Abstract
The objective of this study was to determine the influence of a total-mixed ration including unsalable carrots at 45% DM on the rumen microbiome; and the plasma, rumen and liver metabolomes. Carrots discarded at processing were investigated as an energy-dense substitute for barley grain in a conventional feedlot diet, and improved feed conversion efficiency by 25%. Here, rumen fluid was collected from 34 Merino lambs at slaughter (n = 16 control; n = 18 carrot) after a feeding period of 11-weeks. The V4 region of the 16S rRNA gene was sequenced to profile archaeal and bacterial microbe communities. Further, a comprehensive, targeted profile of known metabolites was constructed for blood plasma, rumen fluid and biopsied liver metabolites using a gas chromatography mass spectrometry (GC–MS) metabolomics approach. An in vitro batch culture was used to characterise ruminal fermentation including gas and methane (CH4) production. In vivo rumen microbial community structure of carrot fed lambs was dissimilar (P < 0.01; PERMANOVA), and all measures of alpha diversity were greater (P < 0.01), compared to those fed the control diet. Unclassified genera in Bacteroidales (15.9 ± 6.74% relative abundance; RA) were more abundant (P < 0.01) in the rumen fluid of carrot-fed lambs, while unclassified taxa in the Succinivibrionaceae family (11.1 ± 3.85% RA) were greater (P < 0.01) in the control. The carrot diet improved in vitro ruminal fermentation evidenced as an 8% increase (P < 0.01) in DM digestibility and a 13.8% reduction (P = 0.01) in CH4 on a mg/ g DM basis, while the control diet increased (P = 0.04) percentage of propionate within total VFA by 20%. Fourteen rumen fluid metabolites and 27 liver metabolites were influenced (P ≤ 0.05) by diet, while no effect (P ≥ 0.05) was observed in plasma metabolites. The carrot diet enriched (impact value = 0.13; P = 0.01) the tyrosine metabolism pathway (acetoacetic acid, dopamine and pyruvate), while the control diet enriched (impact value = 0.42; P ≤ 0.02) starch and sucrose metabolism (trehalose and glucose) in rumen fluid. This study demonstrated that feeding 45% DM unsalable carrots diversified bacterial communities in the rumen. These dietary changes influenced pathways of tyrosine degradation, such that previous improvements in feed conversion efficiency in lambs could be explained.
Similar content being viewed by others
Introduction
Ruminants are well-known for their unique ability to convert inedible plant and plant-derived materials into high quality, human-edible products. Degradation of these feedstuffs occurs predominantly in the rumen, where a consortium of microbiota produces metabolites that are utilised for microbial growth, whole-animal maintenance, muscle, milk and fibre production1,2. Metabolites have been quantified in the rumen2 and are important markers in the identification of cell processes that underpin and influence the function of an organism3. The relationship between the rumen microbiome and the host has mainly been explored in animals fed conventional maize grain diets4. Metabolites including histamine, putrescine and tyramine were correlated with Acetitomaculum and Butyrivibrio in the rumen of maize grain-fed animals4, acting as markers for rumen acidosis5. Other markers, including metabolites present in blood plasma, provide information on performance and yield parameters including growth rate or carcass weight6, while liver metabolites best indicate the interaction of diet on nutrient utilisation7. To our knowledge, no other studies on the influence of alternative feedstuffs on the plasma, hepatic or rumen fluid metabolites using an ovine model have been reported in the literature.
In Australia, vegetable waste comprises approximately 29% of total food wastage8, but its use as livestock feed is seldom employed as an avenue of waste mitigation. Feeding a range of vegetables including beans, cabbage, cauliflower and potato maintained dry matter (DM) intake, average daily gain and DM digestibility in 12–18-month-old Bos indicus bulls at up to 9.7% DM of a wheat bran-based diet9, while the liveweight and average daily gain of Rambouillett × Polypay wethers fed a mixed ration substituting 50% DM sorghum grain for 50% DM onion bulbs was also maintained at 0.14 kg/day10. Vegetables have also demonstrated promise, where feeding whole potatoes at 30% DM of a finishing diet increased final liveweight, and dressing percentage by 5.4 and 2.7% respectively in double-muscled cows11, while carrots fed at 45% DM of a total-mixed ration (TMR) improved feed conversion efficiency in Merino wethers by 25% compared to a conventional grain-based TMR12. Diets containing feedstuffs high in fermentable sugars and readily degradable carbohydrates, such as carrots13 provide an environment rich in substrates readily utilised by rumen microbes, improving microbial activity14 and ruminant performance by extension.
Therefore, the aim of this study was to determine the influence of feeding lambs a TMR including 45% (DM-basis) unsalable carrots on the microbial community structure within the rumen and to profile the plasma, liver, and rumen fluid metabolomes to understand the systemic influence of diet. It was hypothesised that the carrot diet would provide an environment conducive to higher bacterial diversity, resulting in greater variation in the rumen metabolic profile compared to conventional feed ingredients (e.g. control diet).
Materials and methods
Animal experiment and sample collection
The animal study was previously detailed by Forwood et al.12. The methodology was performed in accordance with the guidelines of the Australian code for the care and use of animals for scientific purposes (2013), and was approved by The University of Queensland Animal Ethics Committee (approval #SAFS/035/19). The study is reported in accordance with the ARRIVE guidelines. Thirty-six 7-month-old Merino wether lambs, initial live weight 24.7 ± 0.3 kg were randomly allocated by live weight and assigned to individual indoor pens. After two-weeks adaptation, lambs were fed either control (51.9% barley grain, 40.2% lucerne hay, 7.1% canola meal, 0.8% MegaMin Mineral Blend); or carrot (45.2% carrot, 8.9% barley grain, 30.0% lucerne hay, 15.1% canola meal, 0.8% MegaMin Mineral Blend) experimental diets on a DM-basis for 11-weeks. Diets were formulated to be isonitrogenous. Twenty-four hours after completion of the feeding period, blood was obtained from lambs through jugular venepuncture into 10 mL lithium-heparin vacutainer tubes and placed on ice15. Samples were centrifuged at 2500×g for 15 min with resultant plasma stored at − 80 °C for metabolomics analysis.
Experimental details regarding the commercial slaughter of lambs have been reported12. Here, rumen digesta was collected immediately post-mortem from four locations within the rumen. Contents were pooled, then strained through two layers of muslin cloth, and 1 mL subsamples were immediately frozen in liquid nitrogen and stored at − 80 °C for DNA and metabolomics analysis.
Volatile fatty acids (VFA)
Organic acids and VFA were determined in rumen fluid samples using previously described methods16. Samples were processed and analysed via an Agilent technologies 7820A gas–liquid chromatograph system set up according to described methods17. Total volatile fatty acid concentration were expressed in mM and individual VFA as percentages of total VFA. Predicted CH4 yield was predicted using the following equation18:
where A = acetate; P = propionate and B = butyrate.
Rumen fluid DNA extraction, 16S rRNA sequencing, and diversity
Genomic DNA (gDNA) was extracted from rumen samples using bead-beating and on-column purification, per the methods used by Popova et al.19. DNA extracts were quantified on a Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific, France) and run on a FlashGel System (Lonza, Rockland, Inc.) to check integrity. Approximately 15 µg of gDNA were sent to Génome Québec (Montréal, QC H3B 1S6, Canada) for fluidigm amplification and MiSeq Illumina sequencing. The 515F, paired with 806R primers were selected for amplification targeting the V4 region of 16S rRNA gene of bacteria.
The DADA2 v. 1.820 package was used in R v. 4.0.221 within RStudio 1.3.95922 to process the 16S rRNA gene sequences. Briefly, forward and reverse 16S rRNA gene sequences were trimmed to 220 and 200 bp, respectively, merged, and then chimeras were removed. Taxonomy was assigned to the remaining sequences, referred to amplicon sequence variants (ASVs) at 100% similarity using the RDP naïve Bayesian classifier and the Greengenes database23. The number of ASVs per sample, Shannon diversity index, and inverse Simpson’s diversity index for 16S rRNA gene datasets were calculated in R using Phyloseq v. 1.26.024. All 16S rRNA gene sequences were submitted to the NCBI Sequence Read Archive under BioProject accession number PRJNA772293.
Comprehensive targeted metabolomics
Plasma for comprehensive, targeted metabolomics analyses were prepared following the methods of Zheng et al.25 with adjustments, where 100 µL of sample was combined with 300 µL methanol and vortexed for 15 s. Samples were centrifuged at 16,000×g for 10 min at 4 °C. Subsequently, an aliquot of 50 µL supernatant was placed into 1.5 mL Eppendorf tubes and dried under vacuum (Eppendorf Concentrator Plus, Hamburg, Germany). Rumen fluid was similarly treated, except 30 µL of each sample was pipetted into 1.5 mL Eppendorf tube and dried under vacuum.
Liver samples previously stored at − 80 °C were held on dry ice, then manually pulverised to obtain approximately 20–30 mg frozen liver and combined with 600 µL extraction solution [3:1 (v/v) methanol:water including 13C-sorbitol and 13C,15N-valine as internal standards]. Samples were homogenised via bead-beating on a Bead Ruptor Elite Bead Mill Homogenizer (Omni International, Kennesaw, GA, USA) for 5 cycles at 0 °C, each comprising 30 s bead beating at 4.00 m/s, followed by a dwell period of 45 s. Homogenate was mixed with 120 µL chloroform (chloroform:methanol:water = 1:3:1 v/v monophasic mixture), vortexed vigorously then incubated on ice for 10 min on an orbital shaker at medium speed. Samples were centrifuged at 0 °C for 5 min at maximum speed, and supernatant transferred for GC–MS preparation. Aliquots of 10 and 50 µL supernatant, along with a 200 µL pooled biological quality control were dried under vacuum. A final dry-down step was conducted using 50 µL methanol.
An aliquot of each sample (50 µL) was pooled to generate a pooled biological quality control sample (PBQC) and aliquots (50 µL) of this mixture were evaporated to dryness. Samples and PBQCs were derivatised by methoxyamination by the addition of 25 µL methoxyamine (30 mg/mL in pyridine, 2 h, 37 °C, 900 rpm), followed by trimethylsilylation with 25 µL BSTFA + 1% TMCS (1 h, 37 °C, 900 rpm). Metabolite profiles were acquired on a Shimadzu GCMS-TQ8050 NX system (Shimadzu, Kyoto, Japan).
Approximately 0.5 µL of derivatised sample was injected into the GC inlet set at 280 °C in split mode of 1:10. Chromatographic separation was achieved using an Agilent DB-5 capillary column (30 m × 0.25 mm × 1 µm). Oven conditions were set at 100 °C starting temperature, held for 4 min, then ramped at 10 °C/min to 320 °C and held for 11 min. Helium was used as the carrier gas at a flow rate of 1 mL/min. Compounds were fragmented by electron ionisation and analysed in multiple reaction monitoring (MRM) mode using the Shimadzu Smart Metabolites Database (https://www.shimadzu.com/an/gcms/metabolites/index.html) containing 475 MRM metabolite targets. A high-quality matrix was manually curated using the Shimadzu LabSolutions Insight GCMS program (v.3.7 SP3, Shimadzu Corporation), where metabolite targets were removed from the dataset if they were not present in all samples.
In vitro incubation
In vitro incubations of the carrot and control diets (composition as fed to lambs) were conducted at The University of Queensland (Gatton, QLD). Three Holstein steers were used for these incubations under the approval of The University of Queensland Animal Ethics Committee (approval #AE35581), as donor species does not influence rumen microbial composition26. Rumen digesta was collected from each animal within four distinct regions of the rumen as previously described27. Digesta was pooled and liquor strained through four layers of cheesecloth into a pre-warmed 1 L thermos and immediately transported to the laboratory. The methods of Menke et al.28 were used for the preparation of rumen inoculum, while the methods of Forwood et al.17 were modified for the incubation. Briefly, bottles (n = 18) containing 25 mL inoculum solution (65% buffer; 32% rumen liquor; 3% reducing agent) and ANKOM bags containing 0.5 g DM of each treatment were incubated using an orbital shaker (Ratek Instruments, Boronia, Australia) heated to 39 °C and set to 120 rpm. Moreover, these analyses comprised three 24 h incubation runs, with three replicates of each of carrot and control diet per run.
Samples of gas and methane were collected at 6 h and 24 h incubation. Following the methods of Forwood et al.17 with adjustments, 18 mL gas samples were extracted and transferred into 12 mL evacuated Exetainers (Labco Ltd., High Wycombe, United Kingdom) for CH4 determination. The CH4 concentration was measured using an Agilent model 7890a gas chromatograph with a flame ionization detector (FID) calibrated to 250 °C, air flow 300 mL/min, H2 fuel flow 30 mL/min, makeup flow (N2) 30 mL/min installed with a capillary column (Restek Rt-Q-Bond, 30 m × 0.53 mmID × 20 μm). The Split-Splitless Inlet was heated to 60 °C, 9.526 PSI, with Helium total flow 33 mL/min, septum purge flow 3 mL/min, Split ration 5:1, Split Flow 25 mL/min and an oven temperature of 60 °C. Methane measurements were defined as mg CH4/g DM and mg CH4/g digested DM.
Total gas production was obtained immediately after CH4 sampling using a water displacement apparatus29, while pH of the rumen fluid was measured using a Hanna Edge HI2002 pH meter (Hanna Instruments, Woonsocket, RI, USA). After gas and pH samples were collected, bottles were placed on ice to prevent further fermentation. Bags were removed from the bottles and rinsed thoroughly with water for 1 h in a washing machine, followed by drying in an oven set to 65 °C until a constant weight. In vitro dry matter disappearance was estimated by weighing the dry bags.
Statistical analyses
Alpha diversity and volatile fatty acids were analysed using the MIXED procedure of SAS 9.4 (SAS Institute, Cary NC), with treatment as the fixed effect and sample within treatment as the random effect. In vitro, gas production and methane data were similarly analysed, except for the addition of incubation run and treatment within run as random effects. The effect of diet on the microbial community structures was determined using PERMANOVA (adonis2 function) and Bray–Curtis dis-similarities in R with vegan 2.5-630. Statistical significance for all data analysed via the MIXED procedure was declared if the P-value was < 0.05, and a tendency reported if 0.05 ≤ P ≤ 0.10. Further, significance for log2 fold change data for archaeal and bacterial abundance was declared if false discovery (FDR) adjusted P-value ≤ 0.05.
Univariate and multivariate statistical analyses were conducted on plasma, rumen and liver metabolome data using Metaboanalyst 5.0 (https://www.metaboanalyst.ca). Data were normalised to the median and log-transformed, then pareto-scaled to reduce variability and the likelihood of false discovery. Fold change analyses were conducted at set thresholds of fold change = 1.2 and FDR P-value = 0.05 to determine significantly different metabolites and reduce the potential for false positive readings. Once significantly expressed metabolites were identified, pathway analysis was conducted using Metaboanalyst 5.0 based on the Bos taurus library and Kyoto encyclopaedia of genes and genomes (KEGG) pathway database to assign metabolites to metabolic pathways31. Principal component analyses (PCA) were used to determine separation of differentially expressed metabolites by treatment effect, while orthogonal projections to latent structures discriminant analysis (OPLS-DA) was utilised to ascertain strength of separation. To determine validity of the OPLS-DA model, n = 100 permutation tests were run on the data, where biological significance, thus robustness of the model was declared with Q2 and R2 values > 0.932.
To elucidate the metabolic contribution of rumen microbes to systemic function, functional profiles of rumen microbes were predicted using the CowPI platform (https://www.cowpi.org/33). Subsequently, the influence of functional pathway on systemic differences between carrot and control-fed lambs was ascertained through the linear discriminant analysis effect size (LEfSe; https://huttenhower.sph.harvard.edu/galaxy/34) tool, through the Galaxy platform (version 1.39.5.0 (https://galaxyproject.org/35). Linear discriminant analyses scores were declared significantly different if scores were ≥ 2.0 and P ≤ 0.0536.
The interaction between bacterial and archaeal genera, and metabolites in rumen fluid were analysed by a Spearman’s rank correlation using the “psych” package on R software (v. 4.0.221;37), and visualised using a heat map generated by the packages “ggcorrplot”38 and “corrplot” (v. 0.9039). Pathways from functional analyses derived from differentially expressed rumen microbes were similarly visualised.
Ethics approval and consent to participate
Rumen fluid and liver samples used in this study were obtained from lambs in a companion study under the approval of The University of Queensland Animal Ethics Committee SAFS/035/19.
Results
Rumen fermentation
In vitro fermentation characteristics, gas and CH4 production
In vitro gas production (mL/g DM) was similar between control and carrot diets (P = 0.28; Table 1) despite an 8.8% increase (P < 0.01) in in vitro dry matter digestibility (IVDMD) in carrot compared to control diet. Methane, when expressed as CH4 mg per g DM incubated was 13.8% lower for the carrot diet (P = 0.01) and tended (P = 0.10) to be lower when expressed as a percentage of total gas, compared to the control.
Rumen pH, VFA and predicted CH4 yield from lambs
The pH of rumen fluid collected immediately post-slaughter was higher (P < 0.01; Table 1) for carrot-fed lambs. There was no effect (P ≥ 0.13) of diet on the total concentration of VFA, or individual percentage of acetate, butyrate, caproate acids, or the A:P in rumen fluid. As a proportion of total VFA, branch-chained volatile fatty acids (BCVFA) were twofold greater (P < 0.01) and valerate tended (P = 0.09) to be greater with the carrot diet, whereas, propionate was greater (P = 0.04) in rumen fluid of control lambs. Further, predicted CH4 yield (mol/100 mol TVFA) decreased (P = 0.01) by 3.2% in carrot-fed lambs, compared to control-fed lambs.
Rumen archaeal and bacterial community structure and diversity
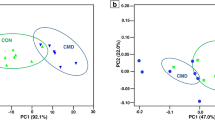
A total of 34 samples of rumen fluid and digesta were sequenced, resulting in 139,642 non-chimeric sequences after quality control and the identification of 2383 unique ASVs from all samples. Rarefaction analysis showed that sequencing depth was sufficient to capture most of the microbial diversity in the samples with rarefaction curves for all samples approaching an asymptote. Richness, based on the Chao1 index, and other alpha diversity metrics were greater (P < 0.01; Fig. 1A; Supplementary Fig. 1) for rumen bacteria from lambs fed the carrot diet. Based on the Bray–Curtis dissimilarity matrix, the carrot and control bacterial communities displayed distinct separation (P ≤ 0.01; PERMANOVA) from one another (Fig. 1B).
Measures of (A) Chao1 and Inverse Simpson alpha diversity and (B) Bray Curtis dissimilarities, and the Log2 fold change difference between significantly different (FDR P-value < 0.05) (C) phyla and (D) genera in the rumen fluid of control- (red) and carrot-fed (blue) lambs. Bray Curtis dissimilarities indicate separation (P = 0.01; PERMANOVA) of the rumen microbial community structure, where each dot represents one lamb. Significant differences are indicated by asterisks ***(P ≤ 0.001) and ****(P ≤ 0.0001). Positive fold change indicates greater abundance of the microbe in the rumen microbial community of carrot-fed lambs, whereas a negative fold change represents greater abundance of the microbe in control-fed lambs.
The three most dominant bacterial phyla comprised Bacteroidetes (63.0 ± 3.1%), Firmicutes (22.7 ± 2.3%) and Synergistetes (3.3 ± 1.3%) for carrot-fed lamb rumen microbial communities, and Bacteroidetes (51.9 ± 4.3%), Firmicutes (22.3 ± 3.7%) and Proteobacteria (14.9 ± 4.2%) for the control-fed lambs, respectively (Supplementary Table 1). However, Synergistetes was 4.26 ± 0.78 log2 fold higher (FDR-adjusted P ≤ 0.04) with the carrot diet, while Actinobacteria and Proteobacteria were greater with the control diet (FDR-adjusted P ≤ 0.01; Fig. 1C). The archaeal phylum, Euryarchaeota did not differ between treatments (FDR-adjusted P = 0.35; Supplementary Table 1).
Prevotella was the most dominant genus in the rumen of both carrot (42.0 ± 2.64%) and control (42.9 ± 3.49%) fed lambs and was unaffected by diet (FDR-adjusted P = 0.79; Supplementary Table 1). In the rumen bacterial community of carrot-fed lambs, an unclassified genus within the order Bacteroidales (15.9 ± 1.59%) and an unclassified genus of the Veillonellaceae family (9.11 ± 1.42) were second and third most dominant genera (FDR-adjusted P ≤ 0.01). However, in the rumen bacterial community of lambs fed the control diet, the second and third dominant genera included an unclassified genus of the Succinivibrionaceae family (11.1 ± 3.85%) and an unclassified genus of the order Bacteroidales (4.89 ± 0.95; adjusted P ≤ 0.01). Further, the relative abundance of unclassified Succinivibrionaceae was 72-fold lower (6.18 ± 0.97 log2 fold change; Fig. 1D) in the rumen bacterial community of lambs fed the carrot diet compared to the control lambs, while the relative abundance of the unclassified Bacteroidales was 2.8-fold higher (1.50 ± 0.47; Fig. 1D).
Blood plasma, rumen and liver metabolome profile
Overall, 170 metabolites were detected in the blood plasma, 355 metabolites were detected in rumen fluid and 212 metabolites were detected in the liver. Univariate analyses of metabolite data and subsequent determination of statistically different metabolites by t-test resulted in the identification of 14 differentially expressed metabolites (P ≤ 0.05; Fig. 2A) in the rumen fluid, and 27 differentially expressed metabolites in the liver (P ≤ 0.05; Fig. 3A). Conversely, there was no influence of diet on the plasma metabolomics profile (FDR-adjusted P > 0.05; Supplementary Fig. 2).
(A) Fold change differences between significantly different (FDR P-value < 0.05) rumen fluid metabolites. GC–MS identified fourteen diet-specific metabolites were detected and reflected by log fold change as determined by t-test (P < 0.05). Positive fold change indicates a greater proportion of the metabolite in the rumen fluid of carrot-fed (blue) lambs, while negative fold change represents a higher proportion in the rumen fluid of control-fed (red) lambs. Error bars were not included as data were normalised by median and log transformed. (B) Principal component analysis of metabolites that are significantly different by t-test (P < 0.05), where each data point represents one lamb. (C) Rumen fluid pathway enrichment analysis on MetaboAnalyst 5.0 associated with the Bos taurus KEGG pathway database for the influence of control or carrot diet on the rumen fluid metabolic profile. Pathways with an impact value ≥ 0.1 and P-value ≤ 0.05 are considered biologically relevant. Circle size indicates the magnitude of the pathway impact value, defined as the cumulative value of significant metabolites. Pathways of the greatest magnitude were labelled A–K, while colour is indicative of P-value, where red = P < 0.05 and ranges to white = P > 0.05 (Supplementary Table S2).
(A) Volcano plot of differentially expressed (FDR adjusted P ≥ 0.05) liver metabolites from lambs fed the carrot (red; positive log2 FC) and control (blue; negative log2 FC) diet. Twenty-seven liver metabolites were influenced by diet and reflected by log2 fold change as determined by t-test (P < 0.05). (B) Separation of liver metabolic profiles via principal component analysis for liver samples from control (red) and carrot-fed (blue) lambs. (C) Pathway analysis of liver metabolites enriched via MetaboAnalyst 5.0 associated with the Bos taurus KEGG pathways. Pathways (A–C) with an impact value ≥ 0.1 and P-value ≤ 0.05 are considered biologically relevant, and tendencies (0.05 ≥ P ≥ 0.10; (D–F)) were illustrated. Circle size indicates the magnitude of the pathway impact value, defined as the cumulative value of significant metabolites. Colour is indicative of P-value, where red = P < 0.05 and ranges to white = P > 0.05 (Supplementary Table S3).
Of the differentially expressed metabolites in rumen fluid, nine were more prevalent in the rumen fluid of lambs fed the carrot diet, compared to five with the control diet (Fig. 2A). There was clear clustering of the carrot samples from the control samples along the second principal component with the PCA analysis of the differentially expressed metabolites, explaining 12.5% of the variance between samples (Fig. 2B). An orthogonal partial least squares discriminant analysis (OPLS-DA), conducted on the total rumen fluid metabolites validated the diet-associated separation of metabolites by n = 100 permutations, which resulted in a P < 0.01 (Q2 = 0.76; R2 = 0.98; Supplementary Fig. 3A,B).
Pathway analyses using MetaboAnalyst 5.0 and the KEGG Bos taurus database (impact value ≥ 0.1; P ≤ 0.05; Fig. 2C) were conducted to identify the dietary influence on the involvement of these metabolites in metabolic pathways. Tyrosine metabolism (dopamine, acetoacetic acid and pyruvate; impact value = 0.13; P = 0.01; Fig. 2C; Supplementary Table 2) was more prevalent in rumen fluid from carrot-fed lambs. Moreover, the synthesis and degradation of ketone bodies (acetoacetic acid) tended (impact value = 0.6; P = 0.06) to be more prevalent in the rumen fluid of carrot-fed lambs. Contrastingly, starch and sucrose metabolism (trehalose and glucose) were the only metabolic pathway of biological relevance in the rumen fluid of control-fed lambs (impact value = 0.42; P ≤ 0.02).
Feeding the carrot diet resulted in the differential expression of twenty-seven liver metabolites between lambs fed the carrot and control diet. Interestingly, 4-hydroxybenzoic acid (FC = 0.62; FDR-adjusted P < 0.01; Fig. 3A) was the only liver metabolite influenced by the carrot diet, compared to 26 metabolites with the control diet (FDR-adjusted P ≤ 0.04). The differentially expressed liver metabolites were not strongly differentiated by the first or second principal component in a PCA (Fig. 3B).
In the enrichment analysis (Fig. 3C), 4-hydroxybenzoic acid was not included in any of the identified metabolic pathways. However, the fructose and mannose metabolism pathway (impact value = 0.22; P < 0.01; Supplementary Table 3) included sorbitol, mannose-6-phosphate and fructose-1-phosphate metabolites which had higher expression in the liver of lambs fed the control diet, as did glycerol and glyceric acid (glycerolipid metabolism pathway; impact value = 0.33; P = 0.03) and xylitol and xylulose (pentose and glucuronate interconversions pathway; impact value = 0.30; P = 0.03).
Relationship between the microbial community, metabolic profile and functional pathways in rumen fluid
Spearman’s correlations between all differentially expressed microbial genera and rumen fluid metabolites in the rumen fluid of carrot or control-fed lambs determined 9 significant correlations in rumen fluid collected from control-fed lambs (P ≤ 0.05; Fig. 4A). Conversely, 45 significant correlations were determined between metabolites, and 1 between the microbial community and rumen fluid metabolites from carrot-fed lambs (P ≤ 0.05; Fig. 4B). An unclassified taxon within the family Succinivibrionaceae was negatively correlated (R2 = − 0.51; P = 0.04) with Selenomonas in the rumen fluid of control-fed lambs, while Bifidobacterium was negatively correlated with Succinivibrio (R2 = − 0.66; P < 0.01). However, all other significant correlations occurred between rumen metabolites in control-fed lambs (R2 ≥ 0.58; P ≤ 0.04).
Spearman’s correlation of differentially expressed bacterial genera and metabolites detected in rumen fluid from lambs fed the (A) control or (B) carrot diet. Colour gradient of squares denotes a strong positive (red), strong negative (purple) or no (white) correlation, while “X” indicates a significant correlation (P ≥ 0.05).
Across genera that were differentially expressed with the carrot diet, an unclassified genus within the order Bacteroidales was negatively correlated with 3-methyl-2-oxovaleric acid (R2 ≥ − 0.49; P = 0.04; Fig. 4B), while no other bacterial genera were significantly correlated with one another or metabolites analysed (P ≥ 0.05). However, with the exception of palmitoleic acid, all rumen metabolites were positively correlated with one another (R2 ≥ 0.63; P ≤ 0.04).
Among significant pathways (LDA log score ≥ 3.62; Fig. 5A), methane, arginine and proline metabolism, valine, leucine and isoleucine, PPAR signalling pathway and carbohydrate metabolism were upregulated in the carrot diet determined through microbial function prediction. Conversely, dioxin degradation, signal transduction mechanisms, protein folding and associated processing and the phosphotransferase system were functional pathways upregulated in the control diet compared to the carrot diet (LDA score log score ≥ 3.70). The phosphotransferase system was negatively correlated (R2 = − 0.75; P < 0.001; Fig. 5B) with ASVs within Bacteroidales, while Selenomonas, Bifidobacterium and Lactobacillus were positively correlated (R2 ≥ 0.56; P ≤ 0.04).
(A) Linear discriminant analysis of the control and carrot diet. LDA scores ≥ 2 indicate the metabolic pathways that may best explain differences observed between diet treatments and (B) Correlation matrix of rumen bacterial and archaeal genera and metabolic pathways identified through CowPI and linear discriminant analysis that were positively (blue) or negatively (red) correlated (− 1 ≤ R2 ≤ 1). Asterisks (*) indicate level of significance, where ***P ≤ 0.001; **P ≤ 0.01 and *P ≤ 0.05.
Moreover, Methanobrevibacter was more abundant with the carrot diet. Branch chained amino acid (valine, leucine and isoleucine) degradation (R2 = 0.61; P ≤ 0.01), as well as arginine and proline metabolism (R2 = 0.56; P ≤ 0.001) pathways were positively correlated with Methanobrevibacter. Further, unclassified family S24-7 was significantly correlated with valine, leucine and isoleucine degradation (P ≤ 0.01), corresponding with a negative correlation between 3-methyl-2-oxovaleric acid and Bacteroidales (Fig. 5B).
Discussion
To our knowledge, this is the first study to utilise comprehensive, targeted metabolomics approach via GC–MS to evaluate the contribution of the rumen microbiota and metabolic profiles to improvements in rumen function and provide a possible explanation to the superior lamb performance12 observed by feeding an unsalable carrot TMR. However, while this study profiled the rumen microbial, plasma and liver metabolites, ruminal tissue metabolites were not profiled. Therefore, functional prediction analyses were conducted utilising the CowPI platform for rumen microbes to predict rumen-associated metabolic pathways40, providing information which may explain the noted superior lamb performance.
The rumen microbial environment comprises a core microbiome, and a consortium of microbes that are readily modified by diet26, seen through the dissimilarity of carrot and control-fed microbial communities in this study. Prior reports have also indicated that diet influences the rumen metabolome, as a grain-based TMR and pasture feeding have shifted their microbial and metabolic profiles, respectively41,42. The replacement of barley grain with unsalable carrots at 45% DM resulted in a greater Bray–Curtis distance and greater α-diversity among the rumen archaeal and bacterial consortia, while consequently modifying the rumen metabolic profile.
The predominant phyla detected irrespective of diet in this study were Bacteroidetes and Firmicutes. In addition, the relative abundance of an unclassified genus within the order Bacteroidales was increased by 1.50 ± 0.47 log2 fold in the rumen fluid of carrot-fed lambs, compared to the control. Knowledge on the taxonomy, function and metabolism of this genus remains uncertain. Although, recent reports have indicated that Bacteroides thetaiotaomicron, a member of Bacteroidales, utilises ribose (d-ribose)43 as a source of carbon and energy for growth. Ribose, found within the lipo- or polysaccharide components of bacterial cell walls44 was greater in the rumen fluid of carrot fed lambs, was negatively correlated with Bacteroidales, and has similarly been detected in hay-concentrate diets45. Therefore, it is possible that the unclassified Bacteroidales utilised ribose as a substrate for cellular maintenance given its greater relative abundance in the microbial community of carrot fed lambs. Bacteroidales may have contributed to feed efficiency, as it was up to 39% higher in the microbial communities of bulls with greater feed efficiency46,47, while similarly increasing by 25% in lambs fed the carrot diet12.
Through the CowPI workflow, a negative correlation (R2 = − 0.75; P < 0.001) was found between the order Bacteroidales and the phosphotransferase system. This is consistent with findings by Ref.48, as some species within the order Bacteroidales do not possess a phosphotransferase system for mannose utilisation. Complementing this, high-level functional interpretation of biological samples inferred a negatively correlated (R2 ≥ − 0.49; P = 0.04) relationship between Bacteroidales and 3-methyl-2-oxovaleric acid, a metabolite of isoleucine produced during branch chain amino acid degradation49 that wasdetected in the rumen fluid of carrot-fed lambs.
In addition, unclassified Bacteroidales were negatively correlated with 4-hydroxybenzoic acid, which may indicate a possible route of utilisation, due to the presence of 4-hydroxybenzoate carboxylase in some facultative anaerobic bacteria50. Consequently, this was also the only metabolite in the liver metabolome of carrot-fed lambs which differed to control-fed lambs. It is possible that greater 4-hydroxybenzoic acid in the liver metabolome of carrot-fed lambs was the result of passive exchange of metabolites between the rumen epithelia and portal blood streams, as previously observed with metabolites such as propionate51. Consequently, 4-hydroxybenzoic acid may have been rapidly hydrolysed into benzoic acid and metabolised in the lamb liver, similar to the metabolism of parabens in the human liver52. Further, carrot cell walls are naturally rich in 4-hydroxybenzoic acid53,54 which accumulates in its esterified form through direct contact of carrots with soil minerals prior to harvest55,56. In this study, carrots were chopped prior to feeding, thereby damaging the carrot cell wall such that accumulation of 4-hydroxybenzoic acid may have occurred in the rumen of carrot-fed lambs. This may provide a possible explanation as to the differential expression of 4-hydroxybenzoic acid in the rumen fluid, and subsequent accumulation in the liver.
The presence of 4-hydroxybenzoic acid in the rumen environment, has previously reduced cellulolytic activity of fibrolytic Fibrobacter succinogenes by up to 38%, while promoting growth of Ruminococcus species57,58,59. Further, Jung et al.60 noted that 4-hydroxybenzoic acid content in lucerne hay increased with lignification by up to 46% between bud and full bloom. This corresponded with a decrease in rumen digestibility, thus accumulation of 4-hydroxybenzoic acid in the rumen of cannulated sheep. Although, a higher abundance of Ruminococcus in the rumen fluid of control-fed lambs in this study was possibly associated with the greater proportion of lucerne hay, thus DM and NDF content in the control compared to the carrot diet12. Instead, fibre within the carrot diet could have undergone degradation via Fibrobacter or by anaerobic fungi and protozoa61,62, which were not characterised in this study. However, our findings demonstrated that the substitution of barley grain for carrot may have coincided with a change in the source of rapidly degradable substrates available to rumen microbes.
In this study, a 20.5% increase in the molar proportion of propionate in the control diet stem from the use of steam-flaked barley grain—up to 78% of which is rumen degradable63. This increases available succinate for conversion into propionate by rumen bacterial genera including Succinivibrio and Roseburia64,65, which were up to 4.13 log fold more abundant in the rumen fluid of control-fed lambs. Starch degradation pathways are utilised by amylolytic rumen bacteria66 such as Succinivibrio, which had a strong positive correlation with propionate when feeding a starch-rich, grain-based diet67. Although, Hatew et al.68 ascertained that the type of grain posed a greater influence on the molar proportion of propionate than starch content. Barley grain is a conventional concentrate rich in starch which rapidly degrades to glucose in the rumen69, and was most prevalent in the control diet.
Amylolytic taxa within the Succinivibrionaceae family (11.1 ± 3.85% RA), Lactobacillus (2.2 ± 0.90%) and Streptococcus (2.3 ± 1.57%) were differentially expressed in the rumen fluid of control-fed lambs, aligning with prior reports detailing the increased abundance of these genera when a grain diet was fed to cattle70,71. Further, functional analysis determined that the carbohydrate phosphotransferase system, associated with the transport of carbohydrates such as mannose was more prevalent in the rumen fluid of control-fed lambs and is a method of carbohydrate uptake utilised by taxa including Selenomonas, Bifidobacterium and Lactobacillus48. Supporting these findings, Spearman correlation of Bifidobacteria and unclassified Succinivibrionaceae with trehalose suggests possible utilisation and production, respectively, as both genera are involved in starch and sucrose metabolism in the rumen of sheep and cattle42,71.
Moreover, the unclassified Succinivibrionaceae was also positively correlated with Selenomonas in rumen fluid from control-fed lambs, which typically decarboxylates succinate, produced by Succinivibrionaceae into propionate by Selenomonas71. Selenomonas taxa present in the rumen are associated with the production of acetate, propionate and lactate72, while lactate is used as a growth substrate in others73,74. Several results in this study, including the proportion of molar propionate or the reduction in rumen pH for control-fed lambs could have been the result of differential expression of Selenomonas. As the rumen pH declines, the proportion of lactic-acid producing bacteria increases, while the proportion of lactic-acid utilising bacteria simultaneously decreases75. However, taxa within the Sharpea genus are minor lactic acid-producing bacteria76 that were differentially expressed in the control diet. These genera possibly contributed to the lower observed in vivo rumen pH, as they have previously been detected in cattle under acidotic challenge when fed high-grain diets77.
Generally, a grain-based diet high in starch will inhibit methanogen activity, thus reducing CH4 emissions by mode of an increase in molar propionate and a decrease in rumen pH. Interestingly, fermentation via in vitro batch culture of the carrot diet resulted in a reduction of CH4 concentration by 13.8% on an mg/g DM basis, despite there being a numerically greater (P = 0.11) proportion of Methanobrevibacter in the rumen fluid of carrot-fed lamb. A reduction in CH4 production was not associated with an effect of diet on gas production nor the percentage of CH4. However, Tapio et al.78 proposed that the composition, rather than the abundance of methanogenic archaea influences the intensity of CH4 emissions, with Methanobrevibacter ruminantium and Methanobrevibacter gottschalkii having been noted as more abundant in ruminants with low CH4 emissions and high CH4 emissions, respectively79. Prior reports have also indicated that Methanosphaera was negatively correlated (R2 = − 0.47; P < 0.02) with CH4 production, and tended to be 4.48 log2 fold more abundant in the rumen fluid of carrot-fed lambs80. Hydrogenotrophic species within Methanobrevibacter may metabolise amino and branched chain amino acids into organic nitrogen in order to proliferate in the rumen environment81,82. This could also be due to the mutualistic relationship of Methanobrevibacter with rumen bacteria83, promoting the oxidation of substrates for use by proteolytic bacteria such as Prevotella84 by utilising free H2. However, with the carrot diet, predicted in vivo CH4 yield was numerically lower than the control by 13.1% indicating that the carrot diet may affect fermentation by rumen microbes and resultant CH4 yield.
Dietary intervention85,86 is a CH4 mitigation strategy that involves the reduction of ruminal CH4 through dietary unsaturated fatty acids, linoleic (n6-18:2; LA), α-linolenic (n6-18:3; ALA) and oleic acids (c9-18:1) as terminal acceptors of H287,88,89. Unclassified ASVs within the order Bacteroidales were linked via MiCA terminal restriction fragments to conjugated linoleic acid as well as saturated fatty acids t11-18:1 and 18:0, indicating a possible role in ruminal biohydrogenation90. Fatty acid biohydrogenation in the rumen is primarily driven by members of Butyrivibrio91,92. However, biohydrogenating taxa isomerise cis-12 bonds and hydrogenated double bonds in conjugated LA to produce stearic acid (18:0)93. A negative correlation between the unclassified Bacteroidales taxa and 18:0, Dewanckele et al.91 suggests a similar capacity for biohydrogenation, as the carrot diet contained 46.4% and 10.6% more total mono- and poly-unsaturated fatty acid substrate compared to the control diet, respectively12. This could possibly explain the decrease in in vitro CH4 production using the carrot diet as fermentation substrate, but future studies should ascertain the specific role of the unclassified members within Bacteroidales to better understand CH4 mitigation via microbial biohydrogenation.
The phylum Synergistetes include anaerobic bacteria that are unable to metabolise sugars, thus obtain energy via the fermentation of aromatic amino acids94,95. The genus TG5, of the Synergistetes phylum was more abundant in the rumen fluid of carrot-fed lambs, which coincided with the differential expression of acetoacetic acid, a product of ketogenic or glucogenic amino acid catabolism96. Connected to tyrosine metabolism via pathway analysis, acetoacetic acid was detected in the rumen fluid of carrot-fed lambs and is a product of tyrosine degradation97, resulting in a ketone body that completes the tyrosine degradation pathway via its influence on fumarylacetoacetate hydrolase98. Similarly, 3-methyl-2-oxovaleric acid, a product of isoleucine metabolism99 was also greater in the rumen fluid of carrot-fed lambs and was negatively correlated with the unclassified Bacteroidales. This suggests a possible route of utilisation for Bacteroidales, as the taxa does not secrete 3-methyl-2-oxovaleric acid and has previously been associated with branched chain amino acid metabolism100,101.
Conclusions
Substituting barley grain for unsalable carrots at 45% DM increased rumen pH by 8.5%, improved in vitro diet digestibility by 8.8% and reduced in vitro CH4 by 13.8% on an mg/g DM basis. The rumen fluid of carrot-fed lambs diverged from control-fed lambs as indicated by a greater Bray–Curtis distance, confirming our hypothesis. Further, a diet-associated shift in the microbiome-metabolome relationship could be seen with the detection of 14 treatment-specific rumen fluid metabolites, and the sole liver metabolite, 4-hydroxybenzoic acid being greater in carrot-fed lambs. Moreover, our results provide evidence toward the possible roles of unclassified taxa within Bacteroidales in improving feed efficiency and fatty acid biohydrogenation, and a potential role of TG5 amino acid metabolism. Such relationships between metabolites that are utilised or produced by the rumen microbiome may explain previously observed improvements in feed efficiency and animal performance. Therefore, replacing barley grain with unsalable carrots at 45% DM is an effective means of eliciting animal performance improvements beyond that of conventional feedlot rations by shifting the rumen microbial and metabolic profiles.
Data availability
All 16S rRNA gene sequences were submitted to the NCBI Sequence Read Archive under BioProject accession number PRJNA772293.
References
Huws, S. A. et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol. 9, 2161 (2018).
Saleem, F. et al. The bovine ruminal fluid metabolome. Metabolomics 9(2), 360–378. https://doi.org/10.1007/s11306-012-0458-9 (2013).
Clish, C. B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 1(1), a000588 (2015).
Mao, S. Y., Huo, W. J. & Zhu, W. Y. Microbiome–metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 18(2), 525–541 (2016).
Wang, D. S., Zhang, R. Y., Zhu, W. Y. & Mao, S. Y. Effects of subacute ruminal acidosis challenges on fermentation and biogenic amines in the rumen of dairy cows. Livest. Sci. 155(2), 262–272. https://doi.org/10.1016/j.livsci.2013.05.026 (2013).
Connolly, S. et al. Relationship of the blood metabolome to subsequent carcass traits at slaughter in feedlot Wagyu crossbred steers. Sci. Rep. 9(1), 1–11 (2019).
Kozloski, G. V., Rocha, J. B. T. D. & Ciocca, M. D. L. S. Visceral metabolism and efficiency of energy use by ruminants. Ciência Rural 31, 909–915 (2001).
Reutter, B., Lant, P. A. & Lane, J. L. The challenge of characterising food waste at a national level—An Australian example. Environ. Sci. Policy 78, 157–166. https://doi.org/10.1016/j.envsci.2017.09.014 (2017).
Das, N. G., Huque, K. S., Amanullah, S. M. & Makkar, H. P. S. Feeding of processed vegetable wastes to bulls and its potential environmental benefit. Anim. Nutr. 5(1), 87–94. https://doi.org/10.1016/j.aninu.2018.04.002 (2019).
Fredrickson, E. L., Estell, R. E., Havstad, K. M., Shupe, W. L. & Murray, L. W. Potential toxicity and feed value of onions for sheep. Livest. Prod. Sci. 42, 45–54. https://doi.org/10.1016/0301-6226(94)00066-g (1995).
Fiems, L. O., De Boever, J. L., Vanacker, J. M. & De Brabander, D. L. Effect of cull potatoes in the diet for finishing Belgian Blue double-muscled cows. Animal 7(1), 93–100 (2013).
Forwood, D. L. et al. Feeding unsaleable carrots to lambs increased performance and carcass characteristics while maintaining meat quality. Meat Sci. 173, 108402 (2020).
Chantaro, P., Devahastin, S. & Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT Food Sci. Technol. 41(10), 1987–1994 (2008).
Asadollahi, S., Ponnampalam, E., Sari, M. & Erfanimajd, N. Effects of sugar beet pulp and roasted canola seed, on performance, rumen and small intestine digesta volatile fatty acid concentrations, and small intestine morphology of Arabian lambs. Livest. Sci. 216, 130–137 (2018).
Santos, A. et al. Liver transcriptomic and plasma metabolomic profiles of fattening lambs are modified by feed restriction during the suckling period1. J. Anim. Sci. 96(4), 1495–1507. https://doi.org/10.1093/jas/sky029 (2018).
Playne, M. J. Determination of ethanol, volatile fatty acids, lactic and succinic acids in fermentation liquids by gas chromatography. J. Sci. Food Agric. 36(8), 638–644 (1985).
Forwood, D. L. et al. Crop sorghum ensiled with unsalable vegetables increases silage microbial diversity. Front. Microbiol. 10, 2599 (2019).
Williams, S. R. O., Hannah, M., Jacobs, J. L., Wales, W. J. & Moate, P. J. Volatile fatty acids in ruminal fluid can be used to predict methane yield of dairy cows. Animal 9(12), 1006 (2019).
Popova, M., Martin, C. & Morgavi, D. P. Improved protocol for high-quality co-extraction of DNA and RNA from rumen digesta. Folia Microbiol. 55(4), 368–372. https://doi.org/10.1007/s12223-010-0060-3 (2010).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13(7), 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
RC Team: R: A language and environment for statistical computing. In R Foundation for Statistical Computing (2021).
RS Team. RStudio: Integrated Development Environment for R (PBC, 2020).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72(7), 5069–5072 (2006).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4), e61217 (2013).
Zheng, P. et al. Plasma metabolomics analysis based on GC-MS in infertile males with Kidney-Yang deficiency syndrome. J. Evid. Based Complementary Altern. Med. 2017, 1–11 (2017).
Henderson, G. et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 5(1), 14567. https://doi.org/10.1038/srep14567 (2015).
Meale, S., Chaves, A., Baah, J. & McAllister, T. Methane production of different forages in in vitro ruminal fermentation. Asian Australas J. Anim. Sci. 25(1), 86 (2012).
Menke, K. et al. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 93(1), 217–222 (1979).
Fedorah, P. M. & Hrudey, S. E. A simple apparatus for measuring gas production by methanogenic cultures in serum bottles. Environ. Technol. 4(10), 425–432 (1983).
Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.5–6. https://cran.r-project.org/web/packages/vegan/ (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30 (2000).
Blasco, H. et al. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 53, 291–299 (2015).
Wilkinson, T. J. et al. CowPI: A rumen microbiome focussed version of the PICRUSt functional inference software. Front. Microbiol. 9, 1095 (2018).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. https://doi.org/10.1186/gb-2011-12-6-r60 (2011).
Jalili, V. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 48, W395–W402. https://doi.org/10.1093/nar/gkaa434 (2020).
Li, R. et al. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 10, 1–14. https://doi.org/10.1038/s41598-020-74958-1 (2020).
Revelle, W. Using the Psych Package to Generate and Test Structural Models. http://bioconductor.statistik.tu-dortmund.de/cran/web/packages/psych/vignettes/psych_for_sem.pdf (2017).
Kassambara, A. Kassambara MAJRpv. Package ‘ggcorrplot’. https://cran.r-project.org/web/packages/ggcorrplot/readme/README.html (2019).
Wei, T. et al. Package ‘corrplot’. Am. Stat. 56(316), e24 (2017).
Amin, N. et al. Host metabolome and faecal microbiome shows potential interactions impacted by age and weaning times in calves. Anim. Microbiome 5(1), 1–9 (2023).
Ametaj, B. N. et al. Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows. Metabolomics 6(4), 583–594. https://doi.org/10.1007/s11306-010-0227-6 (2010).
O’Callaghan, A. & van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 7, 925. https://doi.org/10.3389/fmicb.2016.00925 (2016).
Glowacki, R. W. et al. A ribose-scavenging system confers colonization fitness on the human gut symbiont bacteroides thetaiotaomicron in a diet-specific manner. Cell Host Microbe 27(1), 79–92 (2020).
Sasajima, K.-I. & Yoneda, M. Production of pentoses by micro-organisms. Biotechnol. Bioeng. 2(1), 175–213 (1984).
Martinez-Fernandez, G. et al. Methane inhibition alters the microbial community, hydrogen flow, and fermentation response in the rumen of cattle. Front. Microbiol. 7, 1122 (2016).
McCann, J. C., Wiley, L. M., Forbes, T. D., Rouquette, F. M. Jr. & Tedeschi, L. O. Relationship between the rumen microbiome and residual feed intake-efficiency of Brahman bulls stocked on bermudagrass pastures. PLoS ONE 9(3), e91864 (2014).
Koch, R. M., Swiger, L. A., Chambers, D. & Gregory, K. E. Efficiency of feed use in beef cattle. J. Anim. Sci. 22(2), 486–494 (1963).
Coyne, M. J. et al. A family of anti-bacteroidales peptide toxins wide-spread in the human gut microbiota. Nat. Commun. 10(1), 1–14 (2019).
Matano, L. M., Coyne, M. J., García-Bayona, L. & Comstock, L. E. Bacteroidetocins target the essential outer membrane protein BamA of Bacteroidales symbionts and pathogens. Mbio 12(5), e02285 (2021).
Yoshida, T. & Nagasawa, T. Chapter 5—Biological Kolbe–Schmitt carboxylation: Possible use of enzymes for the direct carboxylation of organic substrates. In Future Directions in Biocatalysis (ed. Matsuda, T.) 83–105 (Elsevier, 2007).
Storm, A. C., Kristensen, N. B. & Hanigan, M. D. A model of ruminal volatile fatty acid absorption kinetics and rumen epithelial blood flow in lactating Holstein cows. J. Dairy Sci. 95(6), 2919–2934. https://doi.org/10.3168/jds.2011-4239 (2012).
Abbas, S. et al. Metabolism of parabens (4-hydroxybenzoic acid esters) by hepatic esterases and UDP-glucuronosyltransferases in man. Drug Metab. 25, 1009280069 (2010).
Ahmad, T. et al. Phytochemicals in Daucus carota and their health benefits—Review article. Foods 8(9), 424. https://doi.org/10.3390/foods8090424 (2019).
Parr, A. J., Ng, A. & Waldron, K. W. Ester-linked phenolic components of carrot cell walls. J. Agric. Food Chem. 45(7), 2468–2471. https://doi.org/10.1021/jf960982k (1997).
Kang, Y.-H., Parker, C. C., Smith, A. C. & Waldron, K. W. Characterization and distribution of phenolics in carrot cell walls. J. Agric. Food Chem. 56(18), 8558–8564 (2008).
Seitz, H., Bach, M., Richter, S., Schnitzler, J.-P. & Steimle, D. Elicitor-induced changes in the phenol metabolism of suspension-cultured carrot cells. In International Symposium on Natural Phenols in Plant Resistance, Vol. 381, 113-120 (1993).
Borneman, W. S., Akin, D. & Van Eseltine, W. Effect of phenolic monomers on ruminal bacteria. Appl. Environ. Microbiol. 52(6), 1331–1339 (1986).
Chesson, A., Stewart, C. S. & Wallace, R. J. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl. Environ. Microbiol. 44(3), 597–603. https://doi.org/10.1128/aem.44.3.597-603.1982 (1982).
Hu, Y. et al. The effect of a diet based on rice straw co-fermented with probiotics and enzymes versus a fresh corn Stover-based diet on the rumen bacterial community and metabolites of beef cattle. Science 10(1), 1–16 (2020).
Jung, H.-J.G., Fahey, G. C. & Merchen, N. R. Effects of ruminant digestion and metabolism on phenolic monomers of forages. Br. J. Nutr. 50(3), 637–651 (1983).
Gijzen, H. J., Lubberding, H. J., Gerhardus, M. J. & Vogels, G. D. Contribution of rumen protozoa to fibre degradation and cellulase activity in vitro. FEMS Microbiol. Lett. 4(1), 35–43 (1988).
Windham, W. & Akin, D. E. Rumen fungi and forage fiber degradation. Appl. Environ. Microbiol. 48(3), 473–476 (1984).
Huntington, G. B. Starch utilization by ruminants: From basics to the bunk. J. Anim. Sci. 75(3), 852–867 (1997).
Hart, E., Creevey, C., Hitch, T. & Kingston-Smith, A. Meta-proteomics of rumen microbiota indicates niche compartmentalisation and functional dominance in a limited number of metabolic pathways between abundant bacteria. Science 8(1), 1–11 (2018).
Ren, Q. et al. Bacterial communities in the solid, liquid, dorsal, and ventral epithelium fractions of yak (Bos grunniens) rumen. MicrobiologyOpen 9(2), e963 (2020).
Cotta, M. A. Amylolytic activity of selected species of ruminal bacteria. Appl. Environ. Microbiol. 54(3), 772–776 (1988).
Iqbal, M. W. et al. Ruminal fermentation and microbial community differently influenced by four typical subtropical forages in vitro. Anim. Nutr. 4(1), 100–108. https://doi.org/10.1016/j.aninu.2017.10.005 (2018).
Hatew, B. et al. Effects of dietary starch content and rate of fermentation on methane production in lactating dairy cows. J. Dairy Sci. 98(1), 486–499 (2015).
Nikkhah, A. Barley grain for ruminants: A global treasure or tragedy. J. Anim. Sci. Biotechnol. 3(1), 1–9 (2012).
Cotta, M. A. Interaction of ruminal bacteria in the production and utilization of maltooligosaccharides from starch. Appl. Environ. Microbiol. 58(1), 48–54 (1992).
Hespell, R. B. The Genera Succinivibrio and Succinimonas. In The Prokaryotes (ed. Balows, A.) 3979–3982 (Springer, 1992).
Bryant, M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J. Bacteriol. 72(2), 162 (1956).
Gilmour, M., Flint, H. J. & Mitchell, W. J. Multiple lactate dehydrogenase activities of the rumen bacterium Selenomonas ruminantium. Microbiology 140(8), 2077–2084 (1994).
Reichardt, N. et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8(6), 1323–1335 (2014).
Mills, J. et al. A dynamic mechanistic model of lactic acid metabolism in the rumen. J. Dairy Sci. 97(4), 2398–2414 (2014).
Kumar, S. et al. Sharpea and Kandleria are lactic acid producing rumen bacteria that do not change their fermentation products when co-cultured with a methanogen. Anaerobe 54, 31–38 (2018).
Petri, R. et al. Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl. Environ. Microbiol. 79(12), 3744–3755 (2013).
Tapio, I., Snelling, T. J., Strozzi, F. & Wallace, R. J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 8(1), 1–11 (2017).
Danielsson, R. et al. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 8, 226 (2017).
Kittelmann, S. et al. Two different bacterial community types are linked with the low-methane emission trait in sheep. PLoS ONE 9(7), e103171 (2014).
Bharathi, M. & Chellapandi, P. Comparative analysis of differential proteome-wide protein–protein interaction network of Methanobrevibacter ruminantium M1. Biochem. Biophys. Rep. 20, 100698 (2019).
Savant, D. V., Shouche, Y. S., Prakash, S. & Ranade, D. R. Methanobrevibacter acididurans sp. Nov., a novel methanogen from a sour anaerobic digester. Int. J. Syst. Evol. Microbiol. 52(4), 1081–1087 (2002).
Patra, A., Park, T., Kim, M. & Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 8(1), 1–18 (2017).
Liu, C. et al. Role of age-related shifts in rumen bacteria and methanogens in methane production in cattle. Front. Microbiol. 8, 1563 (2017).
Kobayashi, Y. Abatement of methane production from ruminants: Trends in the manipulation of rumen fermentation. Asian Australas J. Anim. Sci. 23(3), 410–416. https://doi.org/10.5713/ajas.2010.r.01 (2010).
McAllister, T. A. & Newbold, C. J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 48(2), 7. https://doi.org/10.1071/ea07218 (2008).
Fotouhi, N. & Jenkins, T. C. Ruminal biohydrogenation of linoleoyl methionine and calcium linoleate in sheep. J. Anim. Sci. 70(11), 3607–3614 (1992).
McAllister, T., Cheng, K.-J., Okine, E. & Mathison, G. W. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 76(2), 231–243 (1996).
Polan, C., McNeill, J. & Tove, S. B. Biohydrogenation of unsaturated fatty acids by rumen bacteria. Am. Soc. Microbiol. 88(4), 1056–1064 (1964).
Huws, S. A. et al. As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ. Microbiol. 13(6), 1500–1512 (2011).
Dewanckele, L. et al. Rumen biohydrogenation and microbial community changes upon early life supplementation of 22: 6n–3 enriched microalgae to goats. Front. Microbiol. 9, 573 (2018).
Li, D., Wang, J. Q. & Bu, D. P. Ruminal microbe of biohydrogenation of trans-vaccenic acid to stearic acid in vitro. BMC Res. Notes 5, 97. https://doi.org/10.1186/1756-0500-5-97 (2012).
Jenkins, T., Wallace, R., Moate, P. & Mosley, E. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 86(2), 397–412 (2008).
Jumas-Bilak, E. & Marchandin, H. The phylum Synergistetes. In The Prokaryotes (eds Rosenberg, E. et al.) (Springer, 2014).
Leong, L. E., Denman, S. E., Hugenholtz, P. & McSweeney, C. S. Amino acid and peptide utilization profiles of the fluoroacetate-degrading bacterium synergistetes strain MFA1 under varying conditions. Microbes 71(2), 494–504 (2016).
Litwack, G. Chapter 13—Metabolism of amino acids. In Human Biochemistry (ed. Litwack, G.) 359–394 (Academic Press, 2018).
Stančíková, M. & Rovenský, J. Metabolism of aromatic amino acids. In Alkaptonuria and Ochronosis (eds Rovenský, J. et al.) 9–12 (Springer, 2015).
Carmelo, V. A. O., Banerjee, P., da Silva Diniz, W. J. & Kadarmideen, H. N. J. S. Metabolomic networks and pathways associated with feed efficiency and related-traits in Duroc and Landrace pigs. Science 10(1), 1–14 (2020).
Gómez, E. et al. Metabolomic profiling of Bos taurus beef, dairy, and crossbred cattle: A between-breeds meta-analysis. J. Agric. Food Chem. 68(32), 8732–8743 (2020).
Ogunade, I., Schweickart, H., McCoun, M., Cannon, K. & McManus, C. Integrating 16S rRNA sequencing and LC–MS-based metabolomics to evaluate the effects of live yeast on rumen function in beef cattle. Animals 9(1), 28 (2019).
Henson, M. A. Interrogation of the perturbed gut microbiota in gouty arthritis patients through in silico metabolic modeling. Eng. Life Sci. 21(7), 489–501 (2021).
Acknowledgements
The authors also acknowledge Génome Québec and the Queensland node of Metabolomics Australia (MA) at The University of Queensland, an NCRIS initiative under Bioplatforms Australia Pty Ltd for assistance with sequencing and analysis of metabolites respectively.
Funding
The authors would like to thank Meat & Livestock Australia for their financial support of Daniel Forwood. This research was also funded by The University of Queensland Early Career Researcher Grant scheme.
Author information
Authors and Affiliations
Contributions
D.L.F., A.V.C. and S.J.M. conceived and designed research. D.L.F., M.C.P., T.S. and S.J.M. conducted the experiment. D.L.F., A.V.C. and S.J.M. analysed the data. D.L.F. and A.V.C. conducted statistical analysis. D.L.F., D.J.I. and D.P.d.S. conducted bioinformatics. D.L.F. wrote the original manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forwood, D.L., Innes, D.J., Parra, M.C. et al. Feeding an unsalable carrot total-mixed ration altered bacterial amino acid degradation in the rumen of lambs. Sci Rep 13, 6942 (2023). https://doi.org/10.1038/s41598-023-34181-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34181-0
- Springer Nature Limited