Abstract
Despite many differences, autism spectrum disorder and schizophrenia spectrum disorder share environmental risk factors, genetic predispositions as well as neuronal abnormalities, and show similar cognitive deficits in working memory, perspective taking, or response inhibition. These shared abnormalities are already present in subclinical traits of these disorders. The literature proposes that changes in the inhibitory GABAergic and the excitatory glutamatergic system could explain underlying neuronal commonalities and differences. Using magnetic resonance spectroscopy (1H-MRS), we investigated the associations between glutamate concentrations in the anterior cingulate cortex (ACC), the left/right putamen, and left/right dorsolateral prefrontal cortex and psychotic-like experiences (Schizotypal Personality Questionnaire) and autistic traits (Autism Spectrum Quotient) in 53 healthy individuals (26 women). To investigate the contributions of glutamate concentrations in different cortical regions to symptom expression and their interactions, we used linear regression analyses. We found that only glutamate concentration in the ACC predicted psychotic-like experiences, but not autistic traits. Supporting this finding, a binomial logistic regression predicting median-split high and low risk groups for psychotic-like experiences revealed ACC glutamate levels as a significant predictor for group membership. Taken together, this study provides evidence that glutamate levels in the ACC are specifically linked to the expression of psychotic-like experiences, and may be a potential candidate in identifying early risk individuals prone to developing psychotic-like experiences.
Similar content being viewed by others
Introduction
Already in the earliest clinical reports of schizophrenia and autism spectrum disorder (ASD), their commonalities and associations have been discussed1,2,3. On a phenomenological level, schizophrenia and ASD overlap regarding negative symptoms, social cognition, and perceptual alterations4. Additionally, both disorders display similar cognitive deficits in terms of working memory function, perspective taking, or response inhibition5. Nevertheless, ASD and schizophrenia show distinct clinical profiles and clinical progressions6,7. While ASD is a neurodevelopmental disorder with an early onset, usually diagnosed in childhood, and is characterized by a stable or improving long-term prognosis8, schizophrenia typically develops later in adolescence or early adulthood and is associated with persistent long-term impairment9. The greatest difference between the disorders, however, lies within the type of positive symptoms: while individuals with ASD may show repetitive or restricted behaviors, individuals with schizophrenia may suffer from hallucinations or delusions10.
Despite those differences, both disorders share common environmental risk factors, genetic predispositions as well as neuronal abnormalities4,7. Patients with ASD and schizophrenia show, for example, abnormal development in the anterior cingulate cortex (ACC), striatum and frontal lobe11,12,13. Additionally, shared atypical connections were identified in the default mode network and the salience network14. Both disorders show similar alterations in reward processing15,16 and prediction error learning17,18,19,20, with individuals with ASD showing greater impairment in social reward and prediction error learning. Importantly, both disorders show substantial overlap already at the subclinical and trait level21,22,23.
Potential candidates providing an explanation for the underlying neuronal commonalities and differences are different neurotransmitter systems such as the glutamatergic system. The glutamate hypothesis of schizophrenia is well described24,25, building on the discovery that antagonists of a glutamate receptor (N-methyl-d-aspartate (NMDA) receptor) induced psychotic symptoms. Alterations in neurotransmitter systems may be assessed non-invasively with magnetic resonance spectroscopy (MRS). A recent review24 summarizes that glutamate and Glx (glutamate + glutamine) concentrations are increased in the basal ganglia, thalamus and medial temporal lobe in schizophrenia patients. However, other studies reveal more inconsistent findings, showing increased prefrontal glutamate in anti-psychotic naïve patients26, while another study27 reports glutamate reductions in the ACC in psychosis, but could not detect alterations in the putamen or DLPFC. Reductions in glutamate concentrations have been found more consistently in the ACC in antipsychotic-naïve early psychosis patients28, medicated early psychosis patients29 and a mix of early psychosis and chronic schizophrenia patients30. This supports the theory that increased glutamate is a sign of an acute psychotic or prodromal phase which builds up to a first episode and is linked to inflammatory processes31,32. Interestingly, there are strong links between altered levels of glutamate and neurofunction, which are task-dependent33. In a recent systematic review, Zahid and colleagues34 reported reduced positive associations between ACC glutamate levels and brain activity during resting state conditions, but increased positive associations between ACC glutamate levels and brain activity during cognitive control tasks in early psychosis patients. Wenneberg and colleagues35 and Bojesen and colleagues36 found an association between glutamate and GABA levels and clinical symptoms and cognition in ultra-high-risk individuals in cortical and subcortical regions, supporting the association between ACC glutamate and cognition in the pathophysiology of early psychosis. Together these studies seem to suggest that there are complex interactions, dependent on the brain state, between cortical and subcortical glutamate that may be altered in schizophrenia.
Also, in ASD research there is emerging evidence that an imbalance between different neurotransmitter systems, mainly the excitatory glutamatergic and inhibitory GABAergic system, contributes to the development of the disorder. Alterations with respect to glutamate have been reported in many cortical and subcortical regions. Horder and colleagues37 for example found reduced levels of glutamate in the striatum to be associated with the severity of social symptoms in ASD. Interestingly, Page and colleagues38 found increased concentrations of glutamate/glutamine in the amygdala/hippocampus, but not in parietal regions, while others found increased glutamate in the inferior frontal gyrus39, the sensory motor cortex (children40) or the putamen41. Another study42 found reduced glutamate concentrations in the auditory cortex and increased concentrations in the ACC. Alterations in these regions have also been reported by others43,44. However, also in ASD findings are inconsistent45,46,47. Both disorders are associated with changes in cortico-striatal-thalamic circuits48,49,50 and abnormal excitatory glutamate neurotransmitter concentrations in overlapping cortical and subcortical areas27,37,51,52,53,54,55 including the cingulate cortex, the prefrontal cortex and striatum. This suggests that a complex interaction of altered levels of glutamate in different cortical and subcortical regions may contribute to the development of symptoms within those two disorders.
Consistent with the continuum model of psychotic disorders, such as schizophrenia, and ASD, symptoms of schizophrenia and autism are reflected on a spectrum ranging from subclinical variants of psychotic-like experiences and autistic traits to clinical states of schizophrenia and ASD respectively56 where schizophrenia and autism are seen as extremes of a continuum diverging in opposite directions from normality6,57. Frameworks of subclinical traits allow the investigation of neurobiological mechanisms underlying symptomatology without the influence of potential confounders such as medication, illness duration or age onset. Traits of these disorders may be assessed with the Schizotypal Personality Questionnaire (SPQ) and the Autism Spectrum Quotient (AQ) for psychotic-like experiences and autistic traits, respectively. As for the clinical stages of the disorders, strong associations between both subclinical spectra are reported in the literature. A recent meta-analysis22 revealed a high correlation between autistic and global psychotic-like experiences of 0.48.
Only few studies explored changes of neurotransmitter concentrations associated to psychotic-like experiences and autistic traits. Ford and colleagues58 investigated whether abnormalities in the glutamate/GABA ratio were linked to symptoms shared between the two traits and symptom severity using MRS. They found a positive correlation between glutamate/GABA ratio in the right superior temporal gyrus and the total scores of AQ, SPQ and AQ + SPQ. Additionally, positive correlations with subscales related to social skills and communication58, indicating that alterations in glutamatergic and GABAergic neurotransmitter systems are associated with shared symptoms. Given that the literature reports alterations of neurotransmitter concentrations, mainly glutamate, across a number of different brain regions, it is likely that those regions contribute in an interactive way to the development of clinical symptoms.
The aim of the present study was, therefore, to use MRS to explore if and how glutamate concentrations in five cortical and subcortical areas interact and are associated with autistic traits and psychotic-like experiences in a sample of healthy individuals. The regions included the ACC, the left and right dorsolateral prefrontal cortex (DLPFC), and the left and right putamen. We hypothesized that increased levels of glutamate in the ACC and reduced levels of glutamate in the DLPFC and putamen would be associated with psychotic-like experiences. On the other hand, we hypothesized that reduced levels of ACC glutamate and increased levels of glutamate in the putamen would be associated with autistic traits.
Methods
Participants and subclinical questionnaires
53 healthy subjects (26 women) aged 18–35 years participated in this study. See supplementary materials for recruitment and inclusion criteria. The study was approved by the medical research ethics committee of the Technical University of Munich. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
All participants completed a German version59 of the Schizotypal Personality Questionnaire (SPQ)60 capturing psychotic-like experiences; as well as the Autism Spectrum Quotient (AQ)61 assessing autistic traits (Table 1, Fig. S1; see supplementary material for details on correlations between AQ and SPQ).
MR Image acquisition
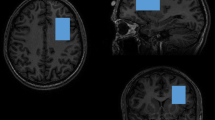
Structural MRI and 1H-MRS data were collected on a 3 T Philips Ingenia Elition X MR-Scanner (Philips Healthcare, Best, The Netherlands) using a 32-channel head coil. For anatomical reference and spectroscopic voxel placement, we acquired a T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence: echo time (TE) = 4 ms, repetition time (TR) = 9 ms, Flip angle (α) = 8°, shot interval = 3000 ms, slice number = 170, matrix size = 240 × 252 and voxel size = 1 × 1 × 1 mm3. MRS data were collected using a 1H-MRS single voxel ECHO volume Point Resolved Spectroscopy Sequence (PRESS) sequence: TR = 2000 ms, TE set to shortest (35.6–41.2 ms), samples = 1024, bandwidth = 2000 Hz, phase cycle steps = 16, and flip angle = 90°. We used the conventional Philips water suppression technique (excitation) that performs Automatic Water Suppression Optimization (AWSO) pre-scans to minimize the residual water (window = 140 Hz, second pulse angle = 300). See supplementary materials for further descriptions. We placed one voxel in the ACC (20 × 20 × 20 mm) and one voxel per hemisphere in the Putamen (20 × 15 × 20 mm) as well as in the DLPFC (30 × 20 × 20 mm); see Fig. 1A–E for voxel placement overlap for each of the five regions.
Voxel placement and representative fitted MRS spectra. Placement of MRS voxels and 1H-MRS spectrum fitted by Osprey of the (A) ACC, (B) DLPFC right, (C) DLPFC left, (D) putamen right, and (E) putamen left. The colors indicate the areas covered by the subjects’ individually placed MRS voxels. The individual voxels were standardized with SPM12, overlapped in MRIcroGL and visualized in FSLeyes. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex.
1H-MRS analysis
The MRS data were analyzed using Osprey version 2.4.0, an all-in-one software for state-of-the-art processing and quantitative analysis of in-vivo MRS data62. As eddy-current correction had already been performed during the automatic pre-processing of the scanner, this was excluded from the automatic preprocessing in Osprey. Subsequently, Osprey executed the remaining processing steps appropriate to the provided data, including frequency-and-phase alignment, water removal, frequency referencing, and initial phasing.
As the TE of our MRS data varied between and within the individual voxels to ensure the shortest acquisition time, we simulated six different PRESS basis sets for each TE (36, 37, 38, 39, 40, 41) with a bandwidth of 2000 Hz and 1024 pts using MARSS in INSPECTOR version 11-202163. Based on recommendations in the LCModel manual64, we selected the following metabolites: alanine, aspartate, creatine (Cr), GABA, glucose, glutamate (Glu), glutamine, glutathione, glycerophosphocholine, lactate, myoinositol, NAA, N-acetyl-aspartylglutamate (NAAG), phosphocholine, phosphocreatine (PCr), scyllo-inositol and taurine. To this basis set we added the default macromolecular and lipid components provided by Osprey.
To analyze our data in Osprey, we utilized the LCModel65 implementation for fitting and quantification. The T1-weighted images were segmented in grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using SPM12 within Osprey. The processing followed standard parameters, with a metabolite fit range of 0.5 to 4.0 ppm and a water fit range of 2.0 to 7.4 ppm. The knot spacing used was 0.4 ppm. Finally, Osprey estimates the tissue and relaxation corrected molal concentration according to the Gasparovic method66. See supplementary materials for a detailed workflow diagram (Supplementary Materials Fig. S2A).
Osprey provides data quality outcomes, including signal-to-noise ratio (Cr SNR; ratio between amplitude of Cr peak and standard deviation of detrended noise in the range of − 2 to 0 ppm62), linewidth for water [full-width half-maximum (FWHM) of single-Lorentzian fit to H2O reference peak62] and Cramer-Rao lower bounds (CRLB) for each estimated metabolite. All spectra were visually inspected for artifacts. An exemplary fit of the spectra can be seen in Fig. 1A–E. Spectral exclusion criteria were therefore either visual failure of the fitting algorithm, a resultant FWHM > 13 Hz in the ACC and DLPFC or FWHM > 10 Hz in the putamen, with thresholds chosen according to recommendations for B0-shimming provided by Juchem et al.67, or a CRLB > 20% of glutamate concentration.
1H-MRS glutamate levels and spectral quality
We measured the tissue and relaxation corrected molal concentration of glutamate (mol/kg) in five voxels of interest, the ACC, the left putamen, right putamen, left DLPFC and right DLPFC. For the ACC and the right DLPFC, the fit of the glutamate spectra were appropriate for all participants. For the left DLPFC, one subject had to be excluded due to visual failure of the fit. Although the visual inspection was appropriate, but generally showing more variation in the residuals, for the left and right putamen, 15 subjects from the left putamen and 20 from the right putamen were excluded due to FWHM > 10 Hz. Results are presented in Table 2. Due to the substantial loss of data in the putamen, we did not include glutamate levels derived from the putamen in any of the following analysis.
Association between glutamate and clinical scores
To understand the contributions of glutamate concentrations in different cortical regions to symptom expression, we first conducted two linear regression models with psychotic-like experiences and autistic traits as outcome variables and levels of glutamate derived from the ACC, left DLPFC and right DLPFC as predictor variables. Furthermore, we corrected for age and sex.
To investigate non-linear relationships between clinical traits and glutamate concentrations, we dichotomized the clinical scores and performed logistic regression analyses. This is important as there has been criticism of the hypothesis of a continuous development of neurobiological changes in psychosis from sub-threshold symptoms to severe psychotic symptoms20,68,69. To dichotomize the clinical scores, we performed a median split, defining a high and low risk group separately for psychotic-like experiences (median = 75) and autistic traits (median = 22). See Fig. 2 for distributions of glutamate by group, and Supplementary Table S3 for demographic and clinical differences. Using a logistic regression, we investigated whether any predictor variables (i.e., levels of glutamate derived from the ACC, left DLPFC and right DLPFC) significantly contributed to a change in the response variable (i.e., low vs high psychotic-like experiences) from 0 to 1 (i.e., from low to high psychotic-like experiences). Also, the logistic regressions were corrected for age and sex. The significance level was set to p < 0.05. Bonferroni multiple comparison corrections were applied, corrected significance levels are reported together with the results. Analyses were performed in R using the stats package version 4.070.
Results
Association between psychotic-like experiences and glutamate concentration in the anterior cingulate cortex
We first fitted two multiple linear regression models to test if absolute glutamate concentrations in the ACC, left DLPFC and right DLPFC predicted symptom scores. The first fitted regression model was: psychotic-like experiences ~ Glu DLPFC_R + Glu DLPFC L + Glu ACC + age + sex. The overall regression was not significant (R2 = 0.15, F(5,46) = 1.63, p = 0.17). Importantly, however we found that levels of glutamate in the ACC significantly predicted psychotic-like experiences (β = 9.72, 95% CI [2.17, 17.27] p = 0.013) (Fig. 3). Autistic traits however were not predicted by levels of glutamate.
Supporting this finding, the binomial logistic regression (PLE-group ~ Glu DLPFC_R + Glu DLPFC L + Glu ACC + age + sex) revealed that while holding all other predictor variables constant, the odds of an individual belonging to the high psychotic-like experiences group increased by 33.1% (β = 0.49, 95% CI [0.01, 2.70], p = 0.014) for a one-unit increase in ACC glutamate. We did not see this effect of glutamate on autistic traits. The effects of the linear and logistic regressions both survived Bonferroni multiple comparison correction (level of significance, p < 0.025, correcting for two tests each). As many studies use Glx (glutamate + glutamine) to measure glutamate, we computed the same analysis using Glx, and replicated our results, which are presented in the Supplementary Materials. Also see Supplementary Table S1 for an overview on levels of Glx. Furthermore, we also computed the same analysis using a novel RStudio based MRS analysis tool—spant71. Again, the results are similar, although only marginally significant, and are presented in the Supplementary Materials, together with a detailed description of the method and analysis (Supplementary Table S2, Supplementary Figs. S2B, S3).
Discussion
In this study, we explored if and how glutamate concentrations in the ACC and left and right DLPFC and putamen are associated with autistic traits and psychotic-like experiences in a sample of healthy individuals. We found that glutamate concentrations in the ACC predicted psychotic-like experiences in healthy individuals, but they did not predict autistic traits. Supporting this finding, we furthermore found that glutamate concentrations in the ACC contributed significantly to the determination of group status (i.e., low psychotic-like experiences vs high psychotic-like experiences) when applying a median split to the clinical data. This was not the case for autistic traits. Regarding the glutamate concentrations in the DLPFC and the putamen, however, we found that whereas the DLPFC did not yield any significant results, levels of glutamate could not be reliably estimated in the putamen.
Association between ACC glutamate and psychotic-like experiences
ACC glutamate levels have been associated with psychotic-like experiences in healthy individuals72, symptoms and structural changes in high-risk individuals73, symptoms in first episode psychosis73,74,75 and chronic psychosis76. Importantly, our results provide support for both a dimensional and a non-linear relationship, showing a positive linear relationship between the severity of psychotic-like experiences and ACC glutamate across the complete sample; importantly however, ACC glutamate is also predictive of whether or not an individual classifies as a high-risk versus a low-risk individual, indicating qualitative differences in the neuropathology of the different disease stages69. This however needs to be explored in future research.
In general, evidence on altered levels of ACC glutamate in the schizophrenia spectrum is inconsistent with some studies reporting higher levels31,74, while others find reductions29,30 or no differences77,78. Although Modinos and colleagues78 did not find different levels of glutamate in the ACC between individuals with high schizotypy and low schizotypy (schizotypy and psychotic-like experiences have overlapping meanings, and are two concepts to describe the liability for psychosis79,80), they found that increased grey matter volume in ACC was negatively related to ACC glutamate. This supports the view that alterations in glutamatergic levels may explain structural changes which are associated with the development of psychotic-like experiences. The association in this, but also in other studies72, however, suggests that decreasing levels of glutamate in the ACC are associated with specific pathologies in psychosis, whereas our study indicates that higher levels of ACC glutamate are associated with the development of psychotic-like experiences. Our results correspond to findings from Demro and colleagues81 who reported that increased subclinical symptoms of grandiosity were linked to increased levels of glutamate in the ACC. Egerton and colleagues82 found higher levels of glutamate/creatine-ratio in the ACC in symptomatic first episode psychosis patients compared to those in remission. In a different study, Egerton and colleagues83 found that higher levels of glutamate metabolites in the ACC predicted treatment response, indicating that higher baseline levels are associated with poorer treatment response, while lower levels of ACC glutamate are predictive of improvements across positive and negative symptoms as well as general functioning in first episode psychosis patients. Relatedly, Godlewska and colleagues27 reported lower levels of glutamate in the ACC in early psychosis patients compared to healthy controls. Importantly, however, the majority of patients included in this study were medicated, which confirms results from a recent meta-analysis84 on the impact of anti-psychotic treatment on frontal glutamate levels. The authors found that antipsychotics were linked to a significant decrease in frontal Glx levels in both first episode and chronic schizophrenia patients, with the effect being stronger in first episode psychosis patients. As our results indicate that ACC glutamate levels are predictive of individuals belonging to the high psychotic-like experience group, the results are in line with the theory that increased glutamate is a sign of an acute psychotic or prodromal phase which builds up to a first episode of psychosis and is linked to inflammatory processes31,32. The theory further suggests that a hypofunction of NMDA receptors reduces the activity of inhibitory GABAergic interneurons, on which they are located. This activity reduction increases glutamatergic neurotransmission of pyramidal cells, and may contribute to the development of, especially, positive symptoms during an acute episode of psychosis32,85.
Lack of effect for other regions and autistic traits
Surprisingly, our data did not reveal any associations between the DLPFC and psychotic-like experiences. Generally, the literatures present an inconsistent picture regarding glutamate concentration in different regions. For example, although Godlewska and colleagues27 reported alterations in glutamate in the ACC in early psychosis patients compared to healthy controls, they did not find differences in the putamen and DLPFC. However, especially the literature with regard to subclinical schizotypal traits is sparse, and mainly concentrates on ACC and hippocampal glutamate72,78. To our knowledge, only one other study investigated prefrontal glutamate in individuals with high schizotypy compared to low schizotypy86, in which the authors reported a reduction of glutamate in high schizotypy.
Furthermore, our data did not reveal any associations between glutamate concentrations and autistic traits despite several studies showing an interaction, which is at odds with results reported in patients suffering from ASD (e.g.,37,38,40,42,43,44,45). Interestingly, studies exploring psychotic-like experiences and autistic traits together found similar patterns between both subclinical scores. For example, results by Ford and colleagues87 suggest that social disorganization is associated with increased glutamate/GABA + ratio in the right superior temporal region across both the autistic and schizotypal spectrum. Whereas, Kondo and colleagues88 report that autistic and schizotypal traits were associated with the Glutamine + Glutamate/GABA ratio in the auditory cortex but not in the frontal areas, including ACC, DLPFC and inferior frontal cortex.
Based on these studies and the fact that psychotic-like experiences and autistic traits are highly correlated, this might not be surprising at all. Our results however clearly indicate no such relationship between glutamate and autistic traits. We hypothesize that the relationship between changes in glutamate and autistic traits is mediated by psychotic-like experiences, which is rooted in their high correlation. To provide some initial evidence we computed an exploratory non-parametric causal mediation model, which is described in the supplementary materials. Indeed, our results revealed that the association between ACC glutamate and autistic traits is fully mediated by psychotic-like experiences. This may suggest that the high correlation between the two subclinical concepts results has a confounding on the relationship, and that underlying and often unassessed psychotic-like experiences may be the driving factor in the relationship between glutamatergic changes and autistic traits. However, more research is needed to explore this hypothesis.
Consistency of results using different measures of glutamate and a novel toolbox
In addition to our analysis using the LCModel implementation in Osprey and extracting absolute water and tissue corrected levels of glutamate (mol/kg), we also replicated our results using Glx as well as a novel r-implemented toolbox (spant71). The replication of our results using different types of concentrations and methods further increases confidence in our results. Nevertheless, studies should focus on consistent ways of measuring metabolite differences, as it is not only the choice of location for voxel placement that differs grossly among studies but also the choice of metabolite representation (e.g., glutamate/GABA ratio, absolute glutamate, glutamate/creatine ratio, etc.) and selection of basis sets.
Limitations
This study has several limitations. First, we did not analyze levels of GABA in the voxels of interest, as no spectral editing was applied to the MRS sequence. Future studies should try to measure both metabolites reliably in order to understand interactions between neurotransmitters and regions. Second, due to high values of FWHM, glutamate concentrations estimated in the left and right putamen had to be excluded from the analysis. One possible explanation for this is that subcortical voxels are usually noisier. Higher resolution (e.g., 7 T) or spectral editing of the sequence should be applied to improve data quality. Third, we were unable to recruit participants with highly increased scores along both spectra. One possible explanation is that many individuals with highly increased scores would already have a clinical psychiatric diagnosis, which was an exclusion criterion in our study. Nevertheless, follow-up studies should recruit larger samples allowing for a larger spectrum. Fourth, our sample does not include many individuals with low SPQ and high AQ scores, and vice versa, which would be necessary in order to clearly differentiate between clinical traits. Yet, our sample represents the distribution of subclinical symptoms within the general population as confirmed by similar correlations of the two scores compared to previous studies22,89. Bigger samples would be favorable to also investigate the clinical subscores (e.g., positive-like experiences, negative-like experiences).
Conclusion
Taken together, this study shows that changes in glutamate in the ACC are associated with the manifestation of psychotic-like experiences but not autistic traits, which indicates that an imbalance in the glutamatergic neurotransmitter system involving the ACC may contribute to the development of psychotic-like experiences specifically. These results suggest that glutamate levels could potentially serve as a promising indicator for identifying individuals at an early stage who are susceptible to experiencing psychotic-like episodes.
Data availability
Data is available upon reasonable request to the corresponding author (franziskaknolle@gmail.com/franziska.knolle@tum.de).
References
Mercier, C. A. Sanity and Insanity (Scott, 1890).
de Lacy, N. & King, B. H. Revisiting the relationship between autism and schizophrenia: Toward an integrated neurobiology. Annu. Rev. Clin. Psychol. 9, 555–587 (2013).
Bleuler E. Dementia praecox, oder, Gruppe der Schizophrenien. Wellcome Collect. (1911). https://wellcomecollection.org/works/v2qqa9g4/items (Accessed 20 Jan 2023).
Jutla, A., Foss-Feig, J. & Veenstra-VanderWeele, J. Autism spectrum disorder and schizophrenia: An updated conceptual review. Autism Res. 15, 384–412 (2022).
Ford, T. C., Apputhurai, P., Meyer, D. & Crewther, D. P. Cluster analysis reveals subclinical subgroups with shared autistic and schizotypal traits. Psychiatry Res. 265, 111–117 (2018).
Nenadić, I. et al. Subclinical schizotypal vs. autistic traits show overlapping and diametrically opposed facets in a non-clinical population. Schizophr. Res. 231, 32–41 (2021).
Zheng, Z., Zheng, P. & Zou, X. Association between schizophrenia and autism spectrum disorder: A systematic review and meta-analysis. Autism Res. 11, 1110–1119 (2018).
Anderson, D. K., Liang, J. W. & Lord, C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry 55, 485–494 (2014).
McCutcheon, R. A., Reis Marques, T. & Howes, O. D. Schizophrenia—An overview. JAMA Psychiat. 77, 201 (2020).
Trevisan, D. A. et al. Autism spectrum disorder and schizophrenia are better differentiated by positive symptoms than negative symptoms. Front. Psychiatry https://doi.org/10.3389/fpsyt.2020.00548 (2020).
Langen, M. et al. Changes in the developmental trajectories of striatum in autism. Biol. Psychiatry 66, 327–333 (2009).
McCutcheon, R. A., Abi-Dargham, A. & Howes, O. D. Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends Neurosci. 42, 205–220 (2019).
Cauda, F. et al. Are schizophrenia, autistic, and obsessive spectrum disorders dissociable on the basis of neuroimaging morphological findings?: A voxel-based meta-analysis: Dissociability of autism, OCD, schizophrenia. Autism Res. 10, 1079–1095 (2017).
Chen, H. et al. Shared atypical default mode and salience network functional connectivity between autism and schizophrenia: Shared atypical FC in ASD and schizophrenia. Autism Res. 10, 1776–1786 (2017).
Scott-Van Zeeland, A. A., Dapretto, M., Ghahremani, D. G., Poldrack, R. A. & Bookheimer, S. Y. Reward processing in autism. Autism Res. 3, 53–67 (2010).
Kesby, J. P., Murray, G. K. & Knolle, F. Neural circuitry of salience and reward processing in psychosis. Biol. Psychiatry Glob. Open Sci. https://doi.org/10.1016/j.bpsgos.2021.12.003 (2021).
Kinard, J. L. et al. Neural mechanisms of social and nonsocial reward prediction errors in adolescents with autism spectrum disorder. Autism Res. 13, 715–728 (2020).
Cannon, J., O’Brien, A. M., Bungert, L. & Sinha, P. Prediction in autism spectrum disorder: A systematic review of empirical evidence. Autism Res. 14, 604–630 (2021).
Montagnese, M. et al. Reinforcement learning as an intermediate phenotype in psychosis? Deficits sensitive to illness stage but not associated with polygenic risk of schizophrenia in the general population. Schizophr. Res. 222, 389–396 (2020).
Ermakova, A. O. et al. Abnormal reward prediction-error signalling in antipsychotic naive individuals with first-episode psychosis or clinical risk for psychosis. Neuropsychopharmacology 43, 1691–1699 (2018).
Dinsdale, N. L., Hurd, P. L., Wakabayashi, A., Elliot, M. & Crespi, B. J. How are autism and schizotypy related? Evidence from a non-clinical population. PLoS ONE 8, e63316 (2013).
Zhou, H. et al. Revisiting the overlap between autistic and schizotypal traits in the non-clinical population using meta-analysis and network analysis. Schizophr. Res. 212, 6–14 (2019).
Larson, F. V., Wagner, A. P., Chisholm, K., Reniers, R. L. E. P. & Wood, S. J. Adding a dimension to the dichotomy: Affective processes are implicated in the relationship between autistic and schizotypal traits. Front. Psychiatry 11, 712 (2020).
McCutcheon, R. A., Krystal, J. H. & Howes, O. D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 19, 15–33 (2020).
Uno, Y. & Coyle, J. T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 73, 204–215 (2019).
Kaminski, J. et al. Glutamate in the dorsolateral prefrontal cortex in patients with schizophrenia: A meta-analysis of 1H-magnetic resonance spectroscopy studies. Biol. Psychiatry 89, 270–277 (2021).
Godlewska, B. R. et al. Brain glutamate concentration in men with early psychosis: A magnetic resonance spectroscopy case-control study at 7 T. Transl. Psychiatry 11, 1–7 (2021).
Bojesen, K. B. et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naïve patients with psychosis. Psychol. Med. 50, 2182–2193 (2020).
Jauhar, S. et al. The relationship between cortical glutamate and striatal dopamine in first-episode psychosis: A cross-sectional multimodal PET and magnetic resonance spectroscopy imaging study. Lancet Psychiatry 5, 816–823 (2018).
Sydnor, V. J. & Roalf, D. R. A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: Implications for studies of psychosis risk. Schizophr. Res. 226, 61–69 (2020).
Kumar, J. et al. Glutathione and glutamate in schizophrenia: A 7T MRS study. Mol. Psychiatry 25, 873–882 (2020).
Moghaddam, B. & Krystal, J. H. Capturing the angel in ‘angel dust’: Twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull. 38, 942–949 (2012).
Haarsma, J. et al. Influence of prior beliefs on perception in early psychosis: Effects of illness stage and hierarchical level of belief. J. Abnorm. Psychol. 129, 581–598 (2020).
Zahid, U. et al. Neurofunctional correlates of glutamate and GABA imbalance in psychosis: A systematic review. Neurosci. Biobehav. Rev. 144, 105010 (2023).
Wenneberg, C. et al. Cerebral glutamate and gamma-aminobutyric acid levels in individuals at ultra-high risk for psychosis and the association with clinical symptoms and cognition. Biol. Psychiatry Cogn. Neurosci. Neuroimaging https://doi.org/10.1016/j.bpsc.2019.12.005 (2019).
Bojesen, K. B. et al. Associations between cognitive function and levels of glutamatergic metabolites and gamma-aminobutyric acid in antipsychotic-naïve patients with schizophrenia or psychosis. Biol. Psychiatry 89, 278–287 (2021).
Horder, J. et al. Glutamate and GABA in autism spectrum disorder—A translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 8, 1–11 (2018).
Page, L. A. et al. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am. J. Psychiatry 163, 2189–2192 (2006).
Sapey-Triomphe, L.-A., Temmerman, J., Puts, N. A. J. & Wagemans, J. Prediction learning in adults with autism and its molecular correlates. Mol. Autism 12, 64 (2021).
He, J. L. et al. Region-specific elevations of glutamate + glutamine correlate with the sensory symptoms of autism spectrum disorders. Transl. Psychiatry 11, 1–10 (2021).
Doyle-Thomas, K. A. R. et al. Metabolic mapping of deep brain structures and associations with symptomatology in autism spectrum disorders. Res. Autism Spectr. Disord. 8, 44–51 (2014).
Joshi, G. et al. Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: A pilot study at 4T. Eur. Arch. Psychiatry Clin. Neurosci. 263, 379–384 (2013).
Brown, M. S., Singel, D., Hepburn, S. & Rojas, D. C. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: A 1H-MRS study: Increased glutamate concentration in autism. Autism Res. 6, 1–10 (2013).
TebartzvanElst, L. et al. Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: Evidence in support of the excitatory/inhibitory imbalance hypothesis. Mol. Psychiatry 19, 1314–1325 (2014).
Kolodny, T. et al. Concentrations of cortical GABA and glutamate in young adults with autism spectrum disorder. Autism Res. Off. J. Int. Soc. Autism Res. 13, 1111–1129 (2020).
Shirayama, Y. et al. The lack of alterations in metabolites in the medial prefrontal cortex and amygdala, but their associations with autistic traits, empathy, and personality traits in adults with autism spectrum disorder: A preliminary study. J. Autism Dev. Disord. https://doi.org/10.1007/s10803-022-05778-7 (2022).
Maier, S. et al. Increased prefrontal GABA concentrations in adults with autism spectrum disorders. Autism Res. 15, 1222–1236 (2022).
Li, W. & Pozzo-Miller, L. Dysfunction of the corticostriatal pathway in autism spectrum disorders. J. Neurosci. Res. 98, 2130–2147 (2020).
Dandash, O., Pantelis, C. & Fornito, A. Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophr. Res. 180, 48–57 (2017).
Sabaroedin, K. et al. Effective connectivity of fronto-striato-thalamic circuitry across the psychosis continuum. Biol. Psychiatry 89, S356 (2021).
Ford, T. C. & Crewther, D. P. A comprehensive review of the 1H-MRS metabolite spectrum in autism spectrum disorder. Front. Mol. Neurosci. https://doi.org/10.3389/fnmol.2016.00014 (2016).
Shukla, D. K. et al. Anterior cingulate glutamate and GABA associations on functional connectivity in schizophrenia. Schizophr. Bull. 45, 647–658 (2019).
Ford, T. C., Abu-Akel, A. & Crewther, D. P. The association of excitation and inhibition signaling with the relative symptom expression of autism and psychosis-proneness: Implications for psychopharmacology. Prog. Neuropsychopharmacol. Biol. Psychiatry 88, 235–242 (2019).
Wenneberg, C. et al. Cerebral glutamate and GABA levels in high-risk of psychosis states: A focused review and meta-analysis of 1H-MRS studies. Schizophr. Res. 215, 38–48 (2020).
Purcell, A. E., Jeon, O. H., Zimmerman, A. W., Blue, M. E. & Pevsner, J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57, 1618–1628 (2001).
Nelson, M. T., Seal, M. L., Pantelis, C. & Phillips, L. J. Evidence of a dimensional relationship between schizotypy and schizophrenia: A systematic review. Neurosci. Biobehav. Rev. 37, 317–327 (2013).
Chisholm, K., Lin, A., Abu-Akel, A. & Wood, S. J. The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence. Neurosci. Biobehav. Rev. 55, 173–183 (2015).
Ford, T. C., Nibbs, R. & Crewther, D. P. Glutamate/GABA+ ratio is associated with the psychosocial domain of autistic and schizotypal traits. PLoS ONE 12, e0181961 (2017).
Klein, C., Andresen, B. & Jahn, T. Erfassung der schizotypen Persönlichkeit nach DSM-III-R: Psychometrische Eigenschaften einer autorisierten deutschsprachigen Übersetzung des ‘Schizotypal Personality Questionnaire’ (SPQ) von Raine [Psychometric assessment of the schizotypal personality according to DSM-III-R criteria: Psychometric properties of an authorized German translation of Raine’s ‘Schizotypal Personality Questionnaire’ (SPQ)]. Diagnostica 43, 347–369 (1997).
Raine, A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 17, 555–564 (1991).
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J. & Clubley, E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17 (2001).
Oeltzschner, G. et al. Osprey: Open-source processing, reconstruction & estimation of magnetic resonance spectroscopy data. J. Neurosci. Methods 343, 108827 (2020).
Landheer, K., Swanberg, K. M. & Juchem, C. Magnetic resonance Spectrum simulator (MARSS), a novel software package for fast and computationally efficient basis set simulation. NMR Biomed. 34, e4129 (2021).
Provencher S. LCModel1 & LCMgui User’s Manual (2021) http://lcmodel.ca/pub/LCModel/manual/manual.pdf.
Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reason. Med. 30, 672–679 (1993).
Gasparovic, C. et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reason. Med. 55, 1219–1226 (2006).
Juchem, C. et al. B0 shimming for in vivo magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. 34, e4350 (2021).
Murray, G. K. & Jones, P. B. Psychotic symptoms in young people without psychotic illness: Mechanisms and meaning. Br. J. Psychiatry 201, 4–6 (2012).
Lawrie, S. M., Hall, J., McIntosh, A. M., Owens, D. G. C. & Johnstone, E. C. The ‘continuum of psychosis’: Scientifically unproven and clinically impractical. Br. J. Psychiatry 197, 423–425 (2010).
R Core Team. R: A Language and Environment for Statistical Computing (2022) https://www.R-project.org/.
Wilson, M. spant: An R package for magnetic resonance spectroscopy analysis. J. Open Source Softw. 6, 3646 (2021).
Modinos, G. et al. Corticolimbic hyper-response to emotion and glutamatergic function in people with high schizotypy: A multimodal fMRI-MRS study. Transl. Psychiatry 7, e1083–e1083 (2017).
Merritt, K., Egerton, A., Kempton, M. J., Taylor, M. J. & McGuire, P. K. Nature of glutamate alterations in schizophrenia: A meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiat. 73, 665–674 (2016).
Sigvard, A. K. et al. Dopamine synthesis capacity and GABA and glutamate levels separate antipsychotic-naïve patients with first-episode psychosis from healthy control subjects in a multimodal prediction model. Biol. Psychiatry Glob. Open Sci. 3, 500–509 (2022).
Reid, M. A. et al. 7T proton magnetic resonance spectroscopy of the anterior cingulate cortex in first-episode schizophrenia. Schizophr. Bull. 45, 180–189 (2019).
Marsman, A. et al. Glutamate in schizophrenia: A focused review and meta-analysis of 1H-MRS studies. Schizophr. Bull. 39, 120–129 (2013).
Maximo, J. O., Briend, F., Armstrong, W. P., Kraguljac, N. V. & Lahti, A. C. Salience network glutamate and brain connectivity in medication-naïve first episode patients—A multimodal magnetic resonance spectroscopy and resting state functional connectivity MRI study. NeuroImage Clin. 32, 102845 (2021).
Modinos, G. et al. Neuroanatomical changes in people with high schizotypy: Relationship to glutamate levels. Psychol. Med. 48, 1880–1889 (2018).
Grant, P., Green, M. J. & Mason, O. J. Models of schizotypy: The importance of conceptual clarity. Schizophr. Bull. 44, S556–S563 (2018).
Lee, K.-W. et al. A systematic review on definitions and assessments of psychotic-like experiences. Early Interv. Psychiatry 10, 3–16 (2016).
Demro, C. et al. Glutamatergic metabolites among adolescents at risk for psychosis. Psychiatry Res. 257, 179–185 (2017).
Egerton, A. et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology 37, 2515–2521 (2012).
Egerton, A. et al. Anterior cingulate glutamate metabolites as a predictor of antipsychotic response in first episode psychosis: Data from the STRATA collaboration. Neuropsychopharmacology 48, 567–575 (2023).
Kubota, M., Moriguchi, S., Takahata, K., Nakajima, S. & Horita, N. Treatment effects on neurometabolite levels in schizophrenia: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophr. Res. 222, 122–132 (2020).
Veerman, S., Schulte, P. & de Haan, L. The glutamate hypothesis: A pathogenic pathway from which pharmacological interventions have emerged. Pharmacopsychiatry 47, 121–130 (2014).
Kozhuharova, P., Diaconescu, A. O. & Allen, P. Reduced cortical GABA and glutamate in high schizotypy. Psychopharmacology 238, 2459–2470 (2021).
Ford, T. C., Nibbs, R. & Crewther, D. P. Increased glutamate/GABA+ ratio in a shared autistic and schizotypal trait phenotype termed Social Disorganisation. NeuroImage Clin. 16, 125–131 (2017).
Kondo, H. M. & Lin, I.-F. Excitation-inhibition balance and auditory multistable perception are correlated with autistic traits and schizotypy in a non-clinical population. Sci. Rep. 10, 8171 (2020).
Ford, T. C. & Crewther, D. P. Factor analysis demonstrates a common schizoidal phenotype within autistic and schizotypal tendency: Implications for neuroscientific studies. Front. Psychiatry 5, 117 (2014).
Acknowledgements
We would like to thank all participants for their time and engagement.
This work was supported by the doctoral program “Translationale Medizin” of the Technical University of Munich funded by Else Kröner-Fresenius-Stiftung (EKFS) to VD.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.K., V.F.D., E.F.S., C.Z. conceptualised the study; F.K., V.F.D., E.F.S. collected the data; F.K., V.F.D., M.W. conducted the formal analysis; F.K., V.F.D. wrote the first manuscript; F.K., V.F.D., E.F.S., C.Z., M.W. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demler, V.F., Sterner, E.F., Wilson, M. et al. Association between increased anterior cingulate glutamate and psychotic-like experiences, but not autistic traits in healthy volunteers. Sci Rep 13, 12792 (2023). https://doi.org/10.1038/s41598-023-39881-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39881-1
- Springer Nature Limited