Abstract
Muscle strength assessment is important in predicting clinical and functional outcomes in many disorders. Manual muscle testing, although commonly used, offers suboptimal accuracy and reliability. Isokinetic dynamometers (IKDs) have excellent accuracy and reliability; but are bulky and expensive, offering limited accessibility. This study aimed to design a portable dynamometer that is accessible, accurate and reliable, and to validate the device in a general population. The portable articulated dynamometry system (PADS) is a portable device with an embedded high-precision load cell, designed to measure muscle strength with optimal accuracy. Seventy-two participants underwent maximal isometric and isokinetic knee extensor torque measurement with the PADS and IKD, respectively. The PADS results were cross-validated against IKD results using change in mean (CIM). Interrater and intra-rater reliabilities were assessed using intraclass correlation coefficients, standard error of measurement, and minimal detectable change. The PADS maximal knee extensor strength results were not significantly different from those by IKD (CIM: − 2.13 Nm; 95% CI − 4.74, 0.49 Nm). The PADS showed interrater reliability (Pearson’s r: 0.958; ICC: 0.979; SEM: 5.51%) and excellent intra-rater reliability (Pearson’s r: 0.912; ICC: 0.954; SEM: 8.38%). The proposed PADS may be an effective alternative to IKD, offering good accuracy, reliability, and potentially better accessibility.
Similar content being viewed by others
Introduction
Muscle strength has been reported to be an independent predictor of clinical and functional outcomes in many disorders1. Even in healthy populations, lower muscle strength was a risk factor of all-cause mortality1,2. In a geriatric population with advanced cancer, better muscle strength was reported to be associated with prolonged survival3, better functional outcomes4, and improved quality of life5. Declining muscle strength was shown to be a risk factor for declining cognition and Alzheimer disease6, and even in early adulthood, muscle strength seems to affect the long-term risk of stroke7.
For muscle assessment, the quadriceps muscles have been identified as an effective muscle group to predict declining muscle mass and strength in various chronic disorders3,8,9,10. In their recently revised guideline in assessing sarcopenia severity, the European Working Group on Sarcopenia in Older People (EWGSOP2) included many gait performance scales (gait speed test, short physical performance battery, timed up and go test, and 400-m walk test), which are highly affected by the quadriceps strength11. In emergency departments and neurocritical care settings, acute deterioration of medical and/or neurological outcomes is often accompanied by a sudden declination in quadriceps strength12,13. In clinical settings, quadriceps strength assessment is most often performed using manual muscle testing (MMT)14. MMT is desirable because it is quick, intuitive, and easy to perform; however, critical drawbacks are that it is qualitative and examiner dependent15. MMT can only be properly executed if the examiner is stronger than the examinee; otherwise, the examiner cannot reliably discern partially impaired from normal muscle strength16. Further, because MMT is only semi-quantitative, it is impossible to reliably track the temporal change in muscle strength. Quantitative muscle testing using hand-held dynamometers (HHDs) allows precise monitoring of a participant’s muscle strength14; however, its reliability is also limited by the examiner strength. When evaluating a powerful muscle group such as the quadriceps, the accuracy and reliability of HHDs are often suboptimal17.
Commercially available isokinetic dynamometers (IKD) such as the Biodex System 4 Pro or BTE PrimusRS are accurate and reliable, and are often used as the “gold standard” for muscle strength testing14,18,19. While IKDs are accurate, they are substantially larger in size than HHDs and therefore not portable. IKDs are also significantly more expensive to use than HHD or MMT. Due to their size and cost, IKD often only offers limited accessibility and are not suitable for routine clinical monitoring of muscle strength18.
Several muscle strength assessment devices have been proposed to overcome the limited accuracy and reliability of MMT and HHDs, as well as the limited accessibility of IKDs. Sung et al.18 proposed an anchoring frame that aides the positioning of HHDs for patients in the supine position, while Hogrel et al.14 developed a portable dynamometer with load cell and in-house user interface to measure the knee extensor torque of participants in the sitting position. Hirano et al.20 used a belt-stabilized HHD to measure the sitting quadriceps strength with maximal portability, and by sacrificing some portability, Padulo et al.21 designed an isometric bench on which participants could sit to assess knee extension power. While these inventions have their respective strengths, they also have drawbacks. Although the HHD anchoring frame can cater to patients in supine position, it is too bulky to be considered portable. Other designs are both portable and accurate, but still require fixing to a bedframe and/or bench; further, they require participants to be able to sit, thus rendering patients with severe muscle weakness unable to be assessed.
The current article proposes a portable articulated dynamometry system (PADS) for routine monitoring of knee extensor torque. The PADS is a foldable frame embedded with a high precision loadcell. The articulated frame and light aluminum body allows maximal portability, and the system can be fastened to the participant’s limb, removing the need for ground fixation and allowing measurement in the supine position. The aim of this study was to design a portable articulated dynamometry device, evaluate the accuracy and reliability of the system via a cross-validation against the Biodex System 4 Pro, and report the results of a user experience survey.
Results
Seventy-two healthy adults (50.0% female; mean age: 29.32 ± 4.57 years) participated in this study; 40 participants (55.6%) were assessed for intra-rater reliability. As all participants were measured twice on each knee to assess interrater reliability, and a subset (n = 40) went through two discrete assessments for intra-rater reliability, a total of 448 observations were gathered for each dynamometer. The age, sex, height, weight, and maximal grip strength of the study participants are presented in Supplementary Table 1.
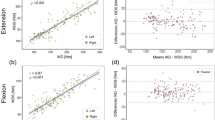
Cross-validation results of the PADS versus IKD are summarized in Tables 1, 2 and 3 and Figs. 1 and 2. After 448 observations, the maximal knee extensor torque measured with the PADS was not significantly different from that measured with IKD (mean of the differences: − 2.13 Nm; 95% CI − 4.74, 0.49 Nm). Data measured with the PADS also demonstrated a strong correlation with data measured using the IKD (Pearson’s r: 0.880; p < 0.0001). The ICC between the PADS and IKD was 0.935, which indicates excellent correlation, and the mean SEM was 13.96 Nm, or 9.75%. The minimal detectable change (MDC) was 38.70 Nm. In terms of interrater reliability, the PADS showed no significant interrater difference (mean of the differences: 0.26 Nm; 95% CI − 1.83, 2.36 Nm), nor did the IKD (mean of the differences: 0.90 Nm; 95% CI: − 0.66, 2.46 Nm). Both systems showed strong interrater correlation (Pearson’s r: PADS, 0.958; IKD, 0.973) and excellent relative reliability (ICC: PADS, 0.979; IKD, 0.986). The mean SEM was 7.94 Nm (5.51%) for the PADS and 6.06 Nm (4.20%) for IKD. The MDC was 22.01 Nm (15.25%) for the PADS and 16.80 Nm (11.64%) for the IKD.
For intra-rater reliability, both the PADS and IKD showed no significant systematic bias between two different test dates (mean of the differences = − 0.28 Nm and 1.72 Nm; 95% CI [− 1.83, 2.36] Nm, [− 0.71, 4.14] Nm, respectively). Both systems showed strong interrater correlation (Pearson’s r: PADS, 0.912; IKD, 0.955) and excellent relative reliability (ICC: PADS, 0.954; IKD, 0.977). The mean SEM was 11.70 Nm (8.38%) for the PADS and 7.83 Nm (5.48%) for IKD. The MDC was 32.43 Nm (23.24%) for the PADS and 21.70 Nm (15.18%) for the IKD.
Table 4 reports the UEQ-S feedback; 69 of 72 participants completed the survey, and results reported significantly more positive overall user experience (p = 0.002) for the PADS. Specifically, participants responded that the PADS was significantly easier (p < 0.001), more efficient (p = 0.018), clearer (p = 0.027), more inventive (p = 0.002), and more leading-edge (p < 0.001) than the IKD.
Discussion
This study evaluated an original dynamometer for the assessment of maximal quadriceps strength in 72 participants. The design of the system was introduced, the agreement with IKD, intra-rater reliability, and interrater reliability were examined, and participants’ experience with the device was investigated. The main results are that the PADS showed excellent correlation with IKD (Pearson’s r: 0.880; p < 0.0001) and excellent intra-rater and interrater reliability (Pearson’s r: 0.958 and 0.912; p < 0.0001 and < 0.0001, respectively).
In clinical settings, manual muscle testing is the most widely employed means of muscle strength assessments15,16; HHDs are also frequently used to quantify muscle strength18. MMT and HHDs are popular because they are easy and quick to administer; however, both methods have been reported to demonstrate suboptimal accuracy and reliability14,17,19. While IKDs provide precise and accurate measurements, they are not suitable for routine examination due to their size and cost17,18. The current study recognized the clinical need to develop a dynamometry system that was portable, easy-to-use, and accessible.
The PADS is a foldable, wearable device that can measure the maximal isometric knee extensor torque in various patient positions. With the weight and size of a typical textbook, the PADS is noticeably smaller and more portable than an IKD. The PADS employs an intuitive design with simple material; the aluminum frame and 3D printed calf/thigh supports are easy to manufacture, and the embedded high precision sensor is easily replaceable. Production of the current prototype only cost about $700 in materials. Good maintainability and cost efficiency are two strengths that the PADS offers, in addition to reliability and portability. Although not yet commercially available, an international patent of the PADS via the Patent Cooperation Treaty (PCT) system is pending, and the production line is in the process of registering a good manufacturing practice (GMP) certificate to make the PADS more readily accessible to researchers, clinicians, and potentially the general public.
The current study results demonstrate that the PADS shows good agreement with IKD. In a study of 56 patients with inclusion body myositis, Hogrel et al.14 reported IKD’s intra-rater SEM to be 8.02%. Our results found the intra-rater SEM of the IKD to be 5.48%, which is comparable with their findings. The intra-rater SEM of the PADS was 8.38%, competing with the results of Hogrel et al. Although the MDC values of the PADS are slightly higher than those of IKD, they are comparable to the corresponding values of existing literature because the MDC is directly proportional to SEM. The interrater reliability of the PADS and IKD were lower than the corresponding results of the intra-rater measurements: the SEM of the PADS and IKD were 5.51% and 4.20%, respectively. The PADS did not exhibit significant differences in the means for interrater reliability or intra-rater reliability evaluations, and with ICC values close to 1, both the PADS and IKD reported excellent relative reliability.
Knee extensor torque, along with maximal grip strength, is reported to be an important clinical indicator for predicting survival22, disease course23, and quality of life24,25,26 in many neurological27,28, musculoskeletal29,30, and geriatric31 conditions. In the most recent definition of sarcopenia by the European Working Group on Sarcopenia in Older People, gait performance tests, such as gait speed test, short physical performance battery, timed up and go test, and 400-m walk test, are essential for assessing the severity of sarcopenia11. Gait performance was reported to be strongly determined by antigravity muscle strength, of which knee extensors are the most important32,33. In a 2008 meta-analysis of nearly two million apparently healthy individuals, Garcia-Hermoso et al.2 found that quadriceps strength was an independent risk factor of all-cause mortality.
In the user experience analysis, participants responded that they felt the PADS was easier, more efficient, clearer, more inventive, and more innovative than the IKD. Not shown in the results section is a preplanned subgroup analysis of participants who were healthcare professionals. About 40% of the cohort (n = 28) were healthcare professionals (e.g. physicians, nurses, physical therapists, and occupational therapists) working for a university hospital. The subgroup analysis showed that they believed the PADS to be significantly easier to use (Supplementary Table 2).
Interpretation of the study results requires caution due to limitations of the study. As only healthy young adults aged between 20 and 39 years were enrolled, the accuracy and reliability of the PADS are not validated in geriatric populations or individuals with very weak quadriceps strengths. Further studies in older adults and patients with neurological or musculoskeletal disorders are therefore warranted. In such studies, validation studies in more positions (e.g. supine, reclined wheelchair) are necessary. Additionally, the present study only evaluated the measurement of knee extensor strength. In certain conditions (e.g. proximal or distal myopathies), simultaneous monitoring of proximal and distal muscle strengths are required. Currently, the PADS for ankle dorsiflexion and plantarflexion strength measurement is in development. Further research of the PADS on ankle strength assessment is planned for more complete lower extremity strength assessment.
Conclusion
Precise monitoring of muscle strength is clinically valuable. However, muscle strength assessment usually relies on MMT with limited reliability, or IKDs with limited accessibility. This study found that the PADS offers good reliability, potentially better accessibility, and cost-efficiency in a healthy adult population. The proposed PADS may be an effective clinical alternative to the MMT or IKD.
Methods
Participants
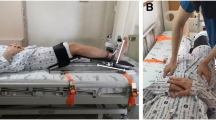
Seventy-two adult participants between the ages 20 and 39 were enrolled in this study. Participants that were pregnant, had a recent history of cardiovascular, musculoskeletal, neurological, or infectious disease, or could not give informed consent, were excluded. This study conformed to the Declaration of Helsinki. This study was approved by Seoul National University Hospital institutional review board (IRB no. 2201-025-1288). All participants provided written informed consent. The participant appearing in Fig. 3 provided written informed consent to publish the figure in an online open-access publication.
Anthropometry
A single board-certified physiatrist performed all anthropometric measurements. Measurements were taken with participants barefoot and without heavy clothing. Height was measured to the nearest millimeter with an ultrasonic stadiometer (PUSH Stadiometer; InBody, Seoul, South Korea), and weight was measured to the nearest 0.1 kg using a digital scale (Mi Smart Scale; Xiaomi, Beijing, China). Maximum grip strength was measured with a hand-held grip dynamometer (EH-101; Camry Scale, CA, USA).
PADS: portable articulated dynamometry system
The PADS incorporates simple and intuitive mechanical designs to ensure optimal usability and portability (Fig. 3A). The foldable main frame was an aluminum 6061 alloy milled with a Numerical Control milling machine (HF 1250-4; Hyubsung Dynamics Co., Gyeonggi, Korea). The main frame was anodized for insulation, and the joint that that couples the distal and proximal limbs of the frame was a stainless-steel six-pin rotator-clamp that allows the system to either fold completely or peg at a predetermined angle (Fig. 3B). The model used in this study could fold or fixate at thirty-degree increments, allowing maximum portability and isometric torque measurement at different angles (Fig. 3C). Attached to both the distal and proximal limbs of the main frame were ergonomically curved calf and thigh supports. The supports were made of carbon fiber-reinforced nylon filaments additively engineered using a 3D printer (Style Neo A22C; Cubicon, Gyeonggi, Korea), and each support was lined with 10 mm-thick silicon (Ecoflex; Smooth-On, Inc., Macungie, PA, USA) for maximal wearability. The device weighed 1.3 kg, and its dimensions were 250 mm × 120 mm × 100 mm. Embedded between the distal limb of the main frame and the calf support was a high precision push–pull loadcell (CBF30-100; CAS Corporation, Seoul, Korea) that could measure force from 0 to 100 kg, with a maximal hysteresis of 0.5% and repeatability of 0.2% over the entire nominal range. The signal output from the sensor was amplified by an HX711-based amplifier (SEN-13879; SparkFun Electronics, Boulder, CO, USA), and flowed to the microcontroller (Arduino UNO; Arduino, Somerville, MA, USA). The microcontroller delivered the data to the end device, which visualized and recorded the data (Fig. 4). The system was calibrated with a set of class M1 weights from 0.1 to 100 kg; a logarithmic scale calibration curve for the PADS is presented in Supplementary Fig. 1.
To remove the effects of gravity, participants sat on a horizontal Bobath table with their height adjusted so that their lower leg would be perpendicular to, but not rest on, the ground. The proximal limb of the frame was placed under the thigh and fixed with two Velcro belts. The distal limb of the frame, where the tension sensor was embedded, was attached to the calf of the participants with another Velcro belt. Other than encouraging them to remain stable, the examiner did not physically secure the participants in any way after the PADS was attached. The distance between the load cell and lateral epicondyle was measured to the nearest millimeter in twenty participants; the mean distance was used as the lever arm length to compute the torque from the measured force. It was postulated that if the lever arm length estimated from a subset of the population can be used to calculate the knee extensor torque of the entire participant population with acceptable accuracy, thus having been internally validated, then the estimated lever arm length could be used to calibrate the PADS so that subsequent users can use the device without having to measure the lever arm length in every participant. Because the PADS was tightly fastened to their legs, participants were allowed minimal compensatory movements, such as grabbing the edge of the table or slightly leaning forward. Although this study measured maximum isometric knee extension torque at 90° of knee flexion with the participant in a sitting position, the PADS can be worn by participants in the supine position or in wheelchairs, with their knee further extended (Fig. 3D–F).
Isokinetic dynamometer
Participants sat on an IKD (Biodex System 4 Pro; Biodex Medical Systems, Shirley, NY, USA) with their hip flexed to 85°, and knee joint aligned with the axis of the lever arm of the system. Participants’ hips were stabilized with a safety strap across the pelvis, and the mid-thigh of the tested leg was fastened to the chair with another strap. Participants were seated so that the center of rotation of the lever arm was directly adjacent to the lateral epicondyle.
Maximal knee extension torque measurement
Three maximal knee extension trials were recorded for each dynamometer; if the last trial showed greater strength than the preceding trials, additional trials were administered until the last measurement was not the greatest record. Before each trial, participants were instructed to execute maximal volition. Strong and continued verbal encouragements were given during the trials, and measurements were performed on both knees; each trial lasted about 3 s, with 30-s breaks in between. To assess the interrater reliability of the two dynamometers, two measurements were performed on each participant by two independent examiners. One examiner was a board-certified physiatrist with 6 years of clinical experience, and the other was an engineer who was part of the development team. Participants were given a 3-min break before switching examiners. To assess the intra-rater reliability of the two dynamometers, a subset of participants (n = 40) went through two repeat assessments, which were at least 24 h apart. All participants performed a 3-min stretching session with a board-certified physiatrist before each assessment, and all assessments were performed under the direct supervision of the same physiatrist.
User experience study
After completing all assessments, participants were invited to complete the user experience questionnaire short form (UEQ-S) regarding their experience with both dynamometers. The UEQ-S is a reliable and valid tool that allows quick assessment of the feelings, impressions, and attitudes that arise when one uses a product34. The questionnaire comprises eight items, and participants rate each item on a 7-point Likert scale, which ranges from − 3 (very negative experience) to + 3 (very positive experience). Completion of the UEQ-S was voluntary, and the responses were anonymous.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess data normality. CIM and paired t-tests were used to detect any systematic bias regarding PADS-IKD cross validation, interrater reliability, and intra-rater reliability. CIM was calculated by taking the mean of differences between the PADS and IKD values of the same participant; CIM was divided by the ratio of standard deviation to the square root of the number of participants to evaluate if there were statistically significant differences in the means between PADS and IKD. To assess reliability and reproducibility, intraclass correlation coefficients were calculated. The agreement between the PADS and the IKD were evaluated using ICC model 2, or tests for absolute agreement. Interrater reliability for each dynamometer was also evaluated using ICC model 2. Intra-rater reliability for each dynamometer was assessed using ICC model 3, or tests for consistency. Because the presented study conducted m repeated measures within n subjects, the observations were not truly independent. Therefore, ICC(2,k) and ICC(3,k) were used. ICC values less than 0.5, between 0.5 and 0.7, between 0.75 and 0.9, and greater than 0.9 were indicative of poor, moderate, good, and excellent relative reliability, respectively35,36,37. To evaluate absolute reliability, standard error of measurement (SEM) was calculated according to the COnsensus-based Standards of the selection of health Measurement INstruments (COSMIN) quality assessment38. Specifically, SEM was calculated using the following formula: \(SEM=SD \sqrt{1-ICC}\), where SD represents standard deviation. To provide a more trackable parameter for clinicians and researchers to use as a valuable guide for interpreting changes in the outcomes over time, minimal detectable change (MDC) at 95% confidence level was calculated using the following formula: \(MDC=1.96*\sqrt{2}*SEM\). Regression analyses were performed, Bland–Altman plots were created39, and the UEQ-S responses for the PADS and IKD were compared using paired t-tests. All statistical analyses were performed using SPSS version 26 for Windows (IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05.
Data availability
The data acquired in this study are not openly available due to the sensitive nature of human data (e.g. age, sex, height and weight). A de-identified dataset containing the full demographic and clinimetric data is available from the corresponding author (S.K.) upon reasonable request.
References
Volaklis, K. A., Halle, M. & Meisinger, C. Muscular strength as a strong predictor of mortality: A narrative review. Eur. J. Intern. Med. 26, 303–310 (2015).
García-Hermoso, A. et al. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 99, 2100–2113 (2018).
Versteeg, K. S. et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. Oncologist 23, 580–585 (2018).
Kilgour, R. D. et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support. Care Cancer 21, 3261–3270 (2013).
Nipp, R. D. et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist 23, 97–104 (2018).
Boyle, P. A., Buchman, A. S., Wilson, R. S., Leurgans, S. E. & Bennett, D. A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 66, 1339–1344 (2009).
Åberg, N. D. et al. Influence of cardiovascular fitness and muscle strength in early adulthood on long-term risk of stroke in Swedish men. Stroke 46, 1769–1776 (2015).
Brønstad, E. et al. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur. Respir. J. 40, 1130–1136 (2012).
Casaña, J. et al. Knee extensor muscle strength is more important than postural balance for stair-climbing ability in elderly patients with severe knee osteoarthritis. Int. J. Environ. Res. Public Health 18, 3637 (2021).
Loprinzi, P. D. & Loenneke, J. P. Lower extremity muscular strength and leukocyte telomere length: Implications of muscular strength in attenuating age-related chronic disease. J. Phys. Act. Health 13, 454–457 (2016).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31 (2019).
Anderson, J. R., Nakhate, V., Stephen, C. D. & Perez, D. L. Functional (Psychogenic) neurological disorders: Assessment and acute management in the emergency department. Semin. Neurol. 39, 102–114 (2019).
Siket, M. S. The fast and focused neurological examination. In Neurologic Emergencies (eds Ganti, L. & Goldstein, J. N.) 1–15 (Springer International Publishing, Cham, 2018). https://doi.org/10.1007/978-3-319-64523-0_1.
Hogrel, J. Y., Benveniste, O. & Bachasson, D. Routine monitoring of isometric knee extension strength in patients with muscle impairments using a new portable device: Cross-validation against a standard isokinetic dynamometer. Physiol. Meas. 41, 015003 (2020).
Bohannon, R. W. Reliability of manual muscle testing: A systematic review. Isokinet. Exer. Sci. 26, 245–252 (2018).
Bohannon, R. W. Grip strength measured by manual muscle testing lacks diagnostic accuracy. Isokinet. Exer. Sci. 26, 253–256 (2018).
Lee, M. Y., Sung, K. S., Ham, H., Yi, Y. G. & Shin, H. I. Knee extensor strength measurement in patients with limited physical activity using a supine dynamometer anchoring frame. Ann. Rehabil. Med. 44, 502–509 (2020).
Sung, K.-S., Yi, Y. G. & Shin, H.-I. Reliability and validity of knee extensor strength measurements using a portable dynamometer anchoring system in a supine position. BMC Musculoskelet. Disord. 20, 320 (2019).
Palmer, T. B., Blinch, J., Farrow, A. C., Agu-Udemba, C. C. & Mitchell, E. A. Real-time measurement of isometric peak torque and rate of torque development using a novel strength testing device: A validity and reliability study. Physiol. Meas. 41, 115005 (2020).
Hirano, M., Katoh, M., Gomi, M. & Arai, S. Validity and reliability of isometric knee extension muscle strength measurements using a belt-stabilized hand-held dynamometer: A comparison with the measurement using an isokinetic dynamometer in a sitting posture. J. Phys. Ther. Sci. 32, 120–124 (2020).
Padulo, J. et al. Validity and reliability of isometric-bench for knee isometric assessment. Int. J. Environ. Res. Public Health 17, 4326 (2020).
Williams, G. R. et al. Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Netw. Open 3, e204783 (2020).
Reeve, T. E. et al. Outpatient grip strength measurement predicts survival, perioperative adverse events, and nonhome discharge among patients with vascular disease. J. Vasc. Surg. 73, 250–257 (2021).
Halaweh, H. Correlation between health-related quality of life and hand grip strength among older adults. Exp. Aging Res. 46, 178–191 (2020).
Chun, S. W., Kim, W. & Choi, K. H. Comparison between grip strength and grip strength divided by body weight in their relationship with metabolic syndrome and quality of life in the elderly. PLoS ONE 14, e0222040 (2019).
Paek, J. & Choi, Y. J. Association between hand grip strength and impaired health-related quality of life in Korean cancer survivors: A cross-sectional study. BMJ Open 9, e030938 (2019).
Ogawa, Y. et al. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front. Neurol. 9, 710 (2018).
Wong-Yu, I. S. K., Ren, L. & Mak, M. K. Y. Impaired hand function and its association with self-perceived hand functional ability and quality of life in Parkinson disease. Am. J. Phys. Med. Rehabil. 101, 843–849 (2022).
Denk, K., Lennon, S., Gordon, S. & Jaarsma, R. L. The association between decreased hand grip strength and hip fracture in older people: A systematic review. Exp. Gerontol. 111, 1–9 (2018).
Hashimoto, S. et al. Preoperative hand-grip strength can be a predictor of stair ascent and descent ability after total knee arthroplasty in female patients. J. Orthop. Sci. 25, 167–172 (2020).
Grigol, M. C. P., Morsch, P. & Bós, Â. J. G. Grip strength is a strong predictor of survival in nonagenarians and centenarians. Geriatr. Gerontol. Aging 16, 1–8 (2022).
Bohannon, R. W. & Andrews, A. W. Correlation of knee extensor muscle torque and spasticity with gait speed in patients with stroke. Arch. Phys. Med. Rehabil. 71, 330–333 (1990).
Osawa, Y., Chiles Shaffer, N., Shardell, M. D., Studenski, S. A. & Ferrucci, L. Changes in knee extension peak torque and body composition and their relationship with change in gait speed. J. Cachexia Sarcopenia Muscle 10, 1000–1008 (2019).
Laugwitz, B., Held, T. & Schrepp, M. in Symposium of the Austrian HCI and usability engineering group 63–76 (Springer).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163 (2016).
Shrout, P. E. & Fleiss, J. L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 86, 420–428 (1979).
Brookshaw, M., Sexton, A. & McGibbon, C. A. Reliability and validity of a novel wearable device for measuring elbow strength. Sensors (Basel) 20, 3412 (2020).
Terwee, C. B. et al. Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Qual. Life Res. 21, 651–657 (2012).
Giavarina, D. Understanding bland Altman analysis. Biochem. Med. 25, 141–151 (2015).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (No-2021R1C1C2095529) and the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute funded by the Korean government (Ministry of Health and Welfare).
Author information
Authors and Affiliations
Contributions
Y.M., S.P., M.C., and S.K. conceptualized and designed the research. Y.M., S.P., M.C., and S.Y.C acquired data. Y.M., S.P., M.C., W.H.L., B.O., and S.K. analyzed and interpreted data. Y.M., S.P., M.C., W.H.L., B.O., and S.K. wrote, reviewed, and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Myong, Y., Park, S., Cho, M. et al. Development and validation of a portable articulated dynamometry system to assess knee extensor muscle strength. Sci Rep 13, 11887 (2023). https://doi.org/10.1038/s41598-023-39062-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39062-0
- Springer Nature Limited
This article is cited by
-
Design and validation of a wearable dynamometry system for knee extension-flexion torque measurement
Scientific Reports (2024)