhance the efficiency of design and optimize the processes because, in this way, the effective variables and their interactions are included over the optimization process19. Using this procedure, the number of tests are decreased and less time is needed, which gives rise to the less consumption of materials needed. Najem et al. investigated the effectiveness of the combined electrocoagulation and photocatalytic process in treating petroleum wastewater. The results showed significant efficiency in water quality purification efficiency in turbidity, color and COD in optimal conditions. The EC process reduces 58% of COD, and the combined process can reduce 70% COD20.
Thus, based on the abovementioned descriptions, a combined AOP method of photocatalytic proxone process was applied for treatment of petrochemical wastewater. To this aim ZnO–Fe3O4 photo-catalyst was synthesized and characterized. This is the first instance that catalyst ZnO–Fe3O4 has been employed in the photocatalytic proxone process, and furthermore, for the first time, the photocatalytic proxone process has been used in the treatment of wastewater with petroleum compounds. The process was modeled and optimized via central composite design. Also, BOD5 and TPH removal assessment, analysis of electrical energy efficiency, kinetic, and synergism studies were also performed at optimum condition.
Materials and methods
Materials and equipment
The chemicals were purchased from Sigma-Aldrich and Merck Co. FeSO4·7H2O (CAS No.: 7782-63-0, 99.8%), NaOH (CAS No.: 1310-73-2, 99%), Zinc acetate [(CH3CO2)2Zn, CAS Number: 557-34-6, ≥ 98%)], Sodium dodecyl sulfate [(SDS, CH3(CH2)11OSO3Na, CAS Number: 863-57-0, 99%)], ethanol [(C2H5OH, CAS Number: 64-17-5)], Hydrogen Peroxide (H2O2, CAS Number: 7722-84-1, 30%), Sulfuric Acid (H2SO4, CAS Number: 7664-93-9), COD vial (High Range 0–15,000 mg/L), deionized water. Also, the used instruments were: UV-C lamp (16-W, λ = 254 nm, Philips), pH meter (MultiMeter K5000-CP, NeoMet, iSTEK, Korea), Magnetic mixer (Alfa P405, ADAK, China), Spectrophotometer (DR6000, HACH, USA), GC-FID (Agilent, 6890 N, USA) by using auto-sample injector (Agilent, 7683B) with a capillary column (25 m × 0.320 mm; 0.17 mm; Agilent, HP-Ultra 2), Ozone generator (350 W, 600 mg/h, Avideh Rayan Alvand co.), Oxygen tank, rotameter (ABB/Fischer & Porter), and Plexi-glass reactor.All solvents and reagents were applied without additional purification.
The wastewater specification
The samples were taken from API refinery of Shahr-e-Rey. The collection and preservation of wastewater samples was carried out according to method No. 1060 recommended in the reference of standard methods for the examination of water and wastewater (23rd edition)21. During the study period, samples were taken weekly from the entrance. Then, the main physicochemical characteristics of the samples were analyzed (Table 1).
Fabrication and characterization of catalyst
ZnO
For the synthesis of ZnO nanoparticles, 20 mL zinc acetate solution (1 mol/L) and 30 mL sodium hydroxide solution (6 mol/L) were mixed in a 100 mL volumetric flask. The resultant mixture was then diluted by deionized water to a Zn2+ concentration of 0.2 mol/L. This solution was subsequently placed in a water bath at 85 °C for 5 h, then the resulting solids were washed with deionized water and ethanol and dried at room temperature for the next 24 h22.
Fe3O4 synthesis
The Fe3O4 nanoparticles were synthesized via the air oxidation method23. In this manner, 0.27 g FeSO4·7H2O was dissolved in 100 mL deionized water to obtain a concentration of 0.001 mol/L of divalent iron. Then, it was stirred vigorously at room temperature and sodium hydroxide (6 M) was gradually added to the solution until its pH reached 11 and then was stirred for 1 h at 25 °C in the presence of airflow. After filtration and washing the solution, the resulting Fe3O4 was dried at 70 °C for 24 h.
ZnO–Fe3O4 synthesis
ZnO–Fe3O4 nanoparticle was synthesized by the layer-by-layer (LBL) self-assembly method24. Briefly, 2 g of ZnO powder was dissolved in 20 mL SDS (8 mol/L). After stirring at room temperature for 1 h, the resulting solution was filtered and washed. Then, the solids were added to 100 mL solution containing Fe3O4 nanoparticles and stirred at room temperature for 5 h. The solids were then filtered and washed with ethanol and finally dried in a vacuum oven at 70 °C for 6 h.
Characterization of synthesis samples
To characterize ZnO, Fe3O4 and ZnO–Fe3O4, FT-IR spectrophotometer (Spotlight 200i FT-IR Microscopy Systems; 4000–400 cm−1) was used to identify relevant created bonds. X-ray diffraction (XRD) patterns were recorded on XRD diffractometer Rigaku-ZSX Primus 400; radiations source: Cu Kα [(λ = 1.54056 Å) monochromatic incident beam between 10° to 80° with the step interval of 0.02°, and a rate of 0.05°/s)] to assess the crystal structure of the samples. Also, the average crystallite size (D) of the prepared nanocomposite has been calculated from the Debye–Scherrer equation (Eq. 1)25:
UV–visible spectrum (UV–Vis DRS) was recorded by Agilent Cary 60 spectrophotometer to study the structural features and optic properties. The surface morphology of the samples was assessed using field-emission Scanning Electron Microscopy (FE-SEM) (UN41219SEM) under vacuum condition of ≥ 1.3 × 10–4 mbar. Energy dispersive spectrum (EDS) was used to analyzes purity and elemental mapping of samples. Transmission electron microscopes (TEM) were recorded on JEOL, JEM1200EX at 200 kV the samples were dispersed in a 1:1 methanol and water solution and deposited on a 3 mm copper grid and dried at ambient temperature after removing the excess solution using filter paper. The special surface area, volume and distribution pore size of catalysts were determined by nitrogen adsorption at 77 K with a Quantachrome Autosorb analyzer (BET). Samples were previously degassed in situ at 200 °C under vacuum for 12 h. Surface areas were calculated using the Brunauer–Emmet–Teller (BET) method over a p/p0 range where a linear relationship was maintained. The Vibrating-sample magnetometry character of ZnO–Fe3O4 was studied via VSM (LBKFB).
Reactor
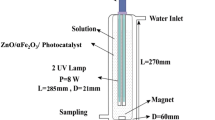
The process was performed in a bench scale photo-reactor. The photo-chemical reactor was constructed with an approximate volume of 500 mL consisted of an ultraviolet lamp (UV-C 16W) and a semi-batch ozonation system. Ozone gas in different concentrations was injected into the chamber by the ozone generator. The light source was placed horizontally inside the quartz sheath in the middle of the reactor and the contents of the reactor were mixed by a magnetic stirrer. The entire system was operated within a pilot which has been shown in Fig. 1.
Procedure
In order to study the process, prepared wastewater sample was transmitted to the laboratory under the transportation and storage conditions specified by the water and wastewater standards. Then, 500 mL of the sample was taken and poured into the reactor vessel and adjusted to the desired pH by 0.1 N sulfuric acid and sodium hydroxide. Afterwards, an amount of the catalyst and hydrogen peroxide was added into the media and the lamp was switched on. The ozone gas, generated via the ozone generator. An ozone-containing oxygen gas was bubbled continuously into the reactor via a ceramic diffuser by passing pure oxygen gas feed through the ozone generator. The concentration of ozone gas in the solution reaction was adjusted by changing the flow rate of the feed oxygen. After that, the process was started and sampling was performed at regular time. In this study, COD, BOD5, and TPH parameters were selected as treatment criteria. The COD was measured based on the close reflux method (No. 5220 D, colorimetric method) by UV–visible spectrophotometer (DR6000) that presented in standard methods of water and wastewater examination. Also, TPH (EPA-821/B-94-004b)26 and BOD5 (No. 5210 D, respirometric Method) are measured based on standard methods for the examination of water and wastewater (23rd edition)21. The decreasing percentage of COD, BOD5, and TPH in the process was calculated as (Eq. 2):
where, C0 and Ct are the concentrations of initial and residual of COD, BOD5, and TPH (mg/L), respectively.
Design of the experiment
Due to the complexity of wastewater treatment processes and the effect of various parameters, recently researchers in this field use statistical modeling methods to investigate and optimize these processes by performing the minimum experiments. One of the valuable methods in this field is the design of experiment (DOE) that for the first time developed by Fisher and Yates in 192027. One of the most popular experimental design methods is the central composite design (CCD) of the response surface method28,29. The CCD method consists of three points: cubic, axial, and center points and the total number of experiments can be calculated as follows (Eq. 3):
where k is the number of factors, 2k is the terms of cubic points, 2k is the axial points, and N0 is the center points.
In this work the parameters of pH (4–8), the ozone concentration (1–3 mg/L-min), the hydrogen peroxide concentration (2–3 mL/L), ZnO–Fe3O4 dose (0.5–1 g/L), and the reaction time (30–60 min) were selected as effective parameters. According to the related literature results, the level of effective parameters was determined. Each variable in this design was examined at five different levels, which are presented in Table 2. It should be mentioned that the UV light (16 W and λ = 254 nm) was used consistently in all runs.
Results and discussion
Characterization of the synthesized catalyst
FESEM, TEM, and EDS
In Fig. 2, FESEM, TEM and EDS images of Fe3O4–ZnO catalyst are presented. As illustrated in the FESEM image (Fig. 2A), the synthesized particles of Fe3O4–ZnO have aggregated. Core–shell structure in TEM images shows that ZnO particles are coated on iron oxide. The formation of spherical structures is observed in the size range of 20–30 nm (Fig. 2B). The results of the present study are consistent with the study of Fernández et al.30. Also, the result of EDS analysis indicates iron, oxygen, zinc and carbon elements in the catalyst structure (Fig. 2C). The results of the study are consistent with Ezzatzadeh et al.31.
XRD and BET
The synthesized samples were structurally characterized by X-ray diffraction measurements (Fig. 3a). Magnetite (Fe3O4, JCPDS PDF-2 card number 19-0629) and zinc oxide (ZnO, JCPDS PDF-2 card number 36-1451) with hexagonal wurtzite structure were identified as the two crystalline phases present in the sample. The sharp peaks at positions 32.1, 34.8, 36.4, 47.9, 56.8, and 62.9 can be assigned to crystal planes (100), (002), (101), (102), (110), and (103) attributed zinc oxide with reference code (JCPDS 1451-36)32. Other peaks correspond well to the planes of the face-centered cubic structure of Fe3O4 (JCPDS 65-3107)33. Significantly, the intensity of ZnO diffraction peaks has decreased due to the core–shell structure.
Based on the Scherrer equation (Eq. 1), the size of the ZnO–Fe3O4 nanocomposite was calculated from the (101) plane as ∼ 14 nm. The results are consistent with the study of Zou et al.34. In Fig. 3B, the results of the adsorption and desorption of nitrogen gas (BET analysis) related to the Fe3O4–ZnO catalyst is presented. As it is clear, the nitrogen gas adsorption and desorption pattern of the catalyst follows the H3 model. Also, the peak diameter of pores is below 10 nm, which indicates the formation of microspores in the catalyst. The highest active surface area of the catalyst is 142 m2/g with a pore volume of 0.26 m3/g. The results of the current study are consistent with the study of Rakati et al.35.
UV–Vis spectra and FTIR
In Fig. 4a, the result of the UV–Vis spectrum is presented. According to the results, the catalyst has the property of absorbing light even in visible wavelengths, which indicates its photocatalytic activity. The absorption spectrum of ZnO shows a steep edge at a wavelength of about 345 nm, which indicates the main absorption in the UV light region. While the primary catalyst shows broad absorption in the wavelength range of 380–700 nm. The results are consistent with the study of Liu et al.24.
Figure 4b presents the FT-IR analysis of Fe3O4–ZnO catalyst. As it is clear from the Fig. 4b, the peak at 585 cm−1 is due to the stretching vibration between oxygen and iron36. The bands at 448, 510, and 610 cm−1 in the spectrum corresponding to ZnO are because of the different stretching modes of the Zn–O bond37. Due to the high concentration of ZnO in Fe3O4–ZnO, a broad band between 450 and 575 cm−1 is observed, which can be attributed to Zn–O vibrations. The broad peaks at 3447 and 1665 cm−1 are because of the stretching and bending vibrations of the O–H bond of the water molecule adsorbed on the nanoparticle surface38. The results of the current study are consistent with the results of Menon et al.39.
VSM
In Fig. 5, the results of the VSM analysis are presented. As it is clear, pure Fe3O4 has more magnetic properties than the Fe3O4–ZnO catalyst, but it still has a significant magnetic property that can be collected by a magnetic field. The saturation magnetization of the catalyst was determined to be 27.7 emu/g. The results of the study are consistent with the study of Abbasi et al.40.
Optimizing the process with CCD
Based on CCD method for 5 effective parameters of pH, the ozone concentration, the hydrogen peroxide concentration, ZnO–Fe3O4 dosage, and the reaction time, 50 experimental test were designed by the software which presented in the Table 3. The designed experiments were performed and COD removal efficiency as response was measured for all experiments. Regression analysis of the data was assessed and a second order polynomial equation (Eq. 3) was suggested by software as an appropriate model to model and predict of the process. The analysis of variance (ANOVA) was used to assess the significance of the model and terms; such that p-values less than 0.05 and greater than 0.10 identify that the model terms are significant and not significant, respectively. ANOVA analysis was presented in Table 4. As seen, the model F-value is 906.87 and the P-value < 0.0001, that implies the model is significant. Also, the F-value of 2.06 and p-value 0.1659 for the lack-of-fit implies the Lack of Fit is not significant relative to the pure error. Totally, R2 = 0.99, predicted R-Squared of 0.97, the Adj R-Squared of 0.98, and the adequate precision of 108.1 show that the model is significant and has well accuracy to predicted the process.
The effect of parameters
For assessment of different parameters effect on COD removal efficiency and interaction between the parameters, 3D graphs were used. The related graphs for pH, ozone concentration, hydrogen peroxide concentration, catalyst dose, and reaction time versus COD removal (%) are presented in Fig. 6. Figure 6 a, illustrated the COD removal variation by pH vs. reaction time. By increasing the solution pH from the acidic condition to the neutral range, the efficiency of the process increases, and the highest efficiency is achieved at pH = 6, and further, as the pH increases from this amount to the alkaline range, the efficiency decreases again. In AOPs, such as processes based on ozonation, ozone molecules are used in order to carry out direct oxidation by the ozone molecule itself and produce highly active hydroxyl radicals, and subsequently carry out oxidation with these active radicals (indirect oxidation). pH has a decisive role in the rate of reaction and the formation of active species41. In the conventional ozonation process, via increasing the pH to the alkaline range, the ozone molecule reacts with the hydroxyl ion (OH−) in the aqueous media and produces the HO2− (Eq. 5). Then HO2− reacts with the ozone molecule and produces hydroxyl radical (Eq. 6)42. The production of HO2- from the reaction between the hydroxyl ion and the ozone molecule (Eq. 7) is considered as a side reaction that reduces the concentration of dissolved ozone in the reaction medium and the reaction rate with H2O2 and HO2− decreases and as a result less hydroxyl radical is produced. On the other hand, due to the decrease in dissolved ozone and the increase in the amount of HO2− compared to ozone in the reaction media, HO2− acts as a radical scavenger and competes with the pollutant in consuming hydroxyl radicals43. In a relatively acidic pH, there is a proportional relationship between hydroxyl ions and ozone molecules, and it causes the production of amounts of hydroxyl radicals, and this increases the efficiency of the process.
The results of this study are consistent with the results of Stanisova Popil and coworkers that investigated the efficiency of the conventional ozonation and the peroxon process in the decomposition of dibutyl sulfide. In this study, the best efficiency of the process was obtained at weak acidic pH44.
The reaction time parameter has an increasing effect on the efficiency of the process. In such a way that the highest efficiency of the process was obtained at high values of this parameter. As it is obvious, by increasing the reaction time, the presence of ozone and UV and subsequently hydroxyl radicals produced in the process and hydrogen peroxide as a source of hydroxyl radical increases that increase the decomposition of pollutants. Over time, the process efficiency was proven due to the complete decomposition of the pollutant occurred. Shahamat et al. studied the UV/O3 process performance in azo red-60 dye degradation in textile wastewater. In this research, reaction time was considered in the range of 10–60 min. The optimum reaction time 60 min reported45.
Figure 6b, shows pH and ozone concentration effect on process performance. By increasing ozone concentration, the efficiency of the process is initially increased and then remains relatively constant. The efficiency of the process has a direct relationship with the concentration of ozone gas entering the reaction chamber. According to the theory of mass transfer, increasing the concentration of ozone entering the reaction media increases the concentration of dissolved ozone in the environment, and since ozone has a dual role as a producer of hydroxyl oxidizing radicals from the reaction with hydrogen peroxide and direct oxidation by itself. So increasing the concentration of ozone increases the efficiency of the process46. A study conducted by Wang and her colleagues on the decolorization of acid orange 2 indicated that increasing the ozone flow rate from 35 to 118 mg/L increased the removal efficiency from 80 to 98 percent47. Also, the results of Asgari et al.'s study on the removal of Butoben and Phenylmethyl ester from aqueous by the processes based on AOPs process, indicated that increasing the concentration of ozone in the UV/ZnO/O3 process, increased the removal efficiency48.
Figure 6c, shows pH and hydrogen peroxide concentration effect on process performance. The results of the effect of the hydrogen peroxide concentration parameter on the efficiency of the process are incremental. This increase starts with a steep slope and then continues with a slow slope and is relatively constant at higher values. As it is clear, by changing the concentration of hydrogen peroxide in the reaction media, the efficiency of the process is also changed. In general, the ozone molecule reacts with the deproteinized form of hydrogen peroxide (Eqs. 8–12)49.
By increasing the optimal ratio of hydrogen peroxide to the ozone gas in the reaction media, secondary series reactions or side reactions started (Eqs. 13–15)49. Finally, it causes the consumption of hydroxyl radicals and the production of compounds that are low activity or are completely inactive compounds50. Feng et al. investigated the pretreatment of mother liquor of gas field wastewater. The results of the ozone/hydrogen peroxide process indicated that the efficiency of the process increased by increasing the concentration of hydrogen peroxide and 6.2 mL was the optimum concentration of H2O251.
Figure 6d, shows the variation of COD removal efficiency versus catalyst dosage and pH changes. As seen, with increasing catalyst dosage, COD removal efficiency increases and with further increase in catalyst dosage, it decreases. To overcome the disadvantages of homogeneous catalysts, solid catalysts with high stability and efficiency have been widely studied in ozonation systems52. The process of heterogeneous catalytic ozonation is complex and involves many reactions and several stages that are influenced by various factors. The catalyst may play various roles in this process, such as creating reaction sites for adsorption and catalysis53.
The organic substances adsorption depends on the polarity of the compounds and the surface characteristics of the catalyst, such as the material charge, which strongly depends on the pH of the solution. Polar compounds may be easily adsorbed on the surface, while nonpolar organic substances are hardly adsorbed on the surface, unless some hydrophobic sites are present.
In addition, the catalyst has a high adsorption capacity for organic ions to some extent, which depends on the surface charge of the catalyst and the dissociation constant of the compounds. Another key factor in determining the catalytic activity of a catalyst is the adsorption and decomposition of ozone, which is believed to occur on the catalyst surface. In addition, ozone adsorption and subsequent decomposition usually lead to surface-bound oxidizing radicals and hydroxyl on catalyst surfaces, which enhance the removal of organic matter53. In the study of Shikhmohammadi and coworkers studied Butyl p-hydroxybenzoate removal from liquid phase by UVC/ZnO/O3/HP process. The results showed that 1 g/L of ZnO had a perfect effect on pollutant degradation54.
Also, ultraviolet rays in the catalyst's presence can lead to the photocatalytic process and improve the efficiency of the process. An excessive increase in the amount of catalyst in a photochemical reactor leads to light scattering and prevents to reach light to other oxidants such as ozone and hydrogen peroxide and will lead to a decrease in efficiency55.
Finally, the predicted optimal conditions for the photocatalytic proxone process were provided by the software. In these conditions (pH = 5.7, reaction time = 56 min., ozone gas concentration = 1.8 mg/min-L, hydrogen peroxide concentration = 2.5 mL/L and catalyst dosage = 0.7 g/L), a COD removal efficiency of 85.3% was predicted. To assess the accuracy of the model, an experiment was performed at optimum condition and a COD removal efficiency of 82 was experimentally obtained.
Further studies
Removal of BOD5 and TPH
In the photocatalytic proxone process, the BOD5 and TPH parameters were simultaneously assessed with the COD removal efficiency in optimal conditions. Based on the results, a BOD5 removal efficiency of 91.1% and a TPH removal efficiency of 89.7% were obtained at the optimum condition.
Reaction kinetic
Another discussion that has a great help in the design and implementation of processes is the study of reaction kinetics. It will help to model and implement the process better in the practical scale. Reactive kinetic models are used to identify the factors affecting the contaminant removal rate and process efficiency. In oxidation processes, pseudo-first-order kinetics is common. So, the reaction kinetic was evaluated based on first-order kinetics under optimal conditions (Eq. 16)49.
where, C0 and Ct: are initial and final (at time) concentration of COD (mg/L), t is the reaction time (min), K1 is the kinetic constant of the first-order reaction.
In studies, reaction kinetics are measured based on various factors. The initial concentration of the pollutant, the amount of the catalyst, the amount of ozone gas, the amount of the oxidizing agent such as hydrogen peroxide, etc., are among the factors that are investigated56. The rate of chemical reaction affects various aspects of the process. The main influencing factor is the EEO, which will be mentioned.
To this aim, an experiment was performed at optimum condition and the obtained data fitting assessment by pseudo-first-order kinetic was performed (Fig. 7). Based on the results, the kinetics of the photocatalytic proxone process follow the first-order kinetics with R2 = 0.95 and rate constant of 0.247 (L/min). In first-order kinetic, the reaction rate has a direct and linear relationship with the amount of reactants, which in this study is the initial concentration of the pollutant. In most of the processes studied in this area, the kinetics of the process follow the first-order kinetic model. According to the study of Kermani et al., the kinetics of peroxon process follow the first-order kinetics12.
The synergistic effect
In the photocatalytic proxone process, the mechanisms are divided into single, binary, triple, and, finally, the fundamental process. The mechanisms of UV, ozone gas, hydrogen peroxide, and catalyst as single mechanisms. UV/O3, UV/H2O2, UV/catalyst, O3/H2O2, O3/catalyst as binary mechanisms. UV/O3/H2O2, UV/O3/catalyst, UV/H2O2/catalyst, and O3/H2O2/catalyst were investigated as triple mechanisms. The results are illustrated in (Fig. 8). Based on the results, it was found that the efficiency of the fundamental process is more than other processes and mechanisms that show the synergistic effect of the mechanisms together. Yang et al. studied the synergistic effects of ozone/peroxymonosulfate for isothiazolinone biocides degradation in aqueous solution. The resulted indicated the synergistic effect of parameters on performance of process57.
Analysis of electrical energy efficiency
The electrical energy required for the photocatalytic proxone process typically has the greatest monetary impact on operating costs. Thus, it is necessary to evaluate the energy efficiency.
Utilizing the figure-of-merit electrical energy per order (EEO) is an appropriate method for computing the electrical energy efficiency. This is a powerful scale-up parameter and evaluation of the rate of purification in a fixed amount of contaminated water according to the energy consumed. The EEO value was used to compare the energy efficiencies of systems. For low pollutant concentrations, the EEO (kW/h-m–3 order–1) value can be derived using Eq. (17)58.
where P is the power (kW) of the light source and ozone generator; V is the volume (L) of solution in the reactor; and k is the pseudo-first-order rate constant (min–1) for mineralization. A higher EEO value corresponds to a lower energy efficiency of the system. Table 5 summarizes the EEO value of various mechanisms and processes in optimum condition.
The results show that the EEO value in the photocatalytic proxone process is more than the other two mechanisms (photolysis and ozonation), but this higher EEO leads to an increase in the process's efficiency in wastewater treatment. In Lester et al.59, and Joardar60 and coworkers studies EEO value calculated. In these studies, different EEO value have been reported, which indicate the different EEO value according to the type of pollutant and the efficiency of the process in wastewater treatment.
Conclusion
The aim of this work was a study of performing photocatalytic proxone process for treatment of petroleum wastewater using ZnO–Fe3O4 nanocatalyst. The ZnO–Fe3O4 catalyst was synthesized and its characteristics were determined by FESEM, EDS, TEM, BET, VSM, XRD, and FTIR analysis. Also, the process was statistically modeled and optimized by CCD method. Based on the model pH = 5.7, ozone concentration = 1.8 mg/L-min, hydrogen peroxide concentration = 2.5 mL/L, reaction time = 56 min, and the catalyst dose = 0.7 g/L were proposed as optimum condition and at these conditions, an efficiency of 82% was experimentally obtained for the removal of COD. Also, a BOD5 removal efficiency of 91.1% and a TPH removal efficiency of 89.7% were obtained at the optimum condition. Based on the results, the kinetics of the process follow the first-order kinetics and it concluded that there is a synergistic effect in the photocatalytic proxone process. The EEO value of photolysis, ozonation and photocatalytic proxone process were calculated 13.29, 34.41 and 113.8 kW/h-m–3-order–1, respectively. Based on the results, the photocatalytic proxone process can treat pollutants in water media, especially industrial wastewaters, because of proper efficiency. The strength of this process is its simplicity in setting up and managing. In this process, hard-to-decompose pollutants, such as slowly decomposable organic compounds, are usually completely and effectively removed and stabilized. Table 6 includes a comparison between the current study and other recent studies regarding petroleum wastewater treatment.
Data availability
The datasets generated and analyzed during the current study available from the corresponding author on reasonable request.
References
Yusoff, M. H. M. et al. Comprehensive review on biodiesel production from palm oil mill effluent. ChemBioEng Rev. 8, 439–462 (2021).
Sundararaman, S., Kumar, J. A., Deivasigamani, P. & Devarajan, Y. Emerging pharma residue contaminants: Occurrence, monitoring, risk and fate assessment—A challenge to water resource management. Sci. Total Environ. 825, 153897 (2022).
Morin-Crini, N. et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 20, 1–43 (2022).
Yegane Badi, M., Vosoughi, M., Sadeghi, H., Mokhtari, S. A. & Mehralipour, J. Ultrasonic-assisted H2O2/TiO2 process in catechol degradation: Kinetic, synergistic and optimisation via response surface methodology. Int. J. Environ. Anal. Chem. 102, 757–770 (2022).
Mehralipour, J. & Kermani, M. Optimization of photo-electro/Persulfate/nZVI process on 2–4 Dichlorophenoxyacetic acid degradation via central composite design: A novel combination of advanced oxidation process. J. Environ. Health Sci. Eng. 19, 941–957 (2021).
Issaka, E. et al. Advanced catalytic ozonation for degradation of pharmaceutical pollutants—A review. Chemosphere 289, 133208 (2022).
Ribeiro, J. P. & Nunes, M. I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 197, 110957 (2021).
Rahmani, A. et al. Bismuth-doped 3D carbon felt/PbO2 electrocatalyst for degradation of diuron herbicide and improvement of pesticide wastewater biodegradability. J. Environ. Chem. Eng. 11, 109118 (2023).
Rahmani, A. et al. The integration of PbO2-based EAOPs with other advanced oxidation processes for improved treatment of water and wastewater. Curr. Opin. Electrochem. 37, 101204 (2022).
Rahmani, A., Shabanloo, A. & Shabanloo, N. A mini-review of recent progress in lead dioxide electrocatalyst for degradation of toxic organic pollutants. Mater. Today Chem. 27, 101311 (2023).
Tian, S.-Q. et al. Heterogeneous catalytic ozonation of atrazine with Mn-loaded and Fe-loaded biochar. Water Res. 193, 116860 (2021).
Kermani, M., Shahsavani, A., Ghaderi, P., Kasaee, P. & Mehralipour, J. Optimization of UV-electroproxone procedure for treatment of landfill leachate: The study of energy consumption. J. Environ. Health Sci. Eng. 19, 81–93 (2021).
Pérez-Lucas, G. et al. Decline of fluroxypyr and triclopyr residues from pure, drinking and leaching water by photo-assisted peroxonation. Process Saf. Environ. Prot. 137, 358–365 (2020).
Kermani, M., Mehralipour, J. & Kakavandi, B. Photo-assisted electroperoxone of 2, 4-dichlorophenoxy acetic acid herbicide: Kinetic, synergistic and optimization by response surface methodology. J. Water Process Eng. 32, 100971 (2019).
Alalm, M. G. et al. Toward scaling-up photocatalytic process for multiphase environmental applications. Catalysts 11, 562 (2021).
Akerdi, A. G. & Bahrami, S. H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 7, 103283 (2019).
Shekofteh-Gohari, M., Habibi-Yangjeh, A., Abitorabi, M. & Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 48, 806–857 (2018).
Lendzion-Bieluń, Z. et al. Effective processes of phenol degradation on Fe3O4–TiO2 nanostructured magnetic photocatalyst. J. Phys. Chem. Solids 136, 109178 (2020).
Galedari, M., Ghazi, M. M. & Mirmasoomi, S. R. Photocatalytic process for the tetracycline removal under visible light: Presenting a degradation model and optimization using response surface methodology (RSM). Chem. Eng. Res. Des. 145, 323–333 (2019).
Al-Rubaiey, N. A., Albrazanjy, M. G. & Kadhim, W. A. Combined electrocoagulation and photocatalytic for oily wastewater treatment using TiO2 nano-catalysts. Egypt. J. Chem. 65, 55–64 (2022).
Rice, E. W., Bridgewater, L. & Association, A. P. H. Standard Methods for the Examination of Water and Wastewater Vol. 10 (American Public Health Association Washington, 2012).
Zagal-Padilla, C., Diaz-Gómez, C. & Gamboa, S. Electrochemical characterization of a plasmonic effect ethanol sensor based on two-dimensional ZnO synthesized by green chemistry. Mater. Sci. Semicond. Process. 137, 106240 (2022).
Zhu, G., Yu, X., Xie, F. & Feng, W. Ultraviolet light assisted heterogeneous Fenton degradation of tetracycline based on polyhedral Fe3O4 nanoparticles with exposed high-energy 110 facets. Appl. Surf. Sci. 485, 496–505 (2019).
Liu, Q. et al. Magnetic ZnO@ Fe3O4 composite for self-generated H2O2 toward photo-Fenton-like oxidation of nitrophenol. Compos. B Eng. 200, 108345 (2020).
Kim, S. P., Choi, M. Y. & Choi, H. C. Photocatalytic activity of SnO2 nanoparticles in methylene blue degradation. Mater. Res. Bull. 74, 85–89. https://doi.org/10.1016/j.materresbull.2015.10.024 (2016).
Environmental, U. Protection Agency. 1990. Quality assurance project plan for characterization sampling and treatment tests conducted for the Contaminated Soil and Debris (CSD) Program: Washington, DC, USEPA Office of Solid Waste (1990).
Hinkelmann, K. & Kempthorne, O. Design and Analysis of Experiments, Introduction to Experimental Design (Wiley, 2007).
Rezaei-Vahidian, H., Zarei, A. R. & Soleymani, A. R. Degradation of nitro-aromatic explosives using recyclable magnetic photocatalyst: Catalyst synthesis and process optimization. J. Hazard. Mater. 325, 310–318. https://doi.org/10.1016/j.jhazmat.2016.12.001 (2017).
Zarei, A. R., Rezaeivahidian, H. & Soleymani, A. R. Investigation on removal of p-nitrophenol using a hybridized photo-thermal activated persulfate process: Central composite design modeling. Process Saf. Environ. Prot. 98, 109–115. https://doi.org/10.1016/j.psep.2015.07.006 (2015).
Fernández, L. et al. Insight into antibiotics removal: Exploring the photocatalytic performance of a Fe3O4/ZnO nanocomposite in a novel magnetic sequential batch reactor. J. Environ. Manag. 237, 595–608 (2019).
Ezzatzadeh, E. & Hossaini, Z. 2D ZnO/Fe3O4 nanocomposites as a novel catalyst-promoted green synthesis of novel quinazoline phosphonate derivatives. Appl. Organomet. Chem. 34, e5596 (2020).
Hong, R., Pan, T., Qian, J. & Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 119, 71–81 (2006).
Feng, J., Mao, J., Wen, X. & Tu, M. Ultrasonic-assisted in situ synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J. Alloy. Compd. 509, 9093–9097 (2011).
Zou, P., Hong, X., Chu, X., Li, Y. & Liu, Y. Multifunctional Fe3O4/ZnO nanocomposites with magnetic and optical properties. J. Nanosci. Nanotechnol. 10, 1992–1997 (2010).
Rakati, K. K., Mirzaei, M., Maghsoodi, S. & Shahbazi, A. Preparation and characterization of poly aniline modified chitosan embedded with ZnO–Fe3O4 for Cu (II) removal from aqueous solution. Int. J. Biol. Macromol. 130, 1025–1045 (2019).
Du, N., Xu, Y., Zhang, H., Zhai, C. & Yang, D. Selective synthesis of Fe 2 O 3 and Fe 3 O 4 nanowires via a single precursor: A general method for metal oxide nanowires. Nanoscale Res. Lett. 5, 1295–1300 (2010).
Qi, S., Zhang, Y.-H., Fan, Y.-J., Xu, Z.-H. & Zhong-Xi, S. Adsorption behavior of heptyl xanthate on surface of ZnO and Cu (II) activated ZnO using continuous online in situ ATR-FTIR technology. Trans. Nonferrous Metals Soc. China 32, 2370–2378 (2022).
Achehboune, M. et al. Microstructural, FTIR and Raman spectroscopic study of Rare earth doped ZnO nanostructures. Mater. Today Proc. 53, 319–323 (2022).
Kulkarni, S. D., Kumbar, S. M., Menon, S. G., Choudhari, K. & Santhosh, C. Novel magnetically separable Fe3O4@ ZnO core–shell nanocomposite for UV and visible light photocatalysis. Adv. Sci. Lett. 23, 1724–1729 (2017).
Abbasi, S., Ahmadpoor, F., Imani, M. & Ekrami-Kakhki, M.-S. Synthesis of magnetic Fe3O4@ ZnO@ graphene oxide nanocomposite for photodegradation of organic dye pollutant. Int. J. Environ. Anal. Chem. 100, 225–240 (2020).
Heebner, A. & Abbassi, B. Electrolysis catalyzed ozonation for advanced wastewater treatment. J. Water Process Eng. 46, 102638 (2022).
Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 37, 1443–1467 (2003).
De Witte, B., Dewulf, J., Demeestere, K. & Van Langenhove, H. Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water. J. Hazard. Mater. 161, 701–708 (2009).
Popiel, S., Nalepa, T., Dzierżak, D., Stankiewicz, R. & Witkiewicz, Z. Rate of dibutylsulfide decomposition by ozonation and the O< sub> 3</sub>/H< sub> 2</sub> O< sub> 2</sub> advanced oxidation process. J. Hazard. Mater. 164, 1364–1371 (2009).
Dadban Shahamat, Y., Masihpour, M., Borghei, P. & Hoda Rahmati, S. Removal of azo red-60 dye by advanced oxidation process O3/UV from textile wastewaters using Box-Behnken design. Inorg. Chem. Commun. 143, 109785. https://doi.org/10.1016/j.inoche.2022.109785 (2022).
Zawadzki, P. & Deska, M. Degradation efficiency and kinetics analysis of an advanced oxidation process utilizing ozone, hydrogen peroxide and persulfate to degrade the dye rhodamine B. Catalysts 11, 974 (2021).
Bakheet, B. et al. Electro-peroxone treatment of Orange II dye wastewater. Water Res. 47, 6234–6243 (2013).
Asgari, E., Sheikhmohammadi, A., Manshouri, M. & Hashemzadeh, B. The investigation of removal performances of UV/ZnO, UV/ZnO/H2O2 and UV/ZnO/O3 processes in the degradation of Butoben and Phenylmethyl ester from aqueous solution. Optik 228, 166208 (2021).
Pastor, M. A. S., Botelho Junior, A. B., Tenório, J. A. S., Espinosa, D. C. R. & Baltazar, M. D. P. G. Use of O3 and O3/H2O2 for degradation of organic matter from Bayer liquor towards new resource management: kinetic and mechanism. Can. J. Chem. Eng. (2022).
Medellin-Castillo, N. A. et al. Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H< sub> 2</sub> O< sub> 2</sub>, O< sub> 3</sub>/H< sub> 2</sub> O< sub> 2</sub> and O< sub> 3</sub>/activated carbon). Sci. Total Environ. 442, 26–35 (2013).
Feng, H., Liu, M., Zeng, W. & Chen, Y. Optimization of the O3/H2O2 process with response surface methodology for pretreatment of mother liquor of gas field wastewater. Front. Environ. Sci. Eng. 15, 1–13 (2021).
Wang, J. & Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 704, 135249 (2020).
Khuntia, S., Sinha, M. K. & Singh, P. Theoretical and experimental investigation of the mechanism of the catalytic ozonation process by using a manganese-based catalyst. Environ. Technol. 42, 632–639 (2021).
Sheikhmohammadi, A., Asgari, E. & Manshouri, M. The synergistic effect of O3 and H2O2 on the Butyl p-hydroxybenzoate photo-catalytic degradability by UVC/ZnO: Efficiency, kinetic, pathway, mechanism. Optik 239, 166673. https://doi.org/10.1016/j.ijleo.2021.166673 (2021).
Gorito, A. M. et al. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 9, 105315 (2021).
Dadban Shahamat, Y., Hamidi, F., Mohammadi, H. & Ghahrchi, M. Optimisation of COD removal from the olive oil mill wastewater by combined electrocoagulation and peroxone process: Modelling and determination of kinetic coefficients. Int. J. Environ. Anal. Chem. 1–14 (2021).
Yang, Z.-W., Wang, W.-L., Lee, M.-Y., Wu, Q.-Y. & Guan, Y.-T. Synergistic effects of ozone/peroxymonosulfate for isothiazolinone biocides degradation: Kinetics, synergistic performance and influencing factors. Environ. Pollut. 294, 118626. https://doi.org/10.1016/j.envpol.2021.118626 (2022).
Wanga, A.-M. et al. Photodegradation of sulfonamides in UV/ozone, UV/oxidant, and UV/ozone/oxidant systems: Comparison in terms of mineralization efficiency and power consumption. Desalin. Water Treat. 220, 255–264 (2021).
Lester, Y., Avisar, D., Gozlan, I. & Mamane, H. Removal of pharmaceuticals using combination of UV/H2O2/O3 advanced oxidation process. Water Sci. Technol. 64, 2230–2238 (2011).
Joardar, I. & Dutta, S. Advanced Oxidation Processes for Wastewater Treatment 107–116 (CRC Press, 2022).
Jiad, M. M. & Abbar, A. H. Treatment of petroleum refinery wastewater by electrofenton process using a low cost porous graphite air-diffusion cathode with a novel design. Chem. Eng. Res. Des. 193, 207–221. https://doi.org/10.1016/j.cherd.2023.03.021 (2023).
Dalanta, F., Kusworo, T. D. & Aryanti, N. Synthesis, characterization, and performance evaluation of UV light-driven Co-TiO2@SiO2 based photocatalytic nanohybrid polysulfone membrane for effective treatment of petroleum refinery wastewater. Appl. Catal. B Environ. 316, 121576. https://doi.org/10.1016/j.apcatb.2022.121576 (2022).
Al Rasbi, A. W. Y. A., Devi, M. G. & Chandrasekhar, G. Synthesis and application of silica and calcium carbonate nanoparticles in the reduction of organics from refinery wastewater. J. Indian Chem. Soc. 99, 100519. https://doi.org/10.1016/j.jics.2022.100519 (2022).
Jiad, M. M. & Abbar, A. H. Treatment of petroleum refinery wastewater by sono fenton process utilizing the in-situ generated hydrogen peroxide. Al-Khwarizmi Eng. J. 19, 52–67 (2023).
Wang, W., Liu, Y., Yu, S., Wen, X. & Wu, D. Highly efficient solar-light-driven photocatalytic degradation of pollutants in petroleum refinery wastewater on hierarchically-structured copper sulfide (CuS) hollow nanocatalysts. Sep. Purif. Technol. 284, 120254. https://doi.org/10.1016/j.seppur.2021.120254 (2022).
Dalanta, F., Kusworo, T. D., Aryanti, N. & Othman, N. H. Optimization of AC/TiO2/CeO2 composite formulation for petroleum refinery wastewater treatment via simultaneous adsorption-photocatalytic process using D-optimal mixture experimental design. J. Environ. Chem. Eng. 9, 106517. https://doi.org/10.1016/j.jece.2021.106517 (2021).
Acknowledgements
This article results from a PhD thesis in environmental engineering, water and wastewater, at West Tehran University. The authors of the article express their gratitude and respect for this university.
Author information
Authors and Affiliations
Contributions
M.A.: Conceptualization, methodology, investigation, formal analysis, writing—original draft. M.B.: Investigation, methodology, writing—review and editing. A.H.H.: Methodology, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aghazadeh, M., Hassani, A.H. & Borghei, M. Application of photocatalytic proxone process for petrochemical wastewater treatment. Sci Rep 13, 12738 (2023). https://doi.org/10.1038/s41598-023-40045-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40045-4
- Springer Nature Limited