Abstract
Chronic rhinosinusitis with nasal polyps (CRSwNP) is defined as a Type 2 eosinophilic disease, while CRSsNP is considered a Type 1 neutrophilic disease. Since neutrophils are also activated in eosinophilic CRSwNP, the eosinophil–neutrophil dualism has been revaluated. Among the inflammatory cells infiltrating sinus-nasal tissues, the role of mast cells (MCs) is not already recognized, although Clinical-Cytological Grading, which defines the severity of CRSwNP, attributes to mixed eosinophil-MC forms of CRSwNP a greater risk of recurrence. We aimed to examine nasal polyps from both a cytological and histopathological point of view, to evaluate the presence and localization of MCs. Cytological and histological examination of 39 samples of nasal polyps were performed. Immunohistochemistry was used to evaluate the presence of Tryptase + CD117 + MCs, which were counted both in the epithelial layer and in the lamina propria. A statistically significant correlation was found between intraepithelial MCs and CRSwNP severity (p < 0.001) and between the total eosinophil count and the total mast cell count (p < 0.001). Cytological examination and immunohistochemistry were comparable in detecting the presence of intraepithelial MCs (p = 0.002). The histological cut-off of 6 intraepithelial MCs was identified to detect severe CRSwNP (p < 0.001). MCs have been shown to be located in the lamina propria of almost all eosinophilic nasal polyps without significantly affecting their severity. Intraepithelial MCs are associated with greater severity of CRSwNP. Histopathological criteria of the eosinophil-MC form of CRSwNP in addition to the eosinophilic one, should be defined to guarantee patients effective and tailored treatments.
Similar content being viewed by others
Introduction
In the era of Precision Medicine (PM) research is focusing on the identification of the molecular pathways underlying Chronic rhinosinusitis (CRS), defined as a persistent sino-nasal inflammation associated with tissue remodelling, dysfunction of the sinuses' natural defence mechanisms, and exacerbation of different inflammatory patterns1.
EPOS 2020 Guidelines define three inflammatory patterns resulting from the epithelial barrier damage that underlies CRS: type 1 immune responses, targeting viruses; type 2 responses, targeting parasites; type 3 responses, targeting extracellular bacteria and fungi. Generally, these inflammatory patterns restore the barrier integrity and eliminate pathogens. However, in CRS, the epithelial barrier damage and the consequential penetration of exogenous agents results in a chronic inflammatory response that fails to resolve, but still typically utilizes the type 1, 2 or 3 pathways alone, or in combinations2.
In Western Countries, Type 2 inflammation is associated with 85% of Chronic rhinosinusitis with nasal polyps (CRSwNP), while Chronic rhinosinusitis without nasal polyps (CRSsNP) is mainly considered a Type 1 mediated disease3,4. Type 2 inflammation is characterized by eosinophilic activation and by increased levels of cytokines IL-4, IL-5 and IL-13. On the contrary, Type 1 and Type 3 responses are both characterized by neutrophilic activation, mediated by IFN-γ/IL-12 and IL-17/IL-22 respectively.
Recently, the eosinophil–neutrophil dualism has been revaluated, since neutrophils, as primary inflammatory cells, have been shown to be highly activated also in nasal polyps eosinophilic tissues5. Among the inflammatory cells that infiltrate the sinus-nasal tissue, the role of mast cells (MCs) is not already recognized, although a Clinical-Cytological Grading, already used to define the severity of CRSwNP as a function of comorbidities and cellular inflammatory infiltrate, attributes to mixed eosinophil-MC forms of CRSwNP a greater risk of recurrence6.
Based on this background, the study aimed to evaluate nasal polyps from a cytological and histopathological point of view, with the aid of immunohistochemistry, in order to evaluate the presence of mast cells, which can be assessed with nasal cytology but not with common stains used for histological examination.
Materials and methods
Thirty-nine consecutive patients suffering from CRSwNP at the Department of Otolaryngology of the University Hospital of Foggia were recruited. Before undergoing endoscopic sinus surgery (EES), all patients underwent careful medical history evaluation, nasal cytology and nasal endoscopy. Then, during ESS, under general anesthesia, endoscopic-guided biopsy samples from nasal polyps were obtained Weil-Blakesley forceps.
Informed written consent was obtained from all participants. The study was approved by the Area 1 Ethics Committee (44/CE/2022 del 4.4.2022 e DCS n. 131 del 19.4.2022) of the University Hospital of Foggia. All experiments were performed in accordance with relevant named guidelines and regulations.
Medical history
Before surgery, the complete medical history of each patient was collected, paying particular attention to atopy, asthma, aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) sensitivity and previous polypectomy procedures. Moreover, each patient completed the Sino-Nasal Outcomes (SNOT)-22 survey to subjectively assess disease-related symptoms7.
Nasal endoscopy
Preoperative nasal endoscopy was carried out by a 3.4 mm diameter flexible fiberscope (Vision-Sciences® ENT-2000) to assess the size of sinonasal polyps using Meltzer endoscopic polyp scores (Total Nasal Polyp Score, TNPS). According to this scoring system, each nasal cavity is scored as grade 0 (no polyps), grade 1 (polyp withinmiddle meatus), grade 2 (occupying the middle meatus), grade 3 (extending beyond the middle meatus, within the sphenoethmoid recess but not totally obstructing, or both) or grade 4 (completely obstructing the nasal cavity). Thus, the total score is the sum of left and right nostril scores, ranging from 0 to 88,9.
Nasal cytology and Clinical-Cytological Grading (CCG)
Cytological samples were collected before ESS by Nasal Scraping® (EP Medica, Italy), under anterior rhinoscopy, from the middle part of the inferior turbinate. Samples obtained were immediately smeared on a glass side, air-dried, stained with May-Grunwald-Giemsa (MGG) and then read at optical microscopy, with a 100× objective with oil immersion. Fifty fields were considered the minimum number to identify a sufficient number of cells. The predominant type of inflammatory cell was considered. Thus, four cytologic phenotypes were identified: neutrophilic, eosinophilic, MCs and mixed cellularity (eosinophil and MCs)10.
Thanks to nasal cytology findings and the medical history of each patient, the severity of CRSwNP can be defined. In particular, a Clinical-Cytological Grading (CCG) has been defined to assess the severity of CRSwNP and the related Prognostic Index of Relapse (PIR) according to both nasal inflammatory infiltrate and co-morbidities such as asthma, allergy and ASA sensitivity. The total CCG is given by the sum of the scores attributed to the phenotype, represented by each comorbidity, and to the endotype, represented by each inflammatory cell pattern. A CCG between 1 and 3 is considered low, 4–6 moderate and ≥ 7 high11.
Histology and immunohistochemistry
The surgical specimens of nasal polyps were fixed in 10% buffered formalin and then subjected to usual processing by paraffin inclusion and hematoxylin–eosin (HE) staining.
Since MCs cannot be marked by HE, also immunohistochemical staining was performed. Indeed, as our intent was to investigate the occurrence and spatial distribution of mast cells in nasal mucosa, we excluded traditional histological stains, such as Toluidine blue and Giemsa, which allow to identify the intact MCs but not those in degranulation. Thus, we evaluated by immunohistochemistry the expression of two markers of MCs12: CD-117 (c-kit receptor), which is strongly expressed on the mast cell membrane and is not affected by the state of degranulation, to determine the presence of MCs and obtain the MC count13; Tryptase, which is considered as the marker of choice for the study of mast cell activation, to evaluate the degree of degranulation and, in a certain extent, mast cells activity 14.
Serial section 4 μm thick were cut from formalin-fixed paraffin-embedded tissue, deparaffinized in xylene, rehydratedin graded ethanol solutions, washed with distilled water and mounted on poly-l-lysine-coated glass slides.
Tryptase and CD117 expression was assessed by standard linked streptavidin–biotin horseradish peroxidase technique (LSAB-HRP) using specific monoclonal antibodies against Tryptase (mouse monoclonal primary antibody, clone G3) and anti-CD117 (rabbit monoclonal primary antibody, clone EP10) delivered by the BenchMark ULTRA (Ventana Medical Systems Inc, Roche Group).
Positive and negative controls were used. For each specimen, the whole section was examined under light microscopy (400× magnification), and the immunohistochemical expression was assessed as number of positive nuclei. In detail, ten high-power field (HPF) for each sample were recorded as mean value per HPF and both eosinophils and MCs were counted by two independent expert pathologists. Moreover, the presence of MCs both in the epithelial layer and in the lamina propria of the nasal mucosa was evaluated.
Data analysis
All analyzes were conducted with SPSS-25 (SPSS, Chicago, USA). By means of the Kolmogorov–Smirnov test we verified that only age had a normal distribution while the other continuous variables had a non-parametric distribution. Continuous parametric variables were expressed as m (mean) ± SD (standard deviation). Continuous nonparametric variables were expressed as median (Interquartile Range (IQ) 25, 75). The dichotomous or non-continuous variables were expressed as%. Student's T test for independent samples was performed to compare the variables continue between subgroups.
For continuous variables with non-parametric distribution, the comparison for independent samples was performed by the Mann – Whitney U test. The Kruskal Wallis test was used for the non-parametric comparison of 3 groups and the ANOVA test for the comparison of continuous variables. The Chi-Square test was used for the comparison of the discontinuous variables.
A ROC curve was constructed to verify the accuracy of intraepithelial MCs in detecting the presence of high CCG. The cutoff was identified using the Youden index.
The Spearman correlation test was performed.
A significance value of p < 0.050 was assumed for all analyzes.
Results
The study group was divided in three groups depending on CCG of CRSwNP (low, n = 10; medium, n = 13; high, n = 16). Patients’ demographic characteristics, comorbidities, CCG and SNOT-22 scores are summarized in Table 1. Table 2 shows nasal cytology and immunohistochemistry findings.
No statistically significant correlations were found between MC distribution and laboratory data.
Charcot-Leyden Crystals (CLCs) were found in 10% of patients with low CCG, in 23% of patients with medium CCG and 31% of patients with high CCG.
No MCs were found in HE-stained samples.
A statistically significant correlation was found between intraepithelial MCs and CCG (R2 = 0.225; p < 0.001) (Fig. 1), as intraepithelial MCs increased with increasing CCG, as well between Tryptase expression and CGG, as MC activity increased with CRSwNP severity. Similarly, a correlation was found between the total eosinophil count and the total mast cell count (R2 = 0.160; p < 0.001) (Fig. 2).
A statistically significant comparison was found between cytological examination and immunohistochemistry, as regards the histological presence of intraepithelial MCs and the finding of MCs at nasal cytology (p = 0.002) (Figs. 3A–C, 4A–C, 5A–E). Moreover, the histological cut-off of 6 intraepithelial MCs was identified to detect severe CCG. The cutoff of 6 had a sensitivity of 62.5%, a specificity of 91.3%, a positive predictive value of 83.3%, a negative predictive value of 77.7% (AUC = 0.874 (0.767–0.980), p < 0.001) (Fig. 6).
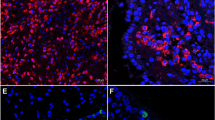
Samples from a patient with medium CCG. (A) Nasal cytology. E eosinophil, M mast cell, De degranulation. MGG staining. Magnification ×1000. (B) Histology. HE staining. Magnification ×200. (C) Immunohistochemistry, using specific monoclonal antibodies against anti-CD117, performed on the closest section to that used for histology. Magnification ×200. The arrows indicate CD117 + MCs.
Samples from a patient with high CCG. (A) Nasal cytology. Magnification ×1000. (B) Histology. HE staining. Magnification ×200. (C) Immunohistochemistry using specific anti-CD117 monoclonal antibodies on the closest section to that used for histology. The arrows indicate CD117 + MCs. Magnification ×200. (D) Histology. HE staining. Magnification ×400 (E) Immunohistochemistry, using anti-CD117 monoclonal antibodies. Magnification ×400.
Discussion
Type 2 CRS is the most extensively studied subset of CRS and, thus, many of the molecular details have been confirmed at the protein level. As a matter of fact, the first data date back to almost 20 years ago, when Van Zele and colleagues described a Th2 polarization in CRSwNP, once defined as nasal polyposis (NP), characterized by abundant eosinophils, high IL-5 levels, and IgE formation. CRSsNP instead was described as Th1 polarized disease, with high levels of IFN-c and TGF-b15.
Since then, several studies have focused on the dualism between Type 1 and Type 2 inflammation, not considering Type 3 inflammation, which has not yet been extensively studied16. Up to now eosinophilic-neutrophilic inflammation has been considered as black and white, almost implying mutual exclusion in CRS. Recently, this dualism has been revaluated, as it trivializes much more complex cellular and cytokines interactions in Type 2 inflammation, which displays also a severe neutrophilic inflammation17. As a matter of fact, neutrophils are traditionally considered primary inflammatory cells, as they are constant defenders and first cellular responders to tissue injury and infection, responsible for maintaining tissue homeostasis18. Since the occurrence of polyps depends on an exaggerated inflammatory reaction, it is not surprising that the inflammatory infiltrate of patients with severe and relapsing forms of CRSwNP, which is considered the inflammatory disease par excellence, may be characterized by abundant neutrophils. In addition, eosinophilic activation is traditionally associated with the presence of Charcot-Leyden crystals (CLCs), the crystallized form of Galectin-10, which is a major constituent of the cytoplasm of eosinophils that characteristically forms bi-piramidal hexagonal crystals when eosinophils are intensely activated and undergo ETosis, causing a crystallopathy19. CLCs have been shown to trigger epithelial cells to recruit neutrophils and produce neutrophil-activating cytokines, such as GM-CSF, TNF-a, and IL-1, that could potentially prime neutrophil effector function20. Actually, a recent study has not only shown that CLCs colocalize strongly with Gal-10 + CD15 + eosinophils and Gal-10 + Tryptase + mast-cells within the lamina propria, but also the strong correlation between the expression of Gal-10, the eosinophilic-mast cell infiltrate and severity of CRSwNP, according to the Clinical-Cytological Grading (CCG)21. Indeed, already in 2009 a CCG based on endotype (inflammatory infiltrate to nasal cytology) and phenotype (comorbidities such as asthma, ASA-sensitivity and allergies) was proposed to establish the severity of CRSwNP and the related Prognostic Index of Relapse (PIR)22. According to the CCG, the eosinophil-mast cell endotype had already been proposed as the most difficult to treat, with the greatest risk of recurrence. The aforementioned study, conducted with immunofluorescence and confocal laser microscopy has confirmed the involvement of MCs in the pathogenesis of the spectrum of Type 2 diseases, such as CRSwNP. In particular, MCs could secrete type 2 cytokines (IL-4, IL-5, IL-13), facilitating eosinophilic inflammation and causing tissue remodeling through the degradation of the extracellular matrix. Therefore, greater pathophysiological dignity should be conferred on MCs and even eosinophilic inflammation should be better defined as mixed eosinophilic-MC inflammation.
As proof of this, as shown in the results, the histological examination of HE-stained specimens did not reveal the presence of MCs in any sample. However, immunohistochemical evaluation revealed the presence of MCs within the lamina propria in 97.4% of cases and within the epithelial layer in 61.4% of cases. Moreover, intraepithelial MC count increased statistically significantly with increasing severity of CRSwNP, according to the CCG and, more in detail, the cut-off of 6 intraepithelial MCs was identified to detect high CCG.
Since eosinophils were found in all analyzed samples, almost all eosinophilic forms of CRSwNP, traditionally considered as Type 2-eosinophilic forms, showed MCs. However, in most cases, the MCs were located at the tonaca propria layer. As the HE staining does not allow to highlight MCs, the latter cytotypes are not described in common histological examinations.
Moreover, this subepithelial localization explains why the nasal cytology also described several eosinophilic forms, without mentioning MCs. In fact, nasal cytology, contrary to what is typically believed, is a non-invasive diagnostic tool that involves sampling by scraping, which is a bloodless procedure, therefore limited to the epithelial level. Indeed, by definition, nasal cytology does not allow to evaluate subepithelial cells but only intraepithelial and infiltrating cells. On the contrary, nasal cytology proved to be a valid tool for identifying cases with intraepithelial MCs, as a statistically significant correlation was found between cytological examination and histological immunohistochemical examination, in detecting intraepithelial MCs.
Interestingly, intraepithelial MCs were directly proportional to CCG and, thus, to the severity of CRSwNP. Indeed, although MCs have always been considered as “children of a lesser god”, these cells MCs likely play a central role in orchestrating immune responses at barrier tissues. As a matter of fact, the characterization of MCs phenotypes in terms of functional heterogeneity has been traditionally underestimated, especially in a setting of mucosal inflammation23. Only recently, several studies have shown that two major human MCs subtypes exist: sub epithelial MCs (MCTC), expressing both tryptase and chymase in conjunction with cathepsin G and carboxypeptidase A3 (CPA3), and mucosal epithelial MCs (MCT), expressing only tryptase24. In CRSwNP and asthma, there is a prevalent expansion of intraepithelial MCT expressing CPA3 and of MCTC infiltrating airway smooth muscle25. These subtypes are responsible for the production of cytokines that not only activate eosinophils but also directly promote the degradation of the extracellular matrix, generating a vicious circle thanks to which eosinophils and MCs attract and activate each other causing exacerbation of inflammation and epithelial damage26. This explains the correlation between total MC and eosinophil count shown in the results, as the two cytotypes influence each other and increase as the severity of CRSwNP increases27. Increased levels of these two cytotypes are also responsible for the increased production of CLCs, which has a correlation with CRSwNP severity. The formation of CLCs is strongly associated with disease severity, since it can induce a crystallopathy by the activation of NLRP 3 inflammasome and the stimulation of innate and adaptative immunity28. This crystallopathy, together with the release of lytic enzymes such as major basic protein (MBP), tryptase and collagenase by both eosinophils and MC induces an exfoliation of epithelial cells and the rupture of desmosomes, allowing pathogens and other exogenous substances to penetrate the mucosal barrier29,30. Among pathogens, Staphylococcus aureus, traditionally associated with the pathogenesis of CRSwNP, has been shown to replicate within the MCs of the nasal mucosa and ultimately induces lysis of MCs and release of intracellular content31,32. As evidence of this, among the histological samples analyzed, those of patients with high intraepithelial MC infiltrate showed a variable de-epithelialization severity (Fig. 6).
The activation of MCs has also been proven by the statically significant correlation between Tryptase and CCG: the expression Tryptase, which is considered a marker of mast cell degranulation, increased with increasing CCG. Indeed, the degranulation of mast cells leads to the release of preformed mediators, such as tryptase, chymase, carboxypeptidase, histamine, and heparin, that in turn cause the release of de novo synthesized mediators such as lipid mediators, chemokines, growth factors and interferons, which exacerbate the disease. Tryptase, in particular, is a neural serine protease that is stored in secretory granules and released following mast cell activation by IgE-dependent and independent processes33. This protease is associated with the development of allergic and inflammatory responses, that are directly associated with tissue remodeling, due to the many biological effects of Tryptase, including the inactivation of fibrinogen, fibronectin, collagens, and lipoproteins, regulation of mesenchymal cell proliferation and survival, upregulation of adhesion molecules, and induction of growth factors and cytokine34. Moreover, it is noteworthy that Tryptases activates circulating eosinophils, inducing the release of granule-associated enzymes and, consequently, generating a vicious circle35.
Since MCs and eosinophils appear to influence each other, it is unclear whether CRSwNP with MC epithelial infiltration represent an evolution of the most severe eosinophilic forms of CRSwNP or it could be considered a stand-alone form of CRSwNP, characterized by abundant CLCs, being therefore more difficult to treat and more prone to relapse36 (Fig. 7). Nevertheless, in light of these evidences, the role of MCs in CRSwNP pathophysiology should be re-evaluated and emphasized37,38.
In this context, we believe that the current classification of CRS inflammatory patterns into Type 1, Type 2 or Type 3 should be revised. In particular, Type 1 and Type 3 endotypes, both characterized by predominant neutrophilic infiltrate, could be considered as subgroups of a single type, Type 1 precisely, which is characterized by neutrophils. These neutrophilic forms of CRSwNP, mainly associated with CRSsNP or CRSwNP with low PIR, could be subclassified into Type 1A and Type 1B depending on the absence (previously defined as Type 1) or presence of IL-17 (previously defined as Type 3), respectively (Table 3).
On the other hand, Type 3 should refer to the eosinophilic-MC forms of CRS, characterized by both abundant eosinophils and intraepithelial MCs, which are typically associated with nasal polyp formation and a high relapse rate. Indeed, while mast cells have been shown to be located in the lamina propria of almost all types of nasal polyps without significantly affecting their severity, their intraepithelial localization determines a greater severity of CRSwNP, according to the CCG.
The concept of intraepithelial infiltration could be used precisely to reformulate the diagnostic criteria of CRSwNP and of its several subtypes, as is already the case for celiac disease or eosinophilic esophagitis (EoE). As a matter of fact, EoE is defined as esophageal dysfunction in the presence of > 15 intraepithelial eosinophils per high-power field (eos/HPF) in either the mid or distal esophagus39. Indeed, the current focus of EoE pathologic evaluation is the peak eosinophil count (PEC), although histologic features other than eosinophilic inflammation are also commonly observed. Moreover, reduced PEC is considered a universal goal in clinical management and constitutes an endpoint in clinical trials of therapies for EoE40. Similarly to EoE, the diagnosis of coeliac disease (CD) is based on several criteria including positive serology, a spectrum of duodenal damage and clinical symptoms and/or risk conditions, and response to a gluten-free diet (GFD) in patients bearing the HLA-DQ2 or DQ8 genotypes. Among the histopathological changes, duodenal intraepithelial lymphocytes (IEL) count is considered a useful tool for CD diagnosis41. In particular, 25–29 IEL/100 enterocytes are considered borderline and > 30 IEL/100 enterocytes are considered diagnostic for CD42,43.
Since CRSwNP has been shown to be characterized by intraepithelial infiltration of both eosinophils and MC, the introduction of their count in the pathology report could be useful and cheap diagnostic tool to characterize nasal polyps and possibly define their severity. Furthermore, since intraepithelial MC are associated with de-epithelialization, the reduction of their count could represent a goal for treatment management.
Conclusion
Further studies are needed to understand whether massive eosinophilic inflammation is responsible for the recall of mast cells at the intraepithelial level or if other factors contribute to the recruitment of MCs at the intraepithelial level. A correct definition of the mixed eosinophil-MC CRSwNP form could have important therapeutic implications, especially after the introduction of biological treatments alongside existing treatments in the management of chronic rhinosinusitis with nasal polyps44. In fact, although biological treatments are now considered valuable therapeutic tools for the management of CRSwNP, only a careful choice of patients suitable for treatment allows to obtain the maximum therapeutic benefit and to reduce the economic burden. In this sense, a close alliance with pathologists is also fundamental, to establish the histopathological criteria that define the eosinophil-MC form of CRSwNP, as well as to possibly confirm the cut-off of intraepithelial MCs associated with greater severity of CRSwNP.
Data availability
Data available on request from the corresponding author.
References
Shaghayegh, G. et al. Chronic rhinosinusitis, S. aureus biofilm and secreted products, inflammatory responses, and disease severity. Biomedicines 10, 1362 (2022).
Fokkens, W. J. et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 58, 1–464 (2020).
Delemarre, T. et al. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: Another relevant endotype. J. Allergy Clin. Immunol. 146, 337-343.e6 (2020).
Ren, L., Zhang, N., Zhang, L. & Bachert, C. Biologics for the treatment of chronic rhinosinusitis with nasal polyps—State of the art. World Allergy Organ. J. 12, 100050 (2019).
Poposki, J. A. et al. Elevation of activated neutrophils in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. S0091–6749(21), 02681–02686. https://doi.org/10.1016/j.jaci.2021.11.023 (2021).
Gelardi, M. et al. Chronic rhinosinusitis with nasal polyps: How to identify eligible patients for biologics in clinical practice. Acta Otorhinolaryngol. Ital. 42, 75–81 (2022).
Racette, S. D. et al. CRS-PRO and SNOT-22 correlations with type 2 inflammatory mediators in chronic rhinosinusitis. Int. Forum Allergy Rhinol. https://doi.org/10.1002/alr.23002 (2022).
Jeong, S. S., Chen, T., Nguyen, S. A., Edwards, T. S. & Schlosser, R. J. Correlation of polyp grading scales with patient symptom scores and olfaction in chronic rhinosinusitis: A systematic review and meta-analysis. Rhinology https://doi.org/10.4193/Rhin22.011 (2022).
Chitguppi, C. et al. Effect of benralizumab in patients with severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps: A case series. Am. J. Rhinol. Allergy 35, 559–567 (2021).
Gelardi, M., Iannuzzi, L., Quaranta, N., Landi, M. & Passalacqua, G. NASAL cytology: Practical aspects and clinical relevance. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 46, 785–792 (2016).
Gelardi, M. et al. Non-surgical management of chronic rhinosinusitis with nasal polyps based on clinical-cytological grading: A precision medicine-based approach. Acta Otorhinolaryngol. Ital. 37, 38–45 (2017).
Panarelli, N. C., Hornick, J. L. & Yantiss, R. K. What is the value of counting mast cells in gastrointestinal mucosal biopsies?. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 36, 100005 (2023).
Caruso, R. A. et al. Intraepithelial infiltration by mast cells in human Helicobacter pylori active gastritis. Ultrastruct. Pathol. 35, 251–255 (2011).
Rujitharanawong, C. et al. Systematic comparisons of various markers for mast cell activation in RBL-2H3 cells. Cell Tissue Res. 390, 413–428 (2022).
Van Zele, T. et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61, 1280–1289 (2006).
Stevens, W. W. et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 7, 2812-2820.e3 (2019).
Delemarre, T., Bochner, B. S., Simon, H.-U. & Bachert, C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J. Allergy Clin. Immunol. 148, 327–335 (2021).
Fine, N., Tasevski, N., McCulloch, C. A., Tenenbaum, H. C. & Glogauer, M. The neutrophil: Constant defender and first responder. Front. Immunol. 11, 571085 (2020).
Mulay, S. R. & Anders, H.-J. Crystallopathies. N. Engl. J. Med. 374, 2465–2476 (2016).
Gevaert, E. et al. Charcot-Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J. Allergy Clin. Immunol. 145, 427-430.e4 (2020).
Gelardi, M. et al. Chronic rhinosinusitis with nasal polyposis (CRSwNP): The correlation between expression of Galectin-10 and clinical-cytological grading (CCG). Am. J. Rhinol. Allergy https://doi.org/10.1177/19458924211049867 (2021).
Gelardi, M. et al. Nasal-sinus polyposis: clinical-cytological grading and prognostic index of relapse. J. Biol. Regul. Homeost. Agents 23, 181–188 (2009).
Boyce, J. A. Advances in mast cell biology. J. Allergy Clin. Immunol. S0091–6749(22), 00487. https://doi.org/10.1016/j.jaci.2022.03.029 (2022).
Gelardi, M., Giancaspro, R., Cassano, M. & Ribatti, D. The underestimated role of mast cells in the pathogenesis of rhinopathies. Int. Arch. Allergy Immunol. https://doi.org/10.1159/000518924 (2021).
Dwyer, D. F. et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci. Immunol. 6, 7221 (2021).
Zhai, G. T. et al. Increased accumulation of CD30 ligand-positive mast cells associates with eosinophilic inflammation in nasal polyps. Laryngoscope 129, E110–E117 (2019).
Takabayashi, T. & Schleimer, R. P. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J. Allergy Clin. Immunol. 145, 740–750. https://doi.org/10.1016/j.jaci.2020.01.027 (2020).
Persson, E. K. et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 364, 4295 (2019).
Persson, C. Primary lysis of eosinophils in severe desquamative asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 44, 173–183 (2014).
Caruso, C. et al. Nasal cytology: A easy diagnostic tool in precision medicine for inflammation in epithelial barrier damage in the nose. A perspective mini review. Front. Allergy 3, 122 (2022).
Boyce, J. A. Mast cells can be commandeered for staphylococcal pathogenicity in patients with chronic rhinosinusitis with nasal polyposis. J. Allergy Clin. Immunol. 145, 103–104 (2020).
Bachert, C. et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J. Allergy Clin. Immunol. 126(962–968), 968.e1–6 (2010).
Jackson, C. W., Pratt, C. M., Rupprecht, C. P., Pattanaik, D. & Krishnaswamy, G. Mastocytosis and mast cell activation disorders: Clearing the air. Int. J. Mol. Sci. 22, 11270 (2021).
Sprinzl, B. et al. Genetic regulation of tryptase production and clinical impact: Hereditary alpha tryptasemia, mastocytosis and beyond. Int. J. Mol. Sci. 22, 2458 (2021).
Vliagoftis, H. et al. Mast cell tryptase activates peripheral blood eosinophils to release granule-associated enzymes. Int. Arch. Allergy Immunol. 135, 196–204 (2004).
Wu, D., Yan, B., Wang, Y., Zhang, L. & Wang, C. Predictive significance of Charcot-Leyden crystal protein in nasal secretions in recurrent chronic rhinosinusitis with nasal polyps. Int. Arch. Allergy Immunol. 182, 65–75 (2021).
Gelardi, M., Giancaspro, R. & Cassano, M. Should the role of mast cells in chronic rhinosinusitis with nasal polyps be revaluated?. Acta Otorhinolaryngol. Ital. Organo Uff. Della Soc. Ital. Otorinolaringol. E Chir. Cerv.-Facc. 41, 576–577 (2021).
Gelardi, M., Giancaspro, R. & Cassano, M. Chronic rhinosinusitis with nasal polyps recurrence: Not only eosinophils and neutrophils. Am. J. Otolaryngol. https://doi.org/10.1016/j.amjoto.2022.103447 (2022).
Collins, M. H. et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 30, 1–8 (2017).
Lin, B., Rabinowitz, S., Haseeb, M. A. & Gupta, R. Usefulness of the eosinophilic esophagitis histologic scoring system in distinguishing active eosinophilic esophagitis from remission and gastroesophageal reflux disease. Gastroenterol. Res. 14, 220–226 (2021).
Ruiz-Ramírez, P. et al. Intraepithelial lymphocyte cytometric pattern is a useful diagnostic tool for coeliac disease diagnosis irrespective of degree of mucosal damage and age—A validation cohort. Nutrients 13, 1684 (2021).
Villanacci, V. et al. Coeliac disease: The histology report. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 43(Suppl 4), S385-395 (2011).
Pai, R. K. A practical approach to small bowel biopsy interpretation: Celiac disease and its mimics. Semin. Diagn. Pathol. 31, 124–136 (2014).
Hopkins, C. Ethical dilemmas associated with the introduction of biologic treatments in chronic rhinosinusitis with nasal polyps. Rhinology https://doi.org/10.4193/Rhin21.477 (2022).
Author information
Authors and Affiliations
Contributions
M.G. Contributed to the research concept, supervised the work and revised the manuscript. R.G. contributed to interpretation of data and wrote the first draft. L.D., C.P., E.M., A.M., F.M.D.C, A.R., A.L., and N.A.A.Q Contributed to collection and interpretation of data. V.N.Q. and F.P. Contributed all data and statistical analysis, and interpretation of data. M.C., and A.M. supervised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gelardi, M., Giancaspro, R., Duda, L. et al. Eosinophil-mast cell pattern of intraepithelial infiltration as a marker of severity in CRSwNP. Sci Rep 13, 12101 (2023). https://doi.org/10.1038/s41598-023-39149-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39149-8
- Springer Nature Limited
This article is cited by
-
Mast Cells in Aspirin-Exacerbated Respiratory Disease
Current Allergy and Asthma Reports (2024)
-
The mast cell: the comfortable third in recent CRS research
European Archives of Oto-Rhino-Laryngology (2023)