Abstract
The relationship between serum uric acid (SUA) and poor cognitive performance in patients with ischemic stroke is unclear. We hypothesized that the severity of renal function mediates the association between SUA and cognitive dysfunction.A retrospective analysis of 608 patients with ischemic stroke was conducted between 2016 and 2020. SUA was obtained from inpatient medical records. Global cognitive function via mini-mental state exam (MMSE) and Montreal Cognitive Assessment (MoCA) was determined one month after hospital discharge. The relationship between SUA and cognitive function was assessed by multiple linear and logistic regression analyses. Patients had a mean age of 66.6 years (SD: 4.1 years), and 52% were male. The mean SUA level was 298.6 ± 75.4 μmol/L. SUA increases were significantly positively associated with lower MMSE and MoCA scores and increased risk of moderate-severe cognitive impairment one month after stroke (p < 0.01), even after adjusting for factors including age, gender, BMI, diabetes and hypertension history. Adding a term for estimated glomerular filtration rate (eGFR) attenuated these associations such that SUA was no longer associated with cognitive performance. A fully adjusted stronger negative association between SUA and cognitive performance was found in those who had lower eGFR, with a significant eGFR interaction for MMSE (p-interaction = 0.016) and MoCA (p-interaction = 0.005). In patients with ischemic stroke, SUA showed an inverse association with cognitive function among those who have lower eGFR. The renal function might mediate the association between SUA and cognitive dysfunction.
Similar content being viewed by others
Introduction
Post-stroke cognitive impairment and dementia is a major source of morbidity and mortality worldwide1. A Chinese community-based study showed that the overall prevalence of post-stroke cognitive impairment (PSCI) is up to 80.97%, including 48.91% for cognitive impairment without dementia and 32.05% for dementia2. A better understanding of modifiable risk factors of cognitive decline or dementia after stroke is of vital importance to develop preventive strategies3. Serum uric acid (SUA) is the final product of purine catabolism and is excreted by the kidneys. Increased SUA is associated with a higher risk of vascular disease including stroke and chronic kidney disease (CKD)4,5,6, which may predispose individuals to cognitive impairment7,8,9.
To date, many cross-sectional or longitudinal studies have been performed to determine the relationship between uric acid and cognitive impairment, but the available data remain controversial and the link with renal function is not clear10,11,12,13. Recently, a meta-analysis found that a higher level of SUA after disease onset may be a marker of PSCI risk in patients with acute ischemic stroke14. A prospective study in patients with ischemic stroke and transient ischemic attack (TIA) in China found a U-shaped association between SUA and PSCI in males, but no association in females15. A cohort study in China found a negative correlation between SUA levels and MoCA scores in patients with lacunar cerebral infarction8. One cross-sectional study in patients with chronic heart failure demonstrated that elevated SUA is independently related to worse cognitive function in men but not in women16. One study in Turkey found uric acid levels are independently and inversely associated with mild cognitive dysfunction in subjects with CKD17. In contrast, a large population-based cohort did not find a relationship between SUA, eGFR (calculated using creatinine or cystatin C) levels and cognitive performance18. In a longitudinal study, hyperuricemia was associated with lower baseline mini-mental state exam (MMSE) scores but not with MMSE change over time, and also that albuminuria is an independent predictor of subsequent cognitive decline in men19.
SUA is commonly elevated in those with CKD and is associated with an increased risk of CKD progression20. A meta-analysis reported that uric acid lowering therapy may be effective in retarding the progression of CKD21. Furthermore, one study suggests that lowering uric acid in subjects with CKD may slow down the progression of renal disease and cardiovascular risk22. Similarly, early reduction in SUA may predict future improvement of renal function, which greatly positive affects the risk of cardiovascular events23 and cognitive dysfunction24.
The exact links between SUA and cognitive dysfunction in stroke patients are not fully understood. The main pathological mechanisms involved in SUA related cognitive dysfunction, such as oxidative stress, neuroinflammation, endothelial dysfunction and excitotoxicity, may collectively affect neuronal and brain function and further implicate SUA-related cognitive decline. SUA metabolism may be a double-edged sword in terms of the inflammatory and/or oxidative responses it induces in brain tissue, although its harmful effects appear to outweigh the benefits of SUA in most cases25,26,27. SUA as the compound of uremic toxicity is more likely to affect cerebro-renal interaction dysfunction, which may be the cause of cognitive impairment with CKD28. It is important to clarify the relationship between SUA and PSCI and whether this relationship is modified by renal function. This study aimed to investigate whether SUA is independently associated with worse cognitive performance in patients with ischemic stroke, and the influence of renal function.
Methods
Data source
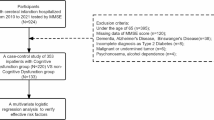
Using a retrospective design, this study analyzed data from 608 patients aged 60 to 80 years with acute ischemic stroke within 72 h of onset between January 2016 and December 2020. Data were obtained from inpatient medical records and a database of cognitive assessment results for outpatient visits one month after hospital discharge. All patients had no history of a severe cognitive disorder or hyperuricemia before the stroke and were able to complete the assessment. Patients were excluded if they had a condition preventing them from completing the examination, such as aphasia or a consciousness disorder.
The study was conducted at the Department of Neurology, Second Hospital of Shanxi Medical University, Taiyuan, a tertiary hospital in Shanxi, China. This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (No. 2019YX214). The research was completed in accordance with the Helsinki Declaration. Due to the retrospective nature of the study, informed consent of the patients was not required.
Clinical and laboratory data
Clinical and laboratory information extracted from hospital records included systolic and diastolic blood pressure, self‐reported demographic characteristics (age, gender, height, weight, years of education, smoking and alcohol consumption status, and diabetes mellitus and hypertension history); and laboratory results obtained on admission. Fasting venous blood was collected for analysis. Biochemical measurements were measured using automatic clinical analyzers (Beckman Coulter) at the core lab of the Second Hospital, Shanxi Medical University, Taiyuan, China. SUA was assayed by an uricase-peroxidase method. Day-by-day variation had to be within 5%. Values were set in international units (µmol/L). The reference range for adult men was 208.3–428.4 µmol/L, whereas for women, this range was cited as 154.7–357.0 µmol/L. Serum glucose was determined by a hexokinase method. Fasting lipids and serum creatinine concentrations were determined by an enzymatic method. Serum folate and vitamin B12 were measured using a chemiluminescent immunoassay. Urine albumin and creatinine levels were determined using an automatic protein analyzer (Alere Afinion AS100) at the lab of nephrology department.
Additionally, data on neural function were taken via the National Institutes of Health Stroke Scale (NIHSS) score. All patients were diagnosed according to the TOAST classification as large artery atherosclerosis, cardioembolism, small artery occlusion, stroke of other determined cause, or stroke of undetermined cause in the medical records29. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The eGFR was calculated using the CKD-EPI equation.
where serum creatinine (Scr) is expressed in mg/dl and age in years; \(\kappa\) is 0.7 for females and 0.9 for males; \(\alpha\) is − 0.329 for females and − 0.411 for males; min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 130.
Cognitive impairment testing
All cognitive function tests were conducted by two neuropsychological assessors trained in consistency using the MMSE and Montreal Cognitive Assessment (MoCA) one month after hospital discharge. The good reliability and validity of MoCA and MMSE were confirmed in China31,32. These two commonly-used cognitive screening tools have similar accuracy for detection of dementia/multidomain impairment33. Compared to the MMSE, the MoCA has a higher sensitivity and specificity for initial cognitive function screening34,35, and has been reported to be particularly useful to discern PSCI in patients whose cognitive deficits were undetectable with the MMSE. The uses of the MoCA and MMSE should be combined to optimize the PSCI screening36,37. An extra point was added to the MoCA test score for patients with < 12 years of formal education. The MMSE and MoCA scores ranged from 0 to 30, with higher scores indicating better cognitive function. The tests assess attention, concentration, orientation, language, the ability to follow simple verbal and written commands, and immediate- and short-term recall. According to a previous study and cognitive scores of the patients, moderate-severe cognitive impairment one month after stroke was defined as a MoCA score < 17 or MMSE < 2037.
Statistical analysis
Retrospective data were analyzed. Patient characteristics were presented as mean ± SD for continuous variables, and categorical variables were reported as frequencies and percentages. Differences between groups for continuous data were tested by one-way ANOVA. Differences between groups for proportions were tested using Chi-square. Multiple linear regression and logistic regression were used to estimate the β and odds ratio (OR) of SUA level with cognitive function. SUA levels were divided into: 58.0–246.9, 247.0–287.9, 288.0–339.9, and 340.0–611.0 μmol/L according to SUA quartiles. Clinical and laboratory variables were analyzed according to SUA grade levels. Crude and adjusted ß and OR with 95% CIs of cognitive scores and moderate-severe cognitive impairment were calculated for quartiles of uric acid (with the lowest quartile as the reference) as well as continuously, per 20 unit increase in uric acid level. Then, we combined Q1–Q3 and Q4 categories for further analysis.
We constructed three models with progressively increased adjustments for confounding variables that could affect the association between uric acid level and cognitive function. The first was crude. Model 1 was additionally adjusted for age, gender, systolic blood pressure, BMI, education, diabetes and hypertension history, stroke subtypes, NIHSS score, smoking and alcohol drinking status, serum total cholesterol, triglyceride, vitamin B12, folate, fasting glucose, and UACR. Model 2 was further adjusted for estimated glomerular filtration rate. A two-tailed p < 0.05 was considered statistically significant. All analyses were performed using Empower® (www.empowerstats.com; X&Y solutions, Inc., Boston, MA) and R (http://www.R-project.org).
Ethics approval and consent to participate
This retrospective research was completed in accordance with Helsinki Declaration. This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (NO. 2019YX214). Due to the retrospective nature of the study, informed consent of the patients was not required.
Results
Population characteristics
The demographic and clinical characteristics of all 608 patients grouped by SUA quartiles are shown in Table 1. Patients had a mean age of 66.6 years (SD: 4.1 years) and 52% were male. The mean SUA level was 298.6 ± 75.4 μmol/L. The ranges of SUA for quartiles 1–4 were 58.0–246.9, 247.0–287.9, 288.0–339.9, and 340.0–611.0 μmol/L, respectively. Patients in the higher SUA categories were more likely to be male and former or current smokers and drinkers. In addition, they were less likely to have diabetes, but more likely have a higher level of education and DBP, lower blood glucose and eGFR on admission, and lower MoCA scores one month post-stroke (Table 1).
SUA levels and cognitive performance
Table 2 contains the results of unadjusted and adjusted models for continuous and categorical relationships between parameters of SUA and cognitive tests. When examined as a continuous variable (per 20-unit increase) in both crude and model 1, SUA increases were also significantly positively associated with lower MMSE and MoCA scores (p < 0.01). However, after eGFR was introduced into the model, the relationship between SUA and cognitive performance disappeared (p = 0.396 for MMSE and p = 0.122 for MoCA). In the category analysis, we found a significant association for patients with the highest SUA quartile compared to patients with the lowest quartile in crude and model 1: MMSE (ß, − 0.49; 95% CI − 0.96, − 0.02 in crude and ß, − 0.60; 95% CI − 1.11, − 0.10 in model 1); and MoCA (ß, − 0.78; 95% CI − 1.30, − 0.26 in crude and ß, − 0.82; 95% CI − 1.38, − 0.26 in model 1). The direction of the relationship between SUA and cognitive function changed once eGFR was introduced into the model (ß, 0.45; 95% CI − 0.03, 0.92 in Model 2 for MMSE and ß, 0.60; 95% CI 0.10, 1.09 in Model 2 for MoCA). Furthermore, when we combined the first three-quarters of SUA as the reference, the outcomes were similar with the fourth quartile of SUA versus the first three-quarters (Table 2).
Table 3 shows the 1-month incidence of moderate-severe cognitive impairment across groups with different SUA levels. A total of 120 (19.7%) and 88 (14.5%) patients had moderate- severe cognitive impairment, as determined by the MoCA and MMSE, respectively. The results were similar to linear regression. In both crude and model 1, compared with the Q1 group, patients in Q4 had a higher risk of moderate-severe cognitive impairment at 1 month (adjusted OR = 2.36, 95% CI 1.09–5.12, P = 0.030 for MMSE; OR = 3.34, 95% CI 1.64–6.80, P = 0.001 for MoCA), whereas the direction of the relationship between SUA and incidence of moderate-severe cognitive impairment changed once eGFR was introduced into the model (OR = 0.64, 95% CI 0.25–1.62, P = 0.350 for MMSE; OR = 0.67, 95% CI 0.28–1.64, P = 0.384 for MoCA in Model 2). The outcomes were similar when SUA was analyzed as a dichotomous and continuous variable.
Stratified analyses by potential effect modifiers
Stratified analyses were performed to further assess the relationship of SUA (the fourth quartile vs. the first three-quarters; Table 4) with cognitive performance in various subgroups. A fully adjusted stronger negative association between SUA and cognitive performance was found in those with lower eGFR with significant eGFR interaction for MMSE (p-interaction = 0.016) and MoCA (p-interaction = 0.005). None of the other variables, including gender, age, systolic blood pressure, NIHSS score, stroke subtypes, or blood glucose significantly modified the association between SUA and cognitive performance.
Discussion
This study found a negative correlation between SUA level and cognitive performance in older adults with ischemic stroke and low eGFR in China; SUA increases were significantly positively associated with elevated risk of moderate-severe cognitive impairment and that severity of renal function influenced this relationship. This effect was present even after controlling for variables known to be associated with both cognition and elevated SUA. This partly supports the finding that, in CKD, the presence of elevated uric acid is associated with worse cognitive function17.
Of note, there are some contradictory data about SUA and cognitive dysfunction. Some studies reported that a higher level of SUA was associated with poorer cognitive performance8, while others suggested a beneficial effect7, and a possible effect of gender16,38. A prospective study in patients with ischemic stroke and TIA in China found a U-shaped association between SUA and PSCI in males but not in females15. Our study found that the relationship between SUA and cognitive function attenuated once eGFR was introduced into the model, in part confirming the association between eGFR, SUA and cognitive function. SUA may be a less informative risk factor of cognitive ability than eGFR in ischemic stroke patients.
The precise pathophysiologic mechanisms that underlie the link between the variations in uric acid, eGFR, and cognitive dysfunction after stroke are not known and require further elucidation. The relationship between eGFR and SUA levels was documented by numerous studies21,39. Hyperuricemia may potentiate the effects of angiotensin II to induce renal vasoconstriction, which could be mediated by its effect to upregulate angiotensin type 1 receptors on vascular smooth muscle cells, leading to arteriopathy, hypertension and kidney dysfunction40. In addition, it needs to be stressed that a low GFR value is a well-known risk factor for cognitive dysfunction41. A possibility may be that coexistence of lower eGFR and higher uric acid may also contribute to the development of vascular cognitive impairment by producing non-crystal associated renal and cerebral vascular injury, which underlies the development of moderate-severe vascular cognitive impairment. Coexistence of hyperuricemia and renal insufficiency may be a risk factor of PSCI. It remains unclear what the additional detrimental effect of these markers is on brain function. More research is needed to elucidate this issue.
The strengths of this study include its evaluation of cognitive function with both MoCA and MMSE, thus providing clinical accuracy to the analyses. In addition, we were able to control for a number of potential confounders while assessing the association, including demographic and clinical indicators.
There were several limitations to the design of our study. First, the SUA level was measured once, which ignored possible intra-individual fluctuations. Another consideration was that cognitive assessment was performed one month after hospital discharge, and the incidence of PSCI is highest three months after stroke42. Although there were no patients with dementia at baseline, since we did not take baseline cognitive score, we could not assess changes in cognitive level. Further long-term follow-up is still needed. Finally, we did not analyze impairment in specific cognitive domains, only compared overall cognitive scores.
Conclusion
In summary, our findings suggest that in patients with ischemic stroke, SUA showed an inverse association with cognitive function in those with lower eGFR. We conclude that current findings support the hypothesis that severity of renal function may modify the relationship between SUA and cognitive performance. The findings have clinical implications. Patients with renal insufficiency should pay attention to uric acid level and actively control hyperuricemia to prevent cognitive dysfunction, or delay disease progression. Further studies are warranted to confirm whether uric acid lowering therapy in patients with renal dysfunction is a possible new determinant for cognitive impairment.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rost, N. S. & Brodtmann, A. Post-stroke cognitive impairment and dementia. Circ. Res. 130(8), 1252–1271 (2022).
Qu, Y. et al. Prevalence of post-stroke cognitive impairment in China: A community-based, cross-sectional study. PLoS ONE 10(4), e0122864 (2015).
Plassman, B. L., Williams, J. W. Jr., Burke, J. R., Holsinger, T. & Benjamin, S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann. Intern. Med. 153(3), 182–193 (2010).
Martínez-Quintana, E., Tugores, A. & Rodríguez-González, F. Serum uric acid levels and cardiovascular disease: The Gordian knot. J. Thorac. Dis. 8(11), E1462-e1466 (2016).
Nishizawa, H., Maeda, N. & Shimomura, I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens. Res. 45(4), 635–640 (2022).
Tariq, M. A., Shamim, S. A., Rana, K. F., Saeed, A. & Malik, B. H. Serum uric acid–risk factor for acute ischemic stroke and poor outcomes. Cureus 11(10), e6007 (2019).
Euser, S. M., Hofman, A., Westendorp, R. G. & Breteler, M. M. Serum uric acid and cognitive function and dementia. Brain 132(Pt 2), 377–382 (2009).
Wanggong, F., Xiang, J., Yang, S., Zhang, W. & Tuerganbieke, R. Correlation of serum uric acid, cystatin C and high-sensitivity C-reactive protein with cognitive impairment in lacunar cerebral infarction. Am. J. Transl. Res. 13(6), 6717–6723 (2021).
Miglinas, M., Cesniene, U., Janusaite, M. M. & Vinikovas, A. Cerebrovascular disease and cognition in chronic kidney disease patients. Front. Cardiovasc. Med. 7, 96 (2020).
Khan, A. A., Quinn, T. J., Hewitt, J., Fan, Y. & Dawson, J. Serum uric acid level and association with cognitive impairment and dementia: Systematic review and meta-analysis. Age 38(1), 16 (2016).
Du, N. et al. Inverse association between serum uric acid levels and Alzheimer’s disease risk. Mol. Neurobiol. 53(4), 2594–2599 (2016).
Alam, A. B., Wu, A., Power, M. C., West, N. A. & Alonso, A. Associations of serum uric acid with incident dementia and cognitive decline in the ARIC-NCS cohort. J. Neurol. Sci. 414, 116866 (2020).
Liu, M., Wang, J., Zeng, J. & He, Y. Relationship between serum uric acid level and mild cognitive impairment in Chinese community elderly. BMC Neurol. 17(1), 146 (2017).
Yan, X. et al. Uric acid and cognitive impairment in patients with acute ischemic stroke: A meta-analysis. Horm. Metab. Res. 54(5), 316–324 (2022).
Liu, Q., Liao, X., Pan, Y., Jin, A. & Zhang, Y. Association between serum uric acid levels and cognitive function in patients with ischemic stroke and transient ischemic attack (TIA): A 3-month follow-up study. Neuropsychiatr. Dis. Treat. 17, 991–999 (2021).
Niu, W., Yang, H. & Lu, C. The relationship between serum uric acid and cognitive function in patients with chronic heart failure. BMC Cardiovasc. Disord. 20(1), 381 (2020).
Afsar, B., Elsurer, R., Covic, A., Johnson, R. J. & Kanbay, M. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am. J. Nephrol. 34(1), 49–54 (2011).
Richard, E. L. et al. Biomarkers of kidney function and cognitive ability: A Mendelian randomization study. J. Neurol. Sci. 430, 118071 (2021).
Richard, E. L. et al. Markers of kidney function and longitudinal cognitive ability among older community-dwelling adults: The Rancho Bernardo Study. J. Alzheimers Dis. JAD 83(1), 319–331 (2021).
Fassett, R. G. et al. Biomarkers in chronic kidney disease: A review. Kidney Int. 80(8), 806–821 (2011).
Liu, X. et al. Effects of uric acid-lowering therapy on the progression of chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 40(1), 289–297 (2018).
Goicoechea, M. et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. CJASN 5(8), 1388–1393 (2010).
Ito, S. et al. Impact of serum uric acid on renal function and cardiovascular events in hypertensive patients treated with losartan. Hypertens. Res. 35(8), 867–873 (2012).
Drew, D. A., Weiner, D. E. & Sarnak, M. J. Cognitive impairment in CKD: Pathophysiology, management, and prevention. Am. J. Kidney Dis. 74(6), 782–790 (2019).
Mijailovic, N. R., Vesic, K. & Borovcanin, M. M. The influence of serum uric acid on the brain and cognitive dysfunction. Front. Psych. 13, 828476 (2022).
Wu, J. X., Xue, J., Zhuang, L. & Liu, C. F. Plasma parameters and risk factors of patients with post-stroke cognitive impairment. Ann. Palliat. Med. 9(1), 45–52 (2020).
Chrysant, S. G. Association of hyperuricemia with cardiovascular diseases: Current evidence. Hosp. Pract. 2023, 1–10 (1995).
Watanabe, K., Watanabe, T. & Nakayama, M. Cerebro-renal interactions: Impact of uremic toxins on cognitive function. Neurotoxicology 44, 184–193 (2014).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1), 35–41 (1993).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Wen, H. B., Zhang, Z. X., Niu, F. S. & Li, L. The application of Montreal cognitive assessment in urban Chinese residents of Beijing. Zhonghua Nei Ke Za Zhi 47(1), 36–39 (2008).
Mitchell, A. J. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J. Psychiatr. Res. 43(4), 411–431 (2009).
Lees, R. et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 45(10), 3008–3018 (2014).
Huang, Y. Y. et al. Post-stroke cognitive impairment: Epidemiology, risk factors, and management. J. Alzheimers Dis. JAD 86(3), 983–999 (2022).
Munthe-Kaas, R. et al. Test accuracy of the montreal cognitive assessment in screening for early poststroke neurocognitive disorder: The nor-COAST study. Stroke 52(1), 317–320 (2021).
Suda, S. et al. Early cognitive assessment following acute stroke: Feasibility and comparison between mini-mental state examination and montreal cognitive assessment. J. Stroke Cerebrovasc. Dis. 29(4), 104688 (2020).
Shi, D., Chen, X. & Li, Z. Diagnostic test accuracy of the Montreal Cognitive Assessment in the detection of post-stroke cognitive impairment under different stages and cutoffs: A systematic review and meta-analysis. Neurol. Sci. 39(4), 705–716 (2018).
Perna, L., Mons, U., Schöttker, B. & Brenner, H. Association of cognitive function and serum uric acid: Are cardiovascular diseases a mediator among women?. Exp. Gerontol. 81, 37–41 (2016).
Tsai, C. W., Lin, S. Y., Kuo, C. C. & Huang, C. C. Serum uric acid and progression of kidney disease: A longitudinal analysis and mini-review. PLoS ONE 12(1), e0170393 (2017).
Johnson, R. J. et al. Essential hypertension, progressive renal disease, and uric acid: A pathogenetic link?. J. Am. Soc. Nephrol. 16(7), 1909–1919 (2005).
Wang, F., Zhang, L., Liu, L. & Wang, H. Level of kidney function correlates with cognitive decline. Am. J. Nephrol. 32(2), 117–121 (2010).
Sun, J. H., Tan, L. & Yu, J. T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2(8), 80 (2014).
Funding
This work was supported by grants from the Scientific Research Foundation for Doctors, the Second Hospital of Shanxi Medical University, China [grant number 201601-9]; the Natural Science Foundation of Shanxi Province, China [grant number 201801D221411]; and the Natural Science Foundation of Shanxi Province, China [Grant Number 20210302124427].
Author information
Authors and Affiliations
Contributions
D.F.L. and C.Y.Z. conceived of the project and devised the methodology. C.Y.Z. and P.F.M. extracted the data and performed the statistical analysis. C.Y.Z., X.P.Z., H.Z.G., and B.B. composed the manuscript. All authors provided contributions to data interpretation, and manuscript revisions and all approved the final version of the manuscript. D.F.L. and C.Y.Z. provided supervision for the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, C., Zhang, X., Meng, P. et al. The association between serum uric acid and cognitive performance in patients with ischemic stroke is modified by estimated glomerular filtration rate. Sci Rep 13, 7097 (2023). https://doi.org/10.1038/s41598-023-34352-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34352-z
- Springer Nature Limited