Abstract
Present knowledge on spawning seasonality of freshwater fishes in tropical Asia and their relationship with environmental factors remains limited. Three Southeast Asian Cypriniformes fishes, Lobocheilos ovalis, Rasbora argyrotaenia and Tor Tambra, found in rainforest streams in Brunei Darussalam were studied on a monthly basis for a period of 2 years. To assess spawning characteristics, seasonality, gonadosomatic index and reproductive phases were examined from 621 L. ovalis, 507 R. argyrotaenia and 138 T. tambra. This study also examined environmental factors such as rainfall, air temperature, photoperiod and lunar illumination that may influence the timing of spawning of these species. We found that L. ovalis, R. argyrotaenia and T. tambra were reproductively active throughout the year but did not find that spawning in these species were associated with any of the investigated environmental factors. Our study showed that the non-seasonal reproductive ecology found in the tropical cypriniform species is distinctly different from that of temperate cypriniforms, which are known to follow spawning seasonality, suggesting an evolutionary adaptation to ensure their survival in an unstable environment. The reproductive strategy and ecological responses found in the tropical cypriniforms might be shifted in response to climate change scenarios in the future.
Similar content being viewed by others
Introduction
Present knowledge on spawning seasonality in freshwater fishes is largely based on temperate species in North America and Europe as well as tropical species in South America. Considerably less information is available on the spawning seasonality of freshwater fishes in tropical Asia, particularly in the Southeast Asian region. In temperate latitudes, fishes often show some degree of seasonality in reproduction and limit their annual spawning within a short but optimal period1,2,3. Spawning in these freshwater fishes often occurs in months with warmer temperatures and longer day lengths4,5,6,7. In the warm and wet tropics, freshwater fishes commonly exhibit continuous reproduction or reduced seasonality2,8,9. Protracted spawning in tropical species often coincides with the wet season10,11,12,13,14.
Cypriniformes is a diverse order of freshwater fishes15 that characterizes the freshwater fish fauna of tropical Asia16,17. In this region, spawning in some cypriniform species extend throughout the year while also displaying peak spawning activities as described in Barbus nigrofasciatus, Barbus dorsalis, Barbus titteya9, Neolissochilus soroides18, and Rasbora tawarensis19. Conversely, seasonal spawning has also been reported in other tropical Asian cypriniform fishes such as in Tor tambroides20, Barbus bimaculatus, Barbus cumingii, Puntius vittatus9 and Barbus lacerta21. These studies have reported that peaks as well as seasonality in spawning coincide with periods of high rainfall, further confirming rainfall as an important environmental cue for reproduction in tropical freshwater fishes. However, few studies on freshwater fishes in tropical Asia have examined the association of spawning with other environmental factors such as temperature, photoperiod and lunar illumination. While air temperature does not show major seasonal variations in the tropical regions, it has been shown to influence reproductive activities in Neotropical and South Asian freshwater fishes12,22,23. Longer photoperiod has been reported to trigger spawning migration of Neotropical riverine fishes24,25,26.
The present study examines the reproductive ecology of Southeast Asian Cypriniformes fishes Lobocheilos ovalis, Rasbora argyrotaenia and Tor tambra belonging to the Cyprinidae family17,27. However, in a recent phylogenetic study the Rasbora genus has been reclassified in the Danionidae family28. Southeast Asia is one of the world’s biodiversity hotspots and represents a region of distinct conservation importance29. It is estimated that 70 of the 205 genera of freshwater fishes belonging to the Cyprinidae family are endemic to this region16. Unprecedented rates of habitat degradation and over exploitation have been recognized as major threats affecting the natural populations of freshwater fishes in Southeast Asia30. Although categorized as ‘Least Concern’ in the IUCN red list of threatened species (IUCN red list)31, the limited geographical range of L. ovalis in Northwest Borneo32 makes this species susceptible to extinction in the face of habitat degradation and climate change33. Similarly, R. argyrotaenia is a species of ‘Least Concern’34 but has recently been recognized as a potential aquaculture species35,36,37. While, T. tambra is categorized as ‘Data Deficient’ according to the IUCN red list, the wild population is known to be declining due to excessive exploitation38.
There is very scarce information available on the reproductive ecology of L. ovalis, R. argyrotaenia and T. tambra. Thus, our aim is to investigate the spawning seasonality in these species using monthly captured specimens. We examined spawning seasonality by evaluating the gonadosomatic index (GSI) and reproductive phases of the gonads. We also examined environmental factors such as rainfall, air temperature, photoperiod and lunar illumination influencing the timing of spawning in these species. Our findings contributed to further understanding of the life history strategies of tropical cypriniform fishes which inhabit flashy and unstable headwater streams. These findings suggest the utility of life history knowledge for interpreting ecological responses in freshwater fishes in the context of habitat and climate changes.
Results
Sex composition, total length (TL) and gonadosomatic index (GSI)
A total of 724 L. ovalis, 609 R. argyrotaenia and 145 T. tambra were collected in this study. There was a total of 353 female and 268 male L. ovalis, while the sexes of 103 specimens could not be determined. For R. argyrotaenia a total of 335 females and 172 males were found, while sexes of 102 specimens could not be determined. A total 120 female and 18 male of T. tambra were found, while the sexes of 7 specimens could not be determined. Gonads that could not be determined were either undeveloped or damaged during processing. Specimens of 621 L. ovalis, 507 R. argyrotaenia and 138 T. tambra were used for further analysis (Table 1). In the three species, overall sex compositions were female dominant (Chi-squared test, P < 0.05).
All species showed sexual dimorphism where the females were larger than males (LME analyses; P < 0.05; Table 1). Female and male GSI were significantly different in all species (LME analyses; P < 0.05; 3.73 ± 0.25 and 3.40 ± 1.30 in female and male L. ovalis respectively, 3.97 ± 0.20 and 5.30 ± 0.28 in female and male R. argyrotaenia respectively, 0.45 ± 0.09 and 0.84 ± 0.12 in female and male T. tambra respectively). All species displayed the highest GSI values during the spawning capable and actively spawning phases (LME analyses, P < 0.05; Table 1).
Monthly GSI and maturity level frequencies
Female GSIs ranged from 0.02 to 18.82 in L. ovalis, 0.01 to 17.66 R. argyrotaenia and 0.05–6.78 T. tambra. Male GSI ranged from 0.05 to 11.04 in L. ovalis, 0.07–16.99 in R. argyrotaenia and 0.12–2.03 in T. tambra (Table 1). The GSI values in female and male L. ovalis and R. argyrotaenia were highly variable between months (Supplementary Fig. S1). Female and male L. ovalis and R. argyrotaenia with advanced gonad development i.e., those in the spawning capable and actively spawning phases were found in every month and showed variable frequencies between months (Fig. 1). Similarly, the GSI values in male T. tambra were highly variable between months, with the spawning capable and actively spawning phases found nearly in every month. On the other hand, the monthly female GSI values in T. tambra were generally low except in March and May 2019, where values above 4 were recorded (Supplementary Fig. S1). Spawning capable and actively spawning female T. tambra were only found between August to September 2018 and March and May 2019 (Fig. 1). However, the presence of regressing and developing female T. tambra in various months provided indications of spawning in previous and subsequent months (Fig. 1). These findings suggest that L. ovalis, R. argyrotaenia and T. tambra were reproductively active throughout the year.
Monthly fluctuations in reproductive phases in: (a) Female L. ovalis, (b) female R. argyrotaenia, (c) female T. tambra, (d) male L. ovalis, (e) male R. argyrotaenia, (f) male T. tambra from September 2017 to August 2019. ns: no study was conducted in the respective month. nf: no fish specimens were collected but study was conducted.
Environmental factors
The 30-day rainfall pattern showed weak seasonal variations (Fig. 2a). The main period of high rainfall occurred in September 2017 and December 2017 to February 2018 (579–837 mm of rainfall). From March 2018, the 30-day rainfall varied between periods of low and moderate rainfall until August 2019. The 7-day rainfall and 30-day rainfall showed similar monthly variations (Fig. 2a,b). The daily rainfall did not correspond to the 30-day or the 7-day rainfall patterns (Fig. 2a–c), indicating variable daily rainfall distributions.
The mean air temperatures were constantly high throughout the study months with small fluctuation from 25 ± 1.14 °C to 28.9 ± 1.48 °C (Fig. 2d). Photoperiod showed a small annual range and varied monthly from 11.8 ± 0.0002 to 12.4 ± 0.0003 h (Fig. 2e). Increasing photoperiod occurred in January to July and decreasing photoperiod occurred in July to December. Lunar illumination showed a cyclical pattern of waxing (0–100% illumination) in and waning (100–0% illumination) phases of the lunar cycle (Fig. 2f). Eleven of the study months (January, February, April, July and September 2018 and November, December, January, February, June and August 2019) occurred during the waxing phases of the lunar cycle.
Effect of environmental factors on spawning
Based on the GLM analyses of, spawning in L. ovalis, R. argyrotaenia and T. tambra were not found to be associated with any of the environmental factors (Table 2) that were studied. In all the three species, the odds of spawning male fishes were significantly higher than in females (OR = 9.960, p < 0.0001 in L. ovalis; OR = 10.489, p < 0.0001 in R. argyrotaenia; OR = 65.756, p < 0.0001 in T. tambra).
Discussion
The present study is the first to describe the spawning periods of L. ovalis, R. argyrotaenia and T. tambra, which were found to be continuous throughout the year as revealed by the assessment of gonad maturity. Although spawning female T. tambra were only found in four months, the extended presence of females that had recently spawned and in preparation of spawning, as well as mature males supported the extended spawning strategy. Compared to the temperate region, considerably less research has examined the reproductive timing of freshwater fishes in tropical Asia. Temperate cypriniform fishes often display spawning seasonality due to the temporally variable temperature and photoperiod as reported in Hybognathus placitus39, Barbus sclateri, Chondrostoma polylepis willkommii4, Cyprinus carpio4,6 and Notropis buccula40. While, non-seasonal spawning reported in tropical cypriniform species such as B. nigrofasciatus, B. dorsalis, B. titteya9, N. soroides18 and R. tawarensis19 are often linked with temporally stable rainfall and temperature.
In this study, the year-round spawning in L. ovalis, R. argyrotaenia and T. tambra also displayed highly variable spawning frequencies between months. Our analyses did not find that these spawning variabilities to be associated with any of the environmental factors that were investigated. Thus, the year-round spawning that was observed in these cypriniform fishes during this study could be related to the absence of distinct seasonal variations in rainfall, air temperature and photoperiod in the study site (Fig. 3).
Reproductive activities in tropical freshwater fishes have often been linked to rainfall pattern. Prolonged spawning has often been reported in freshwater fishes inhabiting regions with temporally stable rainfall such as in B. nigrofasciatus, B. dorsalis, B. titteya found in two river systems in Sri Lanka9. Other tropical fishes such as N. soroides and R. tawarensis Malaysia and Indonesia respectively were reported to display peak spawning activities along with year-round spawning pattern. Although these regions do not have distinct wet and dry seasons, the spawning peaks were found to be linked with periods of high rainfall18,19. On the other hand, in tropical regions of South America with distinct seasonality in rainfall, protracted spawning in freshwater fishes such as Gasteropelecus sternicla, Corynopoma riisei, Astyanax bimaculatus, Hemigrammus unilineatus, Corydoras aeneus41; Hypostomus pusarum, Cichla monoculus, Prochilodus brevis and Cichlasoma orientale42 were reported to occur during the wet season.
Our results also showed how closely related species from distinct geographical locations may display different temporal spawning pattern. For example, T. tambroides which is a sympatric species of T. tambra in Peninsular Malaysia and Indonesia, displayed seasonal spawning in contrast to the continuous spawning that we reported for T. tambra. Ingram et al.43 reported a short spawning season of one to two months in T. tambroides in the Penisular Malaysia. Whereas Wibowo and Kaban20 reported protracted spawning season of six to eight months in T. tambroides in Indonesia. In both geographical locations, the spawning seasons in T. tambroides were linked to the wet season20,43.
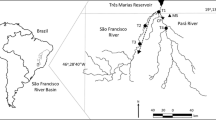
Thus, our finding conforms to the current knowledge of the association between rainfall and spawning in tropical freshwater fishes. In this study, we report that there was no distinct wet and dry season and rainfall was variable on a monthly, weekly and daily scales (Fig. 2). Heavy downpours can occur at any time of the year and due to the location of the study site in the upper catchment of the Temburong basin (Fig. 3), spates in response to heavy rains occur frequently44. The lack of seasonality in spawning in L. ovalis, R. argyrotaenia and T. tambra could be attributed to the flashy and unstable hydrology of the Temburong basin. Magoulick et al.45 and Hitt et al.46 reported the association of unstable hydrology with the abundance of opportunistic strategists which comprised fishes that have prolonged breeding season and continuous or multiple breeding events46,47. Fishes that are opportunistic strategists can recolonise freshwater habitats efficiently following disturbances caused by hydrologic spates2,48. Thus, the non-seasonal reproduction observed in L. ovalis, R. argyrotaenia and T. tambra was possibly an evolutionary adaptation to ensure their survival in an unstable environment.
Air temperature is recognized as an important environmental cue for spawning in freshwater fishes at higher latitudes which show seasonal air and water temperature variations5,7,49,50. The role of air temperature in reproduction in tropical freshwater fishes is not well understood. On the contrary, water temperature has been shown to influence the temporal pattern of reproduction of freshwater fishes in other tropical regions. For instance, in a semi-arid region in Northeast Brazil where water temperature showed variations of 24.5–31 °C, peak spawning activity in Cichla monoculus was found to coincide with lower water temperature23. While, in the tropical wetlands of West Bengal India water temperature range of 29–31 °C was found to be the most favorable for spawning in Channa punctata12. This study only looked at the effect of air temperature on spawning in the three studied species. We did not find significant associations of air temperature with spawning possibly because air temperature was constantly high throughout the study period which provided favorable conditions for continuous spawning in L. ovalis, R. argyrotaenia and T. tambra51. The effect of water temperature on spawning was not investigated in this study and should be a subject of further investigation.
The role of photoperiod as important environmental cue for spawning in fishes is well-known at higher latitudes5,7,49,50. Although less frequently reported, photoperiod have been shown to trigger spawning migrations in neotropical riverine fishes24,25. For example, spawning migration in Prochilodus argenteus was reported to coincide with longer photoperiods10. Variations in photoperiod influence gonad maturation and affect spawning frequencies as seen in Catla catla, Betta splendens52 and Oreochromis niloticus53. However, in this study we did not find evidence of spawning being association with photoperiod which could be attributed to the insignificant temporal differences in photoperiod.
Our result also showed that sex is an important predictor of spawning where males fishes were much more likely to spawn throughout the year in comparison to the females. The continuous presence of the spawning capable male is likely a reproductive strategy which ensures that males were physiologically ready to spawn when the first mature females became available. Similar year-round presence of reproductively active male fishes has been reported in other freshwater fishes such as in live-bearing poecilid fishes in Costa Rica54. This reproductive strategy is thought to be facilitated by the reduced energetic cost of producing spermatozoa compared to oocytes in females14.
This study presents new information on reproductive ecology of L. ovalis and R. argyrotaenia and T. tambra and adds to the knowledge on freshwater fishes in Southeast Asia and the tropical region. The spawning periods of these cypriniform species were found to extend throughout the year and were linked to the temporally stable rainfall, air temperature and photoperiod thus providing further evidence for non-seasonal reproduction in tropical Asian freshwater fishes. Our finding also provided clues on the life history strategies of tropical cypriniform fishes which inhabit flashy and unstable headwater streams and thus suggesting the utility life history knowledge for understanding ecological responses in freshwater fishes in the context of habitat and climate changes. In consideration of the small sample size of male T. tambra, we recommended that further studies are needed to elucidate whether male T. tambra are reproductively active throughout the year. Further, we recommend that water temperature is also examined as one of the environmental factors related to spawning. In addition, experimental studies related to temperature effects on gonadal maturation will aid in better understanding the mechanism of reproductive activities in tropical freshwater fishes.
Materials and methods
Site description
The study site was located in the upper catchment of the Temburong River Basin (1100 km2) in Brunei Darussalam (Brunei) in Northwest Borneo (4° 33′ 8.77″ N, 115° 9′ 24.21″ E; Fig. 3), within Ulu Temburong National Park (UTNP), a protected area that is dominated by the Mixed Dipterocarp Forest. Brunei has a tropical climate, which is weakly influenced by the Southeast Asian monsoon. UTNP receives at least 4000 mm of rainfall annually with weakly seasonal rainfall consisting of two maxima (October to January and May to July) and two minima (February to March and June to August)44,55. However, daily weather in Ulu Temburong is highly variable as a result of localized storms. The rivers are characterized by swift and turbulent water current with coarse substrates, as well as the prevalence of rapids and waterfalls44 The study site comprised the primary channels of the Temburong and Belalong Rivers that were 20–50 m wide, and where flow was perennial. Sampling stations were chosen based on accessibility and were found within 3 km from the confluence of the two rivers and were 40–80 m above sea level.
Sample collection
Sampling for L. ovalis, R. argyrotaenia were conducted monthly for a period of 24 months from September 2017 to August 2019, with the exception of October 2017. While, T. tambra was sampled for 15 months from July 2018 to August 2019. Multi-meshed gill nets were used for sampling L. ovalis and R. argyrotaenia which were constructed by combining gill nets of two different mesh sizes e.g., 2.5 and 3.8 cm inch mesh sizes. The multi-meshed gill nets were 1.5 m deep and 3.5 m wide and mesh sizes 2.5, 3.2, 3.8 and 4.4 cm were used for L. ovalis, and 0.6 and 1.9 cm for R. argyrotaenia. For T. tambra, gill nets were 2.5 m deep, 6.5 m wide and consisted of 6.4, 7.6 and 8.9 cm mesh sizes.
The sampling duration in each month takes about three to five days to collect up to 30 specimens of each fish species. For L. ovalis and R. argyrotaenia, sampling was conducted during day time and the multi-meshed gill nets were deployed in suitable habitats for up to 8 h per day. While for T. tambra sampling was conducted in daylight or overnight where gill nets were deployed in suitable habitats for up to 14 h per day. All captured fishes were recorded and counted and those not sampled were released back to the river. Collected specimens of L. ovalis, R. argyrotaenia and T. tambra were euthanized using MS-222 (Tricaine methanesulfonate), stored on ice and taken to the laboratory for further procedures.
Morphological measurements and GSI
All fishes were measured for total length, body weight and gonad weight. Total length (TL) is the length of the fish from the tip of the snout to the end of the tail and was measured using a scale to the nearest mm. Body weight was measured to the nearest 0.1 g. Whole gonads were carefully removed from the visceral cavity and weighed the nearest 0.01 g. Weight measurement were made using a top pan balance (Nimbus, NBL 2602e). The mean monthly TL ± standard error (SE) was calculated for the females and males of each species. Mean monthly GSI ± SE were calculated for the females and males of each species. GSI is a measure of gonad maturity, where high GSI values indicate advanced of gonad maturation and vice-versa, which was calculated as follows for each fish:
Gonad histology and reproductive phases
Histological preparations of the gonad tissues followed traditional paraffin wax embedding techniques. A transverse section about 1 cm thick, were taken from the anterior, medial and posterior portion of one gonad of each fish. These sections were placed in 10% neutral buffered formalin for about 2 weeks to allow sufficient tissue fixation. Fixed tissues were dehydrated in a series of ethanol dilutions cleared in xylene and embedded in liquid paraffin wax. The embedded samples were sectioned with a microtome to obtain 4–6 μm-thick sections which were mounted on glass slides. The paraffin sections were dewaxed in xylene, stained with Harris haematoxylin and eosin-Y and then cover-slipped with DPX as the mounting medium56. All slides were observed using a microscope (Leica DM 750) to determine the sex and reproductive phases of the gonad.
Gonad development was assessed by classifying ovaries and testes of each species into reproductive phases following Brown-Peterson et al.57 with a slight modification. Six instead of five phases were identified in this study, namely: (1) Immature, (2) developing, (3) spawning capable, (4) actively spawning, (5) regressing, and (6) regenerating. According to Brown-Peterson et al.57 the ‘spawning capable’ phase includes fishes that are physiologically able to spawn within the current reproductive cycle due to advanced gamete development. The spawning capable phase also includes the ‘actively spawning’ subphase, in which a gonad is in the most advanced stage of gamete maturation where spawning is imminent or taking place. In this study, the ‘actively spawning’ was assigned as a main phase in addition to the ‘spawning capable’ phase to distinguish fishes that were spawning from those that were sexually mature but were not spawning, making six phases instead of five. The actively spawning phase was used as an indicator of spawning.
Each gonad was assigned a reproductive phase following the classification above. Monthly frequencies (expressed as percentage) of the maturity levels were calculated for the females and males of each species, as follows:
where Np was the number of fishes in a particular phase of a particular month, and Nt was the total number of fishes in a particular month.
Environmental factors
Environmental factors of interest included rainfall, air temperature, photoperiod and lunar illumination which were recorded on the sampling days of each month. Rainfall and air temperature data from September 2017 to August 2019 were obtained from a weather station (WeatherHawk 240 Signature Wireless Weather Station) at the Kuala Belalong Field Studies Centre which was located within the study site. Data on photoperiod and lunar illumination for Brunei in 2017–2019 were obtained from a website58. Photoperiod was the numbers of daylight hours from sunrise to sunset on each day. While lunar illumination represented the percentage (0–100%) of the moon's disk that appeared illuminated on each day.
Rainfall was expressed as the total amount of rain in millimetres (mm) within a specified period. To capture the variations in rainfall along different temporal scales 30-day rainfall, 7-day rainfall and daily rainfall patterns were examined. The 30-day and 7-day rainfall were calculated by summing the rainfall on the first sampling day of a particular month and the preceding 29 and 6 days, respectively. The daily rainfall was calculated by summing the rainfall on sampling days in a particular month.
The values of each parameter on each sampling day values were used in the GLM analyses. For monthly values of environmental factors, the total values of the three rainfall variables were calculated for each sampling month. While, for air temperature (°C), photoperiod (hours) and lunar illumination (%) monthly mean ± SE were calculated.
Data analyses
All statistical analyses were performed using R Statistical Software, version 4.1.359.
Sex composition
Overall sex compositions were subjected to the Chi-Square test at α = 0.05 to detect significant deviations from the expected 1:1 female to male sex ratio.
TL, GSI and reproductive phases
Differences in the TL and GSI between the male and female of each species and the differences in the GSI between reproductive phases were explored through linear mixed-effects (LME) modelling and the nlme package, version 3.1-15560. Three separate models were explored to assess the effect of sex on: (a) TL of L. ovalis, (b) TL of R. argyrotaenia and (c) TL of T. tambra. Another three separate models were explored to assess the effect of sex and reproductive phases on: (a) GSI of L. ovalis, (b) GSI of R. argyrotaenia and (c) GSI of T. tambra. For all models, the sampling month was modelled as the random effect. Where necessary, the response variable was log 10-transformed to meet the assumption of normal distribution prior to analysis. Following each of the LME analysis, a pair-wise comparison was performed by obtaining the estimated marginal means (a.k.a. least squares means) using the emmeans package, version 1.7.1-161. The pairwise comparison indicated the differences in means between the different groups that were being tested. We performed data exploration before each LME model followed with model validation after executing the LME model according to the protocol by Zuur et al.62. Diagnostic plots of the final LME models in Supplementary Figs. S2–S7 are available online.
Association of environmental factors with spawning
The association of environmental factors with spawning activities were explored through a generalised linear model (GLM) using the binomial family function. This model was used to predict the binary outcome of spawning i.e., the odds of spawning occurring in response to the environmental factors. Three models were explored to examine the effect of environmental factors on spawning in: (a) L. ovalis, (b) R. argyrotaenia and (c) T. tambra. To fit the initial GLM models for each species, the actively spawning phase was modelled as the response variable while sex, 30-day rainfall, 7-day rainfall, daily rainfall, air temperature, photoperiod and lunar illumination were modelled as the predictor variables. The initial model was then checked for multicollinearity and where predictor variables with variable inflation factor (VIF) greater than 3 were removed. Subsequently, the final model was fitted with sex, 7-day rainfall, daily rainfall, air temperature, photoperiod and lunar illumination as the predictor variables and actively spawning phase as the outcome variable. We performed data exploration before each GLM followed with model validation after executing the GLM according to the protocol by Zuur et al.62 Diagnostic plots of the final GLMs in Supplementary Figs. S8–S10 are available online.
Ethics approval
Fish sampling and handling procedures were approved by the University Research Ethics Committee of Universiti Brunei Darussalam (approval reference: UBD/OAVCR/UREC/Feb18-09; 1 March 2018). All animal procedures were performed in accordance with the Universiti Brunei Darussalam guidelines on the use of animals for scientific procedures. All methods were performed in accordance with the ARRIVE guidelines.
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
References
Wooton, R. J. Introduction: Strategies and tactics in fish reproduction. In Fish Reproduction: Strategies and Tactics (eds Potts, G. W. & Wooton, R. J.) 1–12 (Academic Press, 1984).
Winemiller, K. O. & Rose, K. A. Patterns of life-history diversification in North American fishes: Implications for population regulation. Can. J. Fish. Aquat. Sci. 49, 2196–2218 (1992).
Durham, B. W. & Wilde, G. R. Asynchronous and synchronous spawning by smalleye shiner Notropis buccula from the Brazos River, Texas. Ecol. Freshw. Fish 17, 528–541 (2008).
Rodriguez-Ruiz, A. & Granado-Lorencio, C. Spawning period and migration of three species of cyprinids in a stream with Mediterranean regimen (SW Spain). J. Fish Biol. 41, 545–556 (1992).
Mills, C. A. Reproduction and life history. In Cyprinid Fishes: Systematics, Biology and Exploitation (eds Winfield, I. J. & Nelson, J. S.) 483–508 (Chapman and Hall, 1991).
Smith, B. B. & Walker, K. F. Spawning dynamics of common carp in the River Murray, South Australia, shown by macroscopic and histological staging of gonads. J. Fish Biol. 64, 336–354 (2004).
Ma, B.-S., Xie, C.-X., Huo, B., Yang, X.-F. & Chen, S. S. Reproductive biology of Schizothorax o’connori (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River, Tibet. Zool. Stud. 51, 1066–1076 (2012).
Ramasamy, M. & Rajangam, S. Maturation and reproductive biology of Reba Carp Cirrhinus Reba (Hamilton) in Lower Anicut Reservoir, Tamil Nadu, India. Fish. Aquacult. J. 08, (2017).
De Silva, S. S., Schut, J. & Kortmulder, K. Reproductive biology of six Barbus species indigenous to Sri Lanka. Environ. Biol. Fishes 12, 201–218 (1985).
Sato, Y., Bazzoli, N., Rizzo, E., Boschi, M. B. & Miranda, M. O. T. Influence of the Abaeté River on the reproductive success of the neotropical migratory teleost Prochilodus argenteus in the São Francisco River, downstream from the Três Marias Dam, southeastern Brazil. River Res. Appl. 21, 939–950 (2005).
Gurgel, L. L., Verani, J. R. & Chellappa, S. Reproductive ecology of Prochilodus brevis an Endemic Fish from the Semiarid Region of Brazil. Sci. World J. 2012, 1–7 (2012).
Karnatak, G. et al. Understanding the role of climatic and environmental variables in gonadal maturation and spawning periodicity of spotted snakehead, Channa punctata (Bloch, 1793) in a tropical floodplain wetland, India. Environ. Biol. Fishes 101, 595–607 (2018).
de Magalhães Lopes, J., Alves, C. B. M., Peressin, A. & Pompeu, P. S. Influence of rainfall, hydrological fluctuations, and lunar phase on spawning migration timing of the Neotropical fish Prochilodus costatus. Hydrobiologia 818, 145–161 (2018).
López-Rodríguez, N. C., Leão, A. H. F., Rocha, R. M., Prudente, B. S. & Montag, L. F. A. Environmental influence on the reproductive strategy of Helogenes marmoratus (Siluriformes: Cetopsidae) in the Amazonian streams. Neotrop. Ichthyol. 19, 1–20 (2021).
Fricke, R., Eshmeyer, W. N. & Fong, D. Genera/species by family/subfamily. Eschmeyer’s catalog of fishes. https://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (2020).
Rainboth, W. J. Cyprinids of South East Asia. In Cyprinid Fishes: Systematics, Biology and Exploitation (eds Winfield, I. J. & Nelson, J. S.) 175–208 (Chapman and Hall, 1991).
Kottelat, M. & Raffles Museum of Biodiversity Research. The fishes of the inland waters of Southeast Asia : a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. (National University of Singapore, 2013).
Khaironizam, M. Z. & Zakaria-Ismail, M. Spawning period and fecundity of Neolissochilus soroides (Duncker, 1904) (Pisces, Teleostei, Cyprinidae) from a small Malaysian stream. Turk. J. Zool. 37, 65–72 (2013).
Muchlisin, Z. A., Musman, M. & Azizah, M. S. Spawning seasons of Rasbora tawarensis (Pisces: Cyprinidae) in Lake Laut Tawar, Aceh Province, Indonesia. Reproduct. Biol. Endocrinol. 8, 1–8 (2010).
Wibowo, A. & Kaban, S. Reproductive characteristics of Indonesia mahseer (Tor tambroides, Bleeker, 1954), in two different rivers in Western Sumatera. Indones. Fish Res. J. 20, 49 (2014).
Dopeikar, H., Keivany, Y. & Shadkhast, M. Reproductive biology and gonad histology of the Kura Barbel, Barbus lacerta (cyprinidae), in bibi-sayyedan river, tigris basin. North West J. Zool. 11, 163–170 (2015).
Garg, S. K. & Jain, S. K. Effects of photoperiod and temperature on ovarian activity in the Indian murrel, Channa (Ophiocephalus) punctatus (Bloch). Can. J. Zool. 63, (1985).
Chellappa, S., Câmara, M. R., Chellappa, N. T., Beveridge, M. C. & Huntingford, F. A. Reproductive ecology of a neotropical cichlid fish, Cichla monoculus (Osteichthyes: Cichlidae). Braz. J. Biol. 63, 17–26 (2003).
Lowe-McConnell, R. H. Ecological studies in tropical fish communities. Ecol. Stud. Trop. Fish Commun. https://doi.org/10.1017/cbo9780511721892 (1987).
Parkinson, D., Philippart, J. C. & Baras, E. A preliminary investigation of spawning migrations of grayling in a small stream as determined by radio-tracking. J. Fish Biol. 55, (1999).
Sato, Y., Godinho, H., Ross, C. & Baer, A. Migratory fishes of the Sa˜o Francisco River. In Migratory Fishes of South America (eds Carolsfelds, J. & Harvey, B.) 195–232 (IDRC and World Bank, 2003).
Sulaiman, Z., Hui, T. H. & Lim, K. K. P. Annotated checklist of freshwater fishes from Brunei Darussalam, Borneo. Zootaxa 4379, 24–46 (2018).
Tan, M. & Armbruster, J. W. Phylogenetic classification of extant genera of fishes of the order Cypriniformes (Teleostei: Ostariophysi). in Zootaxa vol. 4476 6–39 (Magnolia Press, 2018).
Mittermeier, R. A., Turner, W. R., Larsen, F. W., Brooks, T. M. & Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots (eds Zachos, F. & Habel, J.) 3–22 (Springer, 2011). https://doi.org/10.1007/978-3-642-20992-5_1.
Dudgeon, D. Endangered ecosystems: A review of the conservation status of tropical Asian rivers. Hydrobiologia 248, (1992).
Lumbantobing, D. Lobocheilos ovalis. The IUCN Red List of Threatened Species 2020: e.T91058005A91058010 (2020). https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T91058005A91058010.en.
Kottelat, M. & Hui, T. H. A synopsis of the genus Lobocheilos in Java, Sumatra and Borneo, with descriptions of six new species (Teleostei: Cyprinidae). Ichthyol. Explor. Freshw. 19, (2008).
Malcolm, J. R., Liu, C., Neilson, R. P., Hansen, L. & Hannah, L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 20, (2006).
Torres, A. G. & Lumbantobing, D. Rasbora argyrotaenia. The IUCN Red List of Threatened Species 2021: e.T91071106A91071137. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T91071106A91071137.en (2021).
Rachmatika, I., Nasi, R., Sheil, D. & Wan, M. A First Look at the Fish Species of the Middle Malinau: Taxonomy, Eoclogy, Vulnerability and Importance (Center for International Forestry Research, 2005).
Dina, R., Samir, O., Lukman, Haryani, G. S. & Nasution, S. H. Fish and fisheries of Bada (Rasbora spp.) in Lake Maninjau, West Sumatra. in IOP Conference Series: Earth and Environmental Science vol. 306 (IOP Publishing Ltd, 2019).
Shafrudin, D. et al. Development potential of native fish in Batang Toru Watershed, North Sumatra, Indonesia: Discussion on the impact of dam development and aquaculture efforts. E3S Web Conferences. 322, (2021).
Kottelat, M., Pinder, A. & Harrison, A. Tor tambra. The IUCN Red List of Threatened Species in 2018: : e.T188012A89801879. https://doi.org/10.2305/IUCN.UK.2012-1.RLTS.T188012A1845199.en (2018).
Taylor, C. M. & Miller, R. J. Reproductive ecology and population structure of the Plains Minnow, Hybognathus placitus (Pisces: Cyprinidae), Central Oklahoma. Am. Midland Naturalist 123, 32–39 (1990).
Durham, B. W. & Wilde, G. R. Influence of stream discharge on reproductive success of a prairie stream fish assemblage. Trans. Am. Fish. Soc. 135, 1644–1653 (2006).
Alkins-Koo, M. Reproductive timing of fishes in a tropical intermittent stream. Environ. Biol. Fishes 57, 49–66 (2000).
Chellappa, S., Bueno, R. M. X., Chellappa, T., Chellappa, N. T. & Almeida e Val, V. M. F. Reproductive seasonality of the fish fauna and limnoecology of semi-arid Brazilian reservoirs. Limnologica 39, 325–329 (2009).
Ingram, B., Sungan, S., Tinggi, D., Sim, S. Y. & De Silva, S. S. Breeding performance of Malaysian mahseer, Tor tambroides and T. douronensis broodfish in captivity. Aquacult. Res. 38, 809–818 (2007).
Earl of Cranbook & Edwards, D. Belalong: A Tropical Rainforest. The Royal Geographical Society, London, & Sun Tree Publishing, Singapore. Journal of Tropical Ecology (The Royal Geographical Society and Sun Tree Publishing, 1994). https://doi.org/10.1017/s0266467400008774.
Fox, J. T. & Magoulick, D. D. Hydrologic and environmental thresholds in stream fish assemblage structure across flow regimes. Ecol. Indic. 144, (2022).
Hitt, N. P., Landsman, A. P. & Raesly, R. L. Life history strategies of stream fishes linked to predictors of hydrologic stability. Ecol. Evol. 12, (2022).
Magoulick, D. D. et al. Hydrologic variation influences stream fish assemblage dynamics through flow regime and drought. Sci. Rep. 11, (2021).
Vila-Gispert, A., Moreno-Amich, R. & García-Berthou, E. Gradients of life-history variation: An intercontinental comparison of fishes. Rev. Fish Biol. Fish 12, 417–427 (2002).
Bornestaf, C., Mayer, I. & Borg, B. Melatonin and maturation pace in female three-spined stickleback, Gasterosteus aculeatus. Gen. Comp. Endocrinol. 122, 341–348 (2001).
Mousavi-Sabet, H. et al. Reproductive biology of Alburnus mossulensis (Teleostei: Cyprinidae) in Gamasiab River, western Iran. Iran. J. Ichthyol. 4, (2017).
Dascyllus, D., Sella, A. L. B. & Danilowicz, B. S. The role of temperature in spawning of the damselfish. 57, 624–636 (1995).
Dey, R., Bhattacharya, S. & Maitra, S. K. Importance of photoperiods in the regulation of ovarian activities in Indian major carp Catla catla in an annual cycle. J. Biol. Rhythms 20, 145–158 (2005).
Campos-Mendoza, A., McAndrew, B. J., Coward, K. & Bromage, N. Reproductive response of Nile tilapia (Oreochromis niloticus) to photoperiodic manipulation; effects on spawning periodicity, fecundity and egg size. Aquaculture 231, 299–314 (2004).
Winemiller, K. O. Seasonality of reproduction by liverbearing fishes in tropical rainforest streams. Oecologia 95, 266–276 (1993).
Brunei Darussalam Meterological Department. Brunei Climate. http://bruneiweather.com.bn/climate.
Mumford, S. L. National Wild Fish Health Survey Laboratory Procedures Manual. in (2004).
Brown-Peterson, N. J., Wyanski, D. M., Saborido-Rey, F., Macewicz, B. J. & Lowerre-Barbieri, S. K. A standardized terminology for describing reproductive development in fishes. Mar. Coastal Fisheries 3, 52–70 (2011).
Timeanddate. Bandar Seri Begawan, Brunei—Sunrise, sunset, and moon times for today. https://www.timeanddate.com/astronomy/brunei/bandar-seri-begawan (2021).
R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/ (2022).
Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. https://CRAN.R-project.org/package=nlme (2022).
Lenth, R. V. emmeans: Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeans (2021).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14 (2010).
Acknowledgements
This research was funded by Universiti Brunei Darussalam under the Faculty, Institute and Centre Block Funds (reference: UBD/RSCH/1.13(b)/FICBF/2018/007, UBD/RSCH/1.13(b)/FICBF/2019/013 and UBD/RSCH/1.4/FICBF(b)/2020/029). This work was also supported by Universiti Brunei Darussalam—KBFSC Travel Grant Fund (reference: UBD/AVC-R/1.23). We would like to thank Dr. Faihanna Ching Abdullah for the access to histology facility in Universiti Malaysia Sabah. We are grateful to the Kuala Belalong Field Studies Centre, Universiti Brunei Darussalam and Ministry of Primary Resources and Tourism of Brunei Darussalam for their support throughout the research.
Author information
Authors and Affiliations
Contributions
R.K. and T.A. conceptualization; R.K. designed and performed the study, analyzed the data and prepared the manuscript. T.A. and N.A. reviewed the manuscript for final publication. All authors have reviewed the content, provided critical revisions and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kahar, R., Ahmad, N. & Arai, T. Year-round spawning of three tropical Cypriniformes fishes in Southeast Asia. Sci Rep 13, 8971 (2023). https://doi.org/10.1038/s41598-023-36065-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36065-9
- Springer Nature Limited