Abstract
Heavy metal concentrations in the different tissues of marine turtles are presented; the most frequently monitored elements are mercury, cadmium, and lead. Concentrations of Hg, Cd, Pb, and As in different organs and tissues (liver, kidney, muscle tissue, fat tissue, and blood) of loggerhead turtle Caretta caretta from the southeastern Mediterranean Sea were determined using Atomic Absorption Spectrophotometer, Shimadzu and mercury vapor unite (MVu 1A) for Hg measurements. The highest levels of cadmium and arsenic were found in the kidney (Cd: 61.17 µg g−1; As: 0.051 µg g−1 dry weight). For lead, the highest level was found in muscle tissue (35.80 µg g−1). Mercury tended to be higher in the liver than in other tissues and organs (0.253 µg g−1 dry weight) which showed a higher accumulation of this element. Fat tissue generally displays the lowest trace element burdens. The concentrations of As remained low in all the considered tissues, possibly the result of low trophic levels in sea turtles. In contrast, the diet of loggerhead turtles would result in significant exposure to Pb. This is the first study into metal accumulation in the tissues of a loggerhead turtle from the Egyptian Mediterranean coastline.
Similar content being viewed by others
Introduction
Several studies have been carried out concerning metal accumulation in sea turtles from different areas of the world; studies in the southeastern Mediterranean Sea are limited. The loggerhead sea turtle is suggested to be considered ‘‘endangered’’ in the Mediterranean Sea. A world-wide, reduction of marine turtle populations has been attributed to different factors such as coastal pollution, due to the production of waste and pollutants generated by different human activities (industrial and domestic), which makes places unsuitable for nesting1, accidental capture and the use of eggs and meat as food. Recent reports showed, that sea turtles are affected by marine pollutants, such as debris, tar balls2,3 and toxic chemicals, such as heavy metals4,5. Among the wide range of toxic chemicals, trace metals including both essential and non-essential elements, have a particular significance in ecotoxicology. According to their chemical characteristics (e.g., lipophilia), heavy metals tend to bioaccumulate and biodegrade differently in tissues6 and, unlike organic pollutants such as pesticides, are not biodegradable, since heavy metals are highly persistent and all have the potential to be toxic to living organisms. Moreover, it has been observed that the increase in such contamination could adversely interfere with fertilization and the success of hatching7,8,9,10.
The knowledge of trace metal burden in marine turtles is, therefore, an important focal point to assess the potential impact of these compounds on these endangered organisms. Therefore, the study of toxic metals in C. caretta is fundamental to evaluating their potentially toxic effects and estimating the type and the degree of exposure. In the same context, systemic toxic effects, such as teratogenicity, cytotoxicity, and genotoxicity, can cause multiple damages to the reproductive, immune, and nervous systems, and behaviors, and may cause carcinogenesis. However, the database in terms of such contaminants available for the Mediterranean turtle population is very limited4,11; though the importance of monitoring trace metal burden in marine turtles in an effort to conserve their population is clear12. Because of their solitary lifestyle, duration of the pelagic phase and, long-lasting apneas, sea turtles are in fact among the most difficult marine animals to assess13. Two of the seven species of sea turtles are known to reproduce regularly in the Mediterranean Sea, the loggerhead turtle, Caretta caretta, and the green turtle, Chelonia mydas14.
Turtles feed primarily on sessile or slowly moving benthic prey15 although they do capture organisms throughout the water column16. The loggerhead turtle is currently classified as ‘‘endangered’’ by the UCN (International Union for the Conservation of Nature and Natural Resources).
Small populations of Caretta caretta and C. mydas nesting on the northern Sinai Peninsula are currently under intense pressure from human activities. The fact that 10 nests exhibited signs of human pilfering of eggs is particularly disturbing considering that only 21 egg depositions could be confirmed for the entire season. The combined effects of the capture of adults at sea (caught by trawling and bottom longline fishing), predation of eggs, coastal pollution and development of beaches, threaten to exclude nesting turtles from the Mediterranean coastline of Egypt within the next decade. An intense public awareness campaign and a conservation program of nest protection and/or re-location are required immediately if this population is to survive1.
The primary objective of this study was assess specific metal accumulation in various tissues and organs of loggerhead turtles from the southeastern Mediterranean Sea measuring the concentrations of the potentially toxic elements, Cd, Pb, Hg and As, and to compare our data with those reported from other locations, and improve the knowledge of these contaminants and their potential impact on this important species. Correlations between the elements were determined in order to investigate physiological disorders and the hazard that these contaminants may pose to loggerhead turtles’ survival was discussed. On the other hand, by using the concentrations of trace metals in the tissues of these animals, we can learn about their locations in the ocean and their transmission.
Materials and methods
Sample collection
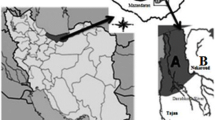
Loggerhead turtles (n = 8) from Alexandria coast, Egypt in June 2019 and August 2019 were caught by fishermen without permission for commercial use near Abu Qir Bay (Fig. 1). Standard length and width were measured. The means of length, width were 75.6 cm (range: 70.6–85.8 cm) and 51.6 cm (46.2–55.3 cm) respectively. Kidney, liver, muscle, fat and blood were collected and were kept at − 4 °C until analysis. Blood samples were taken from the live animals.
Preparation of samples for metal analyses
To avoid contamination, all reagents were handled carefully, polyethylene disposables were thoroughly washed with HNO3 2 N under a fume hood and disposable gloves were worn during the procedure. All of the reagents used were Suprapur (Merck) and the water was bidistilled and deionized (Mili-Q). Different tissues was digested using a high-temperature digestion with a mixture of nitric, perchloric, and sulphuric acids (8:8:1), following the method described by García-Fernández et al.17. The Teflon tubes used (100 mm) for wet digestion were previously washed with 2% nitric acid for 48 h and then rinsed twice with Deionized double distilled water (DDDW) and dried in an oven at 50 °C. Approximately 1.5 g of wet tissue of each sample was dried in an oven at 80 °C until a constant dry weight was obtained. A weight of 0.5 g of these dry tissues was placed in the Teflon digestion tube, to which 5 ml of the acid mixture was added. Samples were digested with a thermostatic apparatus (microwave) equipped for controlling temperatures from 10 to 250 °C and an aluminum-heating block. Thermal digestion was performed by raising the temperature from 100 to 200 °C at a rate of 5 °C/min, followed by digestion at 200 °C for 2 h, and a further temperature increase to 250 °C at a rate of 20 °C/5 min; this temperature was maintained until complete dryness was obtained.
After collection, all blood samples were stored at 4 °C and were analyzed within 2 days. Two hours before sample preparation, the blood samples were brought to room temperature. They were mixed gently for homogenization and 500 µL of the blood sample were diluted with 100µL 0.1% (V/V) Triton-X-100 solution and 500 µL of the internal standard solution. This solution was filled up to 5 mL with a 0.5% (V/V) NH4OH solution in a 10 mL autosampler polypropylene tube using a 5 mL bottle-top dispenser. Finally, 5 mL of conc. HNO3 was added at each sample in a 50-mL closed PTFE tube and heated at 120 ± 1 °C for 1 h for the decomposition of the organic structure. The closed tubes were cooled and opened when the internal temperature of the high-pressure bomb was under 30 °C. The digests were quantitatively transferred to 25-mL volumetric flasks and diluted to volume with de-ionized water.
Analysis of metals
The digested sample was allowed to cool; finally, Deionized double distilled water was added to the resulting samples to bring their volume to 25 mL and they were analyzed with AAS (AA-6800 Atomic Absorption Spectrophotometer, Shimadzu) for Cd, Pb, As and Hg. Concentrations of (As) were measured using Hydride Vapor Generation for As and concentrations of (Hg) were measured by mercury vapor unite (MVu 1A—Mercury vaporizer unite) only used for Hg. Results were expressed as microgram of element per dry weight gram of different tissues, detection limit was estimated in ≤ 0.05 ppp wet weight for Hg, 0.01, 0.02 and 0.02 ppm wet weight for Cd, Pb and As respectively.
Accuracy of this analysis was examined using standard reference materials SRM Dorm-2 (National Research Council Canada). Recoveries of all the elements ranged from 88 to 109%.
Statistical analysis
One way analysis of variance (ANOVA) and Duncan's test (p = 0.05) were used to access whether trace elements concentrations varied significantly. Spearman correlation between heavy metals within the tissues of the loggerheads turtles was determined. All statistical calculations were performed with SPSS 9.0 for windows. A p value of less than 0.05 was considered to indicate statistical significance.
Ethics approval
This is an observational study. The Research Ethics Committee has confirmed that no ethical approval is required.”
Consent to participate
Informed consent was obtained from all individual participants included in the study. (it is single author Maha Ahmed Mohamed Abdallah only).
Results and discussion
Metals in sea turtle tissues and comparison with literature data
Concentrations of Cd, Pb, As and Hg (µg/g dry weight) in muscle, liver kidney, fat and blood of loggerhead turtles are presented in Table 1.
Cadmium
Cadmium concentrations in muscles of loggerhead turtles were slightly higher (0.47 ± 0.09 µg/g) than most of the levels in specimens from the Mediterranean Sea17,18,19,20, Table 2, but lower than that from Italian Adriatic coasts21,22 and from North Tunisian Coasts recorded by Sami et al.23. A number of hypotheses might explain the variability in Cd load among loggerhead turtles from the different areas. Firstly, the environmental contamination that influences in the food chain of these organisms and secondly, the age of the specimens sampled. Cadmium is known to accumulate in marine vertebrate with age. The age is, in fact, known to be one of factors which control concentration of Cd in marine vertebrates24,25 i.e. the change in diet during the different stages of animal growth.
Cd concentrations in liver (6.45 ± 3.95 µg/g) were similar to those detected in blood (6.645 ± 2.55 µg/g) (p > 0.05, Fig. 2), (Table 2) showed that the specimens here analyzed had hepatic levels of Cd lower than those reported for loggerhead turtles in other parts of the Mediterranean Sea10,17,18,23, except for animals from eastern Mediterranean Sea19, which showed comparable levels while animals from western Mediterranean20,22 showed low levels. Thus, the high hepatic of Cd in the organisms in question seems to indicate a recent exposure to a contamination source.
On the other hand, the highest mean concentration of Cd was measured in kidney of loggerhead turtles (Cdkidney/Cdliver = 6.59). This provides evidence of a tendency for large scale accumulation of Cd in kidney tissue. The continuous sorption of small quantities of Cd, which is typical of chronic exposure, gives rise to a prevalence of levels in the kidney over those in the liver20, on account of the large period of time that this metal stays in the kidney due to poor elimination. A comparison with literature data (Table 2) showed that the specimens here analyzed had renal levels of Cd higher than those reported for loggerhead turtles in other parts of the Mediterranean Sea. Meanwhile, the level of Cd to some extent is similar to the levels in specimens from the western and southwestern Mediterranean Sea17,18, and lower than that recorded in West Italy and North Tunisian Coasts by18,23. However, independently from the many factors influencing Cd accumulation in marine turtles, the observed pattern of metal distribution between liver and kidney does not match well with what generally encountered in marine turtles, where kidney exhibits the highest Cd concentrations18,19,26. Studies on fresh water turtles by Rie et al.27 reveal that, liver is the primary site of Cd accumulation upon short-term exposure, whereas during long-term exposure, Cd is redistributed from liver, via the blood, to kidney where it is absorbed and concentrated, this may explain the high level of Cd in the blood in the present study. Accordingly, our results strongly agreed with27 in particular because our search was on the adult animals. Furthermore, the continuous sorption of small quantities of Cd which is typical of chronic exposure, gives rise to a prevalence of levels in the kidney over those in the liver, on account of the large period of time that this metal stays in the kidney due to poor elimination. Low accumulation of Cd (0.391 ± 0.09 µg/g) was observed in fat (Fig. 2). Few data are available as to the levels of this metal in turtle’s fat. As reported in in this study, cadmium tends to bioaccumulated more in kidneys and liver than in muscles and in fat. In addition, it is estimated that, in case of chronic exposure, the renal concentration of cadmium (poorly eliminated) would be higher than that of the liver. Concerning toxic effects, a cadmium chloride exposure in kidneys were high enough to adversely affect the health of sea turtles such as C. caretta.
It is difficult to put forward any detailed hypothesis which could explain these differences between contamination levels in loggerhead turtles from different locations. Marine environment quality differences between the locations are one possibility, leading to an unequal intake of Cd via the trophic route. Alternatively we should also consider the variation in the capacity to accumulate Cd which may be related to age.
Lead
In the present study, we found that the concentration of lead in the muscles of Caretta caretta is approximately two times higher than that in the liver and kidneys, and about 5 times higher than that in fat and blood. The mean for Pb was five and 11.5 times, respectively, higher in fat and muscle than that of Cd (Table 1, Fig. 2). Generally, Pb exhibited high mean concentration in all studied tissues. Storelli et al.19 were of the opinion that lead concentrations below 0.5 µg/g (w.w.) should be considered low. In the present study, 100% of all studied tissues analyzed (n = 8) showed Pb concentrations much higher than this level. Comparison of lead concentrations between our study and previous data regarding loggerhead turtles sampled in the Adriatic Sea and different areas of the Mediterranean Sea (Table 2) reveals a higher contamination degree in animals analyzed here. Lead is a multi-targeted toxicant, and inorganic lead is carcinogenic in animals28,29. In all loggerhead turtle tissues reported from different authors, in Table 2, Pb levels were of the same order of magnitude, except those reported by17,20 for Liver and kidney respectively. Lam et al.30 reveal that, the muscle of adults from South China had relatively higher Pb concentrations than the other samples.
According to these authors, this drop was mainly due to the reduction of leaded petrol in many European countries since the 1970s19. Confirmation of31 that the low concentrations of Pb in animals tissues confirms the decrease in Pb pollution in the Mediterranean, due to the limitation of its use as an additive in gasoline. In the present study, lead concentrations in all tissues were much higher than those described by Storelli et al. 19; however, given that we have no data prior to the abolishment of leaded petrol in Egypt, we cannot know if the same tendency towards a decrease has also taken place.
Mercury
Mercury concentrations in all tissues and organs of the specimens analyzed were, to some extent, similar to the levels in loggerhead turtles from Mediterranean Sea. Sea turtles, in comparison with marine mammals32,33 , which, similar to marine reptiles, are at the top of food chains, contained low mercury concentrations, particularly in fat. The low body burden of mercury in these high trophic level organisms could be mainly attributed to the nature of their diet.
However, the lowest mercury levels were found in the fat tissue (0.04 ± 0.01 µg/g, dry wt) in agreement with what was seen by Sakai et al.34. This finding is surprising if one considers the lipophilic nature of methyl mercury, organic form of mercury primarily accumulated by marine organisms. Nevertheless, a suitable explanation for low levels of mercury in fat tissue may be provided by the large chemical affinity of methyl mercury for the—SH group of some proteins that, resulting in the loss of lipophilicity of the compound, makes the distribution of methyl mercury in tissues not more affected by their lipid content35. This ensures with the present study, where the relatively high concentrations of Hg was found in the tissues of liver (1.16 ± 0.76), kidney (0.88 ± 0.34) and muscle (0.47 ± 0.09) (Fig. 2).
The stability of Hg in the different tissues (as muscles, kidney, fat and liver) makes this tissue preferable for approximating long-term exposure, while blood Hg levels can be affected by recent changes in Hg intake. A large range in blood Hg concentrations (1.44–4.75 µg/ml) was found among species (Table 1), with concentrations rising for all species. This suggests that there is an elevation of bioavailable Hg in near shore habitats where terrestrial influences and anthropogenic impacts are high. The variability in environmental Hg may explain the high intra specific variability and occasional highly contaminated turtle seen in this and previous studies in the eastern Mediterranean Sea.
Arsenic
The average levels of As found in our study are much lower than those, from Mediterranean Sea, reported in specimens from the east coast of Italy by Storelli et al.36 and4, and also from the east coast of Spain by Andreani et al.22 with regards to liver, kidney and muscle tissue. We observed the same pattern of distribution among the tissues as the latter (blood > muscle > liver > kidney > fat) (Fig. 2). In this pattern, the relatively high concentration of As in muscle tissue may indicate a specific metabolic mechanism of this element. The fluctuation in the results suggests differences between the base levels of As contamination in the different areas studied or in the feeding habits of the specimens34.
Some marine animals, such as molluscs and crustaceans, which are a part of the loggerhead sea turtle’s diet, may retain concentrations of this element37. In the case of As, one must take into account that only a small percentage of this metal (2–10%) is present in inorganic form and thus potentially toxic for organisms38.
The differences in concentration profiles in various tissues indicate sources of contamination related to diet and geographical area. In this context, the Mediterranean Sea can be considered a contamination hotspot as it is a semi-closed basin surrounded by 22 countries whose human activities and heavy maritime traffic lead to high pollution.
Correlation between elements in the different tissues and organs
The correlation between the elements in the different tissues is shown in Table 3. In blood, arsenic was positively correlated with Pb (p < 0.001). In muscle tissue a positive correlation between As and Pb (p < 0.001) and between Cd and As (p < 0.05) and a negative correlation between Hg and both Cd and As (p < 0.05) were found to exist.
The concentrations of Cd and both Pb and As were correlated in kidney tissue (p < 0.05), while a negative correlation between Hg and As (p < 0.001) was observed. In fat tissue a positive correlation between Cd and Pb and between Hg and As (p < 0.001) and between As and Pb (p < 0.05) was detected. This last correlation was also observed in liver tissue between As and Hg (p < 0.001). Finally, a statistically significant negative correlation between Pb in liver and kidney (p < 0.001), and also in fat and muscles, a statistically significant positive correlation between Cd in muscle and liver tissue (p < 0.001) exist.
Chronic exposure should give evidence of a parallel accumulation of As and both of Cd and Pb in tissues (Table 3), although we can observe a negative correlation between Hg and As in kidney and muscular tissue. This may be due to the lack of older specimens, whose tissue concentrations of As and Hg would allow us to observe a positive correlation between these two elements due to chronic exposure. On the other hand, the positive correlation between all elements in liver and fat may be indicative of chronic exposure.
Potential health risk by turtle consumption
Sea turtle tissues are commonly used as food items in many communities worldwide, despite national regulations prohibiting such consumption. Although regulations prohibit the capture and consumption of sea turtles in Egypt, there are many people in coastal cities accept the consumption of sea turtle meat and also drink their blood. Blood-drinking was carried out mainly by women, mostly in the belief that it increases fertility. Thus, it is interesting and necessary to evaluate the quality of turtle’s meat as food. Levels of Pb found in muscle tissues from this study are above the established food safety guidelines from several nations (0.3–2.0 µg/g wet weight)39,40,41. Besides, the results reveal that Cd and Hg has the same manner of Pb i.e. they are above the established food safety guidelines from several nations (0.05 and 0.5 µg/g wet weight respectively). Consequently, from these results it is difficult to anticipate a health risk to indigenous communities that consume turtle meat. On the other hand, the serious damage comes from the continued drinking of sea turtles blood of some peoples, where the highest concentrations of toxic metals (in the present study) were concentrated in the turtle’s blood.
Alexandria, Egypt's fish market is one area where sea turtles are killed for meat or international trade. However, sea turtle conservationists and the Egyptian government are trying to monitor and secure sea turtle populations over the entire coast of Egypt. This will not be done only in the case of building a partnership with the fishermen, so is of crucial importance to provide a guideline for the planning process, decentralize the decision-making process, provide better communication with the fishermen, and enhance collaborative relationships with other local actors. At the same time helping to increase awareness, and motivating them to contribute in innovative ways that are characteristically of a social and economic nature, all of this may lead to quit with this habit.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to [after acceptance by the journal, it can be publicly available only] but are available from the corresponding author on reasonable request.
References
Clarke, M., Campbell, A. C., Hameid, W. S. & Ghoneim, S. Preliminary report on the status of marine turtle nesting populations on the Mediterranean coast of Egypt. Biol. Cons. 94, 363–371. https://doi.org/10.1016/S0006-3207(00)00005-7 (2000).
Tomas, J., Guitar, R., Mateo, R. & Raga, J. A. Marine debris ingestion in loggerhead sea turtles, Caretta caretta, from Western Mediterranean. Mar. Pollut. Bull. 44, 211–216. https://doi.org/10.1016/s0025-326x(01)00236-3 (2002).
Gavrilescu, M., Demnerová, K., Aamand, J., Agathos, S. & Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 32, 147–156. https://doi.org/10.1016/j.nbt.2014.01.001 (2015).
Storelli, M. M. & Marcotrigiano, G. O. Chlorobiphenyls, HCB, and organochlorine pesticides in some tissues of Caretta caretta (Linnaeus) specimens beached along the Adriatic Sea, Italy. Bull. Environ. Contam. Toxicol. 64, 481–488. https://doi.org/10.1007/s001280000029 (2000).
Corsolini, S., Aurigi, S. & Focardi, S. Presence of polychlobiphenyls (PCBs) and coplanar congeners in the tissues of the Mediterranean loggerhead turtle Caretta caretta. Mar. Pollut. Bull. 40, 952–960 (2000).
Novillo, O., Pertusa, J. & Tomás, J. Exploring the presence of pollutants at sea: Monitoring heavy metals and pesticides in loggerhead turtles (Caretta caretta) from the western Mediterranean. Sci. Total Environ. 598, 1130–1139. https://doi.org/10.1016/j.scitotenv.2017.04.090 (2017).
Sinaei, M. & Bolouki, M. Metals in blood and eggs of green sea turtles (Chelonia mydas) from nesting colonies of the northern coast of the Sea of Oman. Arch. Environ. Contam. Toxicol. 73, 552–561. https://doi.org/10.1007/s00244-017-0421-x (2017).
Abdallah, M. A. M. Trace element levels in some commercially valuable fish species from coastal waters of Mediterranean Sea Egypt. J. Mar. Syst. 73(1–2), 114–122. https://doi.org/10.1016/j.jmarsys.2007.09.006 (2008).
Abdallah, M. A. M. Bioaccumulation of heavy metals in mollusca species and assessment of potential risks to human health mollusca. Bull. Environ. Contam. Toxicol. 90, 552–557. https://doi.org/10.1007/s00128-013-0959-x (2013).
Dario, S. et al. Impact of heavy metals in eggs and tissues of C. caretta along the sicilian coast (Mediterranean Sea). Environments 9, 88. https://doi.org/10.3390/environments9070088 (2022).
Storelli, M. M. & Marcotrigiano, G. O. Heavy metal residues in tissues of marine turtles. Mar. Pollut. Bull. 46, 397–400. https://doi.org/10.1016/S0025-326X(02)00230-8 (2003).
Sakai, H. et al. Species-specific distribution of heavy metals in tissues and organs of loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) from Japanese coastal waters. Mar. Pollut. Bull. 40, 701–709 (2000).
Witherington, B. E. Biological conservation of loggerheads: challenges and opportunities. In Loggerhead Sea Turtles (eds Bolten, A. B. & Witherington, B. E.) 295–312 (Smithsonian Institution, 2003).
Groombridge, B. Marine turtles in the Mediterranean: distribution, population status, conservation. Conservation. Nature and Environmental Series, 48. Council of Europe Environment Conservation and Management Division, Strasbourg (1990).
Bentivegna, F., Ciampa, M., Mazza, G., Paglialonga, A., Travaglini, A. Loggerhead turtle (Caretta caretta) in Tyrrhenian Sea: Trophic role of the Gulf of Naples. In: Proceedings of the 1st Mediterranean Conference on Marine Turtles, (Rome, Italy, 2001).
Bolten, A. Active swimmers-passive drifters: the Oceanic juvenile stage of loggerheads in the Atlantic system. In The Loggerhead Turtles (eds Bolten, A. & Wintherington, B. E.) 63–78 (Smithsonian Institution, 2003).
García-Fernández, A. J., Gómez-Ramírez, P., Martínez-López, E., Hernández-Garcíaet, A., María-Mojica, P., Romero, D., Jiménez, P., Castillo, J. J., Bellido, J. J. Heavy metals in tissues from loggerhead turtles (Caretta caretta) from the southwestern Mediterranean (Spain). Echotoxicology and Environmental Safety 72: 557–563. https://doi.org/10.1016/j.ecoenv.2008.05.003 (2009)
Maffucci, F., Caurant, F., Bustamante, P. & Bentivegna, F. Trace elements (Cd, Cu, Hg, Se, Zn) accumulation and tissue distribution in loggerhead turtles (Caretta caretta) from Western Mediterranean Sea (southern Italy). Chemosphere 58, 535–542. https://doi.org/10.1016/j.chemosphere.2004.09.032 (2005).
Storelli, M. M. et al. Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: Overview and evaluation. Environ. Pollut. 135, 163–170. https://doi.org/10.1016/j.envpol.2004.09.005 (2005).
Jerez, S., Motas, M., Cánovas, R. Á., Talavera, J., Ramon Miguel Almela, R. M., Bayón del Río, A. Accumulation and tissue distribution of heavy metals and essential elements in loggerhead turtles (Caretta caretta) from Spanish Mediterranean coastline of Murcia. Chemosphere 78, 256–264. https://doi.org/10.1016/j.chemosphere.2009.10.062 (2010).
Franzellitti, S., Locatelli, C., Gerosa, G., Vallini, C. & Fabbri, E. Heavy metals in tissues of loggerhead turtles (Caretta caretta) from northwestern Adriatic Sea. Compd. Biochem. Physiol. Part C 138, 187–194. https://doi.org/10.1016/j.cca.2004.07.008 (2004).
Andreani, G. et al. Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci. Total Environ. 390, 287–294. https://doi.org/10.1016/j.scitotenv.2007.09.014 (2008).
Sami, K., Hedia Attia, E. H., Nadia, M. & Chouba, L. Distribution of trace metals (Cd, Hg, Pb, Cu) and polycyclic aromatic page 2 of 6 hydrocarbons (PAH) in loggerhead turtles (Reptilia: Testudines: Cheloniidae: Caretta Caretta (Linnaeus, 1758)) tissues stranded along the north Tunisian coasts. Int. J. Mar. Biol. Res. 3(3), 1–6. https://doi.org/10.1007/s11356-021-12499-4 (2018).
Stewart, F. M., Thompson, D. R., Furness, R. W. & Harrison, N. Seasonal variation in heavy metals in tissues of common guillemots, Uria algae, from northwest Scotland. Arch. Environ. Contam Toxicol. 27, 168–175. https://doi.org/10.1007/BF00214259 (1994).
Dietz, R., Riget, F. & Johansen, P. Lead, cadmium, mercury and selenium in Greenland marine animals. Sci. Total Environ. 186, 67–93. https://doi.org/10.1016/0048-9697(96)05086-3 (1996).
Gardner, S. C., Fitzgerald, S. L., Vargas, B. A. & Rodrيguez, L. M. Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. Biometals 19, 91–99. https://doi.org/10.1007/s10534-005-8660-0 (2006).
Rie, M. T., Lendas, K. A. & Callard, I. P. Cadmium: Tissue distribution and binding protein induction in the painted turtle, Chrysemys picta. Compd. Biochem. Physiol. Part C 130, 41–51. https://doi.org/10.1016/s1532-0456(01)00219-8 (2001).
USEPA. EPA Integrated Risk Information System (IRIS) electronic database. Available from <http://www.epa.gov/iris/> (1996).
ATSDR. Toxicological Profile for Cadmium (Public Health Service, Agency for Toxic Substances and Disease Registry, 1999).
Lam, J. C. W. et al. Trace element residues in tissues of green turtles (Chelonia mydas) from South China waters. Baseline/Mar. Pollut. Bullet. 48, 164–192. https://doi.org/10.1016/j.marpolbul.2003.09.003 (2004).
Esposito, M., De Roma, A., Sansone, D., Capozzo, D., Iaccarino, D., Di Nocera, F., Gallo, P. Non-essential toxic element (Cd, As, Hg and Pb) levels in muscle, liver and kidney of loggerhead sea turtles (Caretta caretta) stranded along the southwestern coasts of Tyrrhenian sea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 231, 108725. https://doi.org/10.1016/j.cbpc.2020.108725 (2020)
Law, R. J. Metals in marine mammals. Environmental contaminants in wildlife. In Society of Environmental Toxicology and Chemistry (SETAC) Special Publications Series (eds Byer, W. N. et al.) 357–376 (Lewis Publishers, 1996).
Storelli, M. M., Ceci, E. & Marcotrigiano, G. O. Comparison of total mercury, methylmercury, and selenium in muscle tissues and in the liver of Stenella coeruleoalba (Meyen) and Caretta caretta (Linnaeus). B Environ. Contam. Toxicol. 61, 541–547. https://doi.org/10.1007/s001289900796 (1998).
Sakai, H., Ichihashi, H., Suganuma, H. & Tatsukawa, R. Heavy-metal monitoring in sea-turtles using eggs. Mar. Pollut. Bull. 30, 347–353. https://doi.org/10.1016/0025-326X(94)00185-C (1995).
Cardellicchio, N., Decataldo, A., Di Leo, A. & Misino, A. Accumulation and tissue distribution of mercury and selenium in striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea (Southern Italy). Environ. Pollut. 116, 265–271. https://doi.org/10.1016/S0269-7491(01)00127-0 (2002).
Storelli, M. M., Ceci, E. & Marcotrigiano, G. O. Distribution of heavy metal residues in some tissues of Caretta caretta (Linnaeus) specimens beached along the Adriatic Sea (Italy). B Environ. Contam. Toxicol. 60, 546–552. https://doi.org/10.1007/s001289900660 (1998).
Broderick, A. C., Godley, B. J. & Hays, G. C. Trophic status drives inter annual variability in nesting numbers of marine turtles. Process. Biol. Sci. R. Soc. 268, 1481–1487 (2001).
Ozretic, B., Ozretic, M. K., Santin, J., Medjugorac, B. & Kras, M. As, Pb, Cd and Hg in benthic animals from the Kvarner, Rijeka Bay region, Yugoslavia. Mar. Pollut. Bull. 21, 595–598 (1990).
Nauen, C.E. Compilation of legal limits for hazardous substances in fish and fishery products. In: Food and Agriculture Organization of the United Nations. 100 (FAO Fisheries Circular No. 764, Roma, 1983).
Day, R. D., Christopher, S. J., Becker, P. R. & Whitaker, D. W. Monitoring mercury in the loggerhead sea turtle, Caretta caretta. Environ. Sci. Technol. 39, 437–446. https://doi.org/10.1021/es049628q (2005).
Canzanella, S. et al. Concentrations of trace elements in tissues of loggerhead turtles (Caretta caretta) from the tyrrhenian and the ionian coastlines (Calabria, Italy). Environ. Sci. Pollut. Res. 28(21), 26545–26557. https://doi.org/10.1007/s11356-021-12499-4 (2021).
Acknowledgements
The author want to thank the National Institute of Oceanography and Fisheries (NIOF) for giving the examples utilized in this exploration which was important for an arrangement of work under title of feasible improvement of fisheries in regions that completely unexploited
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The author declares that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The author contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by [M.A.M.A.]. The first draft of the manuscript was written by [M.A.M.A.]. Also she read and approved the final manuscript. The affirms author that her research does not contain any experiments conducted on humans or animals.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdallah, M.A.M. Bioaccumulation and biomagnifications of toxic metals in tissues of loggerhead turtles (Caretta caretta) from the Mediterranean Sea coast, Egypt. Sci Rep 13, 7995 (2023). https://doi.org/10.1038/s41598-023-33972-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33972-9
- Springer Nature Limited