Abstract
Permeability is a critical parameter of tight reservoir rocks and one of the important parameters for characterizing fluid flow and production from reservoirs. It determines the feasibility of its commercial development. SC-CO2 has been used in shale gas exploitation for efficient fracturing and the added benefit of CO2 geo-storage. And SC-CO2 plays an important role in permeability evolution of shale gas reservoirs. In this paper, Firstly, the permeability characteristics of shale under CO2 injection are discussed. The experimental results show that the relationship between permeability and gas pressure is not a single exponential relationship, but there is an obvious segmentation phenomenon, which is particularly obvious when it is close to the supercritical state, and the overall trend is first decreased and then increased. Subsequently, other specimens were selected for SC-CO2 immersion, and nitrogen was used to calibrate and compare shale permeability before and after treatment to assess changes in shale permeability after SC-CO2 treatment at pressures from 7.5 to 11.5 MPa and X-ray diffraction (XRD) analysis and scanning electron microscopy (SEM) were used to analyze the raw and CO2-treated shale particle sample, respectively. Results indicate the permeability increases significantly after SC-CO2 treated, and permeability growth is a linear function of SC-CO2 pressure. According to (XRD) analysis and (SEM) analysis, SC-CO2 not only can act as a solvent and dissolve carbonate minerals and clay minerals, but also can complex chemical reactions with mineral components in shale, Further dissolution of carbonate minerals and clay minerals, widened gas seepage channels and enhancing the permeability.
Similar content being viewed by others
Introduction
Shale gas is an unconventional resource. Recoverable shale gas in China ranks first in the world, and the reserve has reached 1115 trillion cubic meters(EIA, 2015). Shale reservoirs have the characteristics of low porosity and permeability1,2,3,4. The common technique, hydraulic fracturing achieves great production while the production decreases gradually a few months later. Moreover, it also causes problems, such as pollution, waste of water5,6,7, and because of fracturing and excessive fracturing fluid into the reservoir, after many years may cause formation collapse and earthquake8,9,10. Because of the greater affinity of shale to CO2 rather than CH411, injection of CO2 in shale formation can achieve the storage of CO2 and further enhance shale gas production12.
The average buried depth of shale gas reservoirs in China is about 3000 m13. CO2 is easy to reach supercritical state (critical temperature of 31.1℃ and critical pressure of 7.38 MPa) under the condition of high temperature and high pressure. Over the years, numerous studies have been conducted to investigate the impacts of CO2 on shale. Thickened liquid carbon dioxide fracturing fluid, reflux better, less damage to the reservoir, and low adsorption performance, can more effectively penetrate into the reservoir and thickened liquid carbon dioxide fracturing fluid also prevents leakage of methane in the reservoir14, Adsorption of SC-CO2 in shale specimens caused expansion15, which can break cross links between aromatic nuclei in coal16 and lead to the reduction in permeability17. The interaction with SC-CO2 caused the greater changes in mechanical properties compared with sub-CO218. Due to characteristics of high diffusion, low viscosity and surface tensile19, SC-CO2 is able to alter structural properties of organic matters acting as solvent. For example, the interaction of CO2 with coal induced the formation of new carbon structures20. Changes in surface characteristics have been investigated in various methods such as low-pressure N2 adsorption (LP-N2A), FE-SEM, XRD and FTIR21,22,23. Some researchers performed the simulation to study CS-EGR2,24,25,26, while there are still many unsolved problems due to the weak maturity of the technique.
In summary, the interaction with SC-CO2 is complex, including adsorption, seepage, and extraction, and most studies have been conducted in the macro- or micro- scale rather than multi-scale. Further, the permeability is a key parameter for reservoir estimation and exploitation, but less attention was paid on permeability variation of shale after exposed to SC-CO2 at various pressures. Therefore, in this work, permeability tests were performed using N2 before and after SC-CO2 treatment to assess changes. Additionally, the changes of mineral composition, surface microstructure and elements in shale under supercritical carbon dioxide were studied by X-ray diffraction (XRD) analysis and scanning electron microscopy (SEM).
Materials and experimental methods
Materials
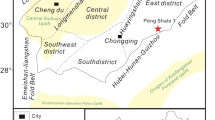
Shale blocks were obtained from an outcrop of Longmaxi Formation in Yanzi Village, Changning County, Yibin City, Sichuan province. The area is located in the west of Hubei and Chongqing, Structurally located in the eastern margin of the Sichuan Basin, according to Chinese system partition of oil and gas reservoirs. The sampling site is under the lower Cambrian single-source and single-source accumulation system in southern Sichuan-southeast Sichuan, It belongs to the shale of Lower Silurian Wufeng Formation-Longmaxi Formation series. Shale specimens and sampling location are shown in Fig. 1.
After using the drilling machine to drill the cut rock sample, place the rock sample on the cap on the double-sided grindstone machine for both ends Polished, then remove residual moisture on the surface of the shale specimen after drilling and cutting, wrap with plastic wrap to keep it moist, finally packing for classification. Specimen parameters are shown in Table 1. A small specimen cut from the same block was prepared for XRD analysis and SEM analysis. Remove the particle sample from the remaining portion of each specimen and crush to a size less than 200 mesh (< 75 μm). Fully mix crushed samples to reduce non-uniformity for XRD analysis and SEM analysis.
Experimental apparatus and methods
Experimental apparatus
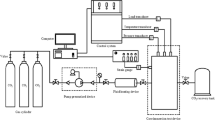
The device diagram is shown in Fig. 2. Mainly divided into three parts, included as pressure control system, temperature control system and main device. Pressure control system included high pressure vessels, air compressor, booster pump, etc. Temperature control system is water bath (the upper limit is 100 ℃ and the accuracy is 0.01 ℃). Main device included core holder (the ultimate pressure is 60 MPa), pressure sensor (with an accuracy of 0.01%, the ultimate pressure is 100 MPa), upstream and downstream chambers, pipes, valves, etc.
Experimental methods
Carbon dioxide permeation experiment
Vacuum and volume correction of experimental device before experiment7,27. Flow behavior of CO2 in shale under injection pressure ranging from 5.5 to 11.5 MPa was measured by pressure pulse permeability experiment method. The experimental program is shown in Table 2.
Carbon Dioxide infiltration experiment
Firstly, N2 permeability tests were performed with the pore pressure from 2 to 8 MPa, and subsequently, specimens were subjected to SC-CO2 with pore pressures of 7.5–11.5 MPa respectively under confining pressure of 12 MPa for 48 h. Finally, N2 was passed through the treated specimen again and permeability changes were estimated in each scheme. The SC-CO2 treatment scheme shown in Table 3.
Figure 3 shows the flowchart of permeability experiment, (a) shows the flowchart of CO2/N2 infiltration experiment, (b) shows the flowchart of Enhance Permeability with SC-CO2.
The experimental conditions for influencing the SC-CO2 treatment on microstructure and mineral constituent of shale were as follows: the treatment time was 48 h with a SC-CO2 pressure of 7.5 MPa, 8.5 MPa, 9.5 MPa, 10.5 MPa, 11.5 MPa.
XRD
The mineral composition of the shale crushed samples before and after SC-CO2 treatment was determined using an x-ray diffractometer. Determination of mineral changes caused by SC-CO2 injection into shale by XRD analysis.
SEM
Surface microstructure characterization of shale crushed samples before and after SC-CO2 treatment using a scanning electron microscope equipped with an energy dispersive X-ray spectrometer (EDS).
Permeability theory
In the mathematical model of traditional pressure pulse permeability experiment28.
The governing equation, boundary conditions and initial conditions are:
p (x,t) in Eq. (1) is the pressure inside the shale sample. p1 and p2 are the upstream and downstream pressure. The pressure (△pD) between the upstream and downstream chambers is expressed as:
The general dimensionless solution can be expressed as28:
θ is the the nth positive root of the Eq. (8).
The later solution can be simplified to28,29
where f0 is constant and s1 is equal to:
f1 is equal to:
Finally, can get the permeability formula as:
If the upstream and downstream volumes are equal, can get28.
Substitute it into the above equation:
where V is the volume of both the upstream and the downstream chambers (m3).
F1 can be expressed as:
ω1 can be obtained by the Eqs. (16)
Ethical statement
I certify that this manuscript is original and has not been published and will not be submitted elsewhere for publication. And the study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time. No data have been fabricated or manipulated (including images) to support your conclusions. No data, text, or theories by others are presented as if they were our own. Te submission has been received explicitly from all co-authors. And authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Results and discussion
CO2 permeability
Figure 4a shows CO2 density changes with increasing fluid pressure for different phases. The red arrows indicate the sharp increase in CO2 density at the phase transition. Figure 4b shows CO2 phase diagram. Figure 4c–e shows CO2 permeability in shale at different confining pressures, 10 MPa, 11 MPa, and 12 MPa. When approaching the supercritical state, there is an obvious segmentation phenomenon, and there is a more obvious trend of sudden drop. The curve of permeability of shale injected with CO2 versus pore pressure is appro -ximately W-shaped. In the early stage, the permeability shows a downward trend with the increase of pore pressure, and the decline begins to slow down when the phase state changes, and after the critical pressure is exceeded, the downward trend began to be obvious again, but the overall trend showed a downward and then upward trend. For example, compared with gaseous CO2, the SC-CO2 stage permeability is reduced by nearly 40%, at the confining pressure of 12 MPa, CO2 permeability decreased from 3.91 × 10−4 μD to 2.45 × 10−4 μD with upstream pressures increasing from 5.5 to 9.5 MPa, while it increases by 1.26 × 10−4 μD with the increase of pressures from 9.5 to 11.5 MPa. The permeability curve can be divided into four stages, a: when the pore pressure is low, permeability decrea -ses gradually, Ranathunga et al.30 thought the reduction of CO2 permeability is a result of matrix swelling. In the present work, we infer the reduction is the result of Klinkenberg effect and swelling. Rather than only the swelling; b: as the pressure of the universe increases, the Klinkenberg effect weakens but expansion still exists, the effect of effective stress is greater than adsorption expansion, the downward trend of permeability slows down; c: corresponding to the CO2 density curve, when approaching the phase change, the density increases sharply and the carbon dioxide adsorption capacity increases, decrease in penetration rate increases, SC-CO2 has the characteristics of high viscosity and density like the liquid, and this may cause a decrease of the flow rate. However, SC-CO2 also has high solubility, the increase of carbon dioxide density will lead to the increase of SC-CO2 solubility and generates carbonic acid with water in shale specimens, it will broaden the pores in shale and increase pore connectivity, with the increase of pore pressures, the density curve tends to be flat when it is close to 9.5 Mpa, at this time the permeability drop of the corresponding seepage curve tends to be flat; d: With the increase of pore pressures, at this time, the effect of effective stress on permeability is dominant, the effective stress reduces and the permeability shows an augment. This confirms that SC-CO2 can significantly change the permeability of shale.
Comparison in N2 permeability before and after shale exposure to SC-CO2
Figure 5a summarizes the original permeability plotted with pore pressures. For the sample T2101, at the confining pressures of 10 MPa, the permeability declines by 0.41 × 10−4 μD with N2 pressure increasing from 2 to 3 MPa, while it increases by 1.33 × 10−4 μD with the increase of N2 pressures from 3 to 8 MPa. The overall trend showed a downward and then upward trend. Figure 5b–f depicts the permeability enhancement after SC-CO2 treated at pressures of 7.5–11.5 MPa. Can be clearly found, the permeability of shale after CO2 treatment is significantly higher than that before treatment. And the relationship between shale permeability and pore pressure is approximately exponential.
Figure 6a shows that differences in the permeability vary with N2 pressures after SC-CO2 treated. The permeability growth, ΔKi, can be calculated based on original permeability, K0, and final permeability, Ki, after exposure to SC-CO2 at pressures, i. After exposed to SC-CO2 at the same pressure, ΔK with injecting 2 MPa N2 is much lower than others, and ΔK with injecting 3 MPa N2 is observed the greatest. We calculate ΔK at various N2 pressures. The relationship between SC-CO2 treatment pressure and differences in permeability and porosity are listed in Table 4. Figure 6b–h shows ΔK is a linear function of SC-CO2 pressures with great determination, indicating that high pressure CO2 has a positive effect on permeability enhancement.
The permeability variation is expressed as:
In the transient pressure pulse test, part of the gas will be adsorbed by the matrix, causing the final equilibrium pressure of the system to drop. Therefore, according to the initial equilibrium pressure and final equilibrium pressure of the system, it can be calculated by Boyle’s law Determine the porosity.
where z can be obtained by Eq. (19)31 Due to the small pulse pressure, the pores of the shale test parts can be regarded as approximately unchanged, which is \(\varphi_{{p_{0} }} = \varphi_{{p_{f} }}\).
where C and D can be obtained by Eq. (20) and Eq. (21)
where \(a = \frac{{0.4572R^{2} T_{c}^{2} }}{{p_{c} }}\), \(b = \frac{{0.0778RT_{c} }}{{p_{c} }}\), R is Gas Universal Constant, Tc is critical temperature, Pc is critical pressure.
The porosity variation is expressed as:
In the Eq. (18), z is the gas compression factor,\(p_{f}\) is the final equilibrium pressure, A is the cross-sectional area of the rock sample, and L is the length of the rock sample. It can be seen that there is only an unknown quantity of Φ, and the porosity can be calculated. Figure 7a–e shows that differences in the porosity vary with N2 pressures after SC-CO2 treated, it can be seen from Fig. 7f that the average growth rate of porosity is proportional to CO2 pressure and to be linear.
The dissolving capability of SC-CO2 for matters in shale is related to the densities32,33,34. When the supercritical fluid pressure rises to the critical pressure, The solubility c* of solid solutes in supercritical fluid increases with the increase of supercritical fluid pressure35, The relationship between the solubility and relative density of the reference solid solute in SC-CO2 is as follows35:
where \( \rho_{r1} = \frac{\rho }{{\rho_{c1} }}\); \( T_{r} = \frac{T}{{T_{c1} }}\)
Darrell, Okamoto Ikuo, Xiaoqiang et al.35,36,37 suggested that Eq. (23) could reflect the relationship between the solubility of solid solute in SC-CO2 and the relative density, where c* is the solubility, ρr,1 is the relative density, ρc,1 is the critical density(467.6 kg/m3), Tr is the relative temperature, Tc,1 is the critical temperature(304.2 K), e0, e1 and e2 are formula parameters, α, β and γ are the molecular mass and enthalpy associated with the supercritical fluid. Figure 8 shows the relationship between the solubility of naphthalene in SC-CO2 and the relative density of SC-CO2 at 313.98 K. The formula parameters are given by Table 5, it can be found that with the increase of SC-CO2 pressure, the solubility of naphthalene in SC-CO2 increased. Through the analysis of Eqs. (23) and Fig. 8, in Fig. 7, SC-CO2 pressure increased from 7.5 to 11.5 MPa, CO2 density increased, caused an increase in the solubility of SC-CO2, increased porosity change rate of shale.
There is a power-law relationship between permeability and porosity, The most commonly used model can be defined as: \(\frac{k}{{k_{0} }} = \left( {\frac{\varphi }{{\varphi_{0} }}} \right)^{3}\)38,39, Take \({\varphi \mathord{\left/ {\vphantom {\varphi {\varphi_{0} }}} \right. \kern-0pt} {\varphi_{0} }}\) as the abscissa and Take \({k \mathord{\left/ {\vphantom {k {k_{0} }}} \right. \kern-0pt} {k_{0} }}\) as the ordinate. The fitting result is shown in Fig. 9, it is found from the fitting results that the value of α is close to 3, but not equal to 3 or 638,39,40, The internal pore distribution and seepage path of shale specimens treated with different pressures of SC-CO2 and the value of α are different, The lower the SC-CO2 pressure, the larger the α value. A larger α corresponds to a higher sensitivity of the permeability to the porosity41.
XRD analysis
Table 6 lists the composition of shale samples before and after CO2 treatment. XDR analysis shows that the mineral composition of shale samples before and after SC-CO2 treatment changes significantly, mainly due to complex chemical reactions between SC-CO2 and mineral components in shale42,43,44,45.
As can be seen from Table 6, as the SC-CO2 pressure increases, the proportion of quartz increases and the content of clay minerals and carbonate minerals decreases. Ao et al. and Lyu et al. found that the change in the proportion of mineral content is mainly due to the reaction of carbon dioxide with water to form carbonic acid and carbonic acid reacts with carbonate minerals in shale, as shown in Eqs. (24)–(26)46,47. In addition, the formation of carbonic acid also reduces the content of clay minerals in shale, as shown in Eqs. (27)–(29)48. Illite can consume hydrogen ions(H+) to form kaolinite and quartz, the newly formed kaolinite and the original kaolinite and original chlorite can continue to consume hydrogen ions to further form quartz49.
In addition, SC-CO2 pressure also affects the degree of dissolution of carbonate and clay minerals in shale50, the solubility of CO2 in water increases with increasing pressure and is more pronounced above the critical state, high solubility will produce high concentration of hydrogen ions to accelerate the reflection of minerals and hydrogen ions(H+), resulting in significant changes in mineral content after SC-CO2 treatment.
SEM analysis
The changes of shale microstructure before and after 11.5 MPa SC-CO2 treatment were observed by SEM. As shown in Fig. 10, the shale surface is heterogeneous, and the original shale fragment samples are significantly different from those treated at 11.5 MPa. After SC-CO2 treatment, many pores were generated on the surface of shale fragment samples, some of the material on the shale surface disappeared (Fig. 10, marked A), and generate some new substances (Fig. 10, marked B), which may be caused by mineral dissolution. Yongdong Jiang et al. found that the average pore size of shale increases after SC-CO2 treatment51. Therefore, the permeability is enhanced.
Pores are defined as micropores (< 2 nm), mesopores (2–50 nm) and macropores (> 50 nm) based on the classification reported by the International Union of Pure and Applied Chemistry (IUPAC)52. Gas adsorption is related to the specific surface area of small pores, and larger mesopore and macropores are significant for the gas diffusion and seepage behaviors34,53,54. Previous literature21,55 reported SC-CO2 exposure can cause the reduction of micropores and mesopores, while the increase of macropores induced can promote the pore connection (Fig. 10, marked a and b) and enhance the permeability.
EDS analysis
Element analysis was performed on eight spots (Fig. 10, marked 1–8) before and after SC-CO2 treated at the pressure of 11.5 MPa, and the comparisons are listed in Table 7, in which minus values indicate the reduction of element portion and positive values show the increase of elemental percentages after SC-CO2 treated. Substances contain mainly C, O, Mg, Al, Ca and Si, indicating the existences of minerals includes kaolinite, montmorillonite, illite, anorthite, and silicate minerals. Significant changes in the portion of elements C, O, Al and Ca are observed. For example, the portion of element C and O increases by 35.76% and 53.39% in spot 3 respectively, and decreases by 45.11% and 10% in spot 6. The reduction of element Al, Si and Ca and the increase of element C reveal the dissolution of SC-CO2 on inorganic matters.
EDS analysis was performed on the whole surface after SC-CO2 treated at pressures of 7.5–11.5 MPa, and results are shown in Table 8. Si and Ca account for the great proportion, and C and Al account for the lowest proportion, it can be found from the proportion of elements, indicating clay minerals are the main components. Few variations in the portion of Si on the whole surface reveal SC-CO2 has few effects on quartz (SiO2). The portion of C increases slightly while portions of O, Ca and Mg decrease gradually with the increase of SC-CO2 pressure. Figure 11 shows the trends of some elements in shale after different SC-CO2 treatments, it can be found that the reduction rate of O, Ca, Mg increases with the increase of SC-CO2 pressure, and the increasing trend is from gentle to increasing. Compared with Fig. 6, it can be seen that the change trend of O, Ca, Mg content with SC-CO2 pressure is consistent with the change trend of SC-CO2 solubility with pressure. The densities change markedly with the increase of pressures and therefore, the dissolving capability enhances. In general, at pressures lower than 10 MPa, SC-CO2 can extract lipophilic, low boiling point components, and especially substances with molecular weights below 30056. Yin et al. observed that carbonate and clay minerals (montmorillonite, kaolinite, and calcite) reduced while quartz increases because of the dissolution of organic matter or other minerals by SC-CO222. The finding of Kutchko et al. indicated that dry SC- CO2 did not alter coal properties to the same extent as with organic solvents57. The interaction with dry SC-CO2 induces physical changes rather than chemical changes. However, when CO2 is dissolved in formation water, a series of chemical reactions take place in a liquid medium, forming carbonic acid43,44,45. Therefore, mineral composition can undergo chemical and physical reactions in such environment.
SC-CO2 treatment time is an important factor. With the increase of treatment time from 1 to 5 days, both the specific surface area and porosity show slightly increasing trends58. Pan et al. performed similar experiments for 30 days and the amount of micropores increased significantly21. However, Kutchko et al.57 cannot see obvious changes in pore areas after SC-CO2 treatment in coal for 104 days possibly because of solubility factor of SC-CO2 exceeding that of coal. Long-term geological storage of CO2 has been revealed to reduce the compressive strength and elastic modulus15,59, and this loss becomes greater with the increase of time. The Brazilian splitting strength, splitting modulus and absorbed energy reduced by 46%, 22%, and 50% respectively after exposing to SC-CO2 for 60 days60. The reduction in mechanical properties is risky in the engineering. Further studies are required to investigate changes in micro-, mecro- and macro-scale for CS-EGR.
Conclusion
In the present work, CO2 flow behaviour is investigated at first and subsequently, shale permeability is compared before and after SC-CO2 treatment using N2. Surface characteristics are studied with the help of XRD and SEM. Several conclusions are drawn in the paper.
1. The curve of permeability of shale injected with CO2 versus pore pressure is appro -ximately W-shaped, and it is more obvious in the depressurization stage, the curve can be divided into four stages: a: when the pore pressure is low, permeability decrea -ses gradually under the combined action of Klinkenberg effect and CO2 adsorption expansion; b: before the critical point, with the continuous increase of pore pressure, the slippage effect gradually weakens, and the adsorption tends to be saturated, the effect of effective stress is greater than adsorption expansion, the downward trend of penetration slows down; c: CO2 undergoes a phase change,induced penetration rate dropped sharply again; d: with the further increase of pore pressure, the effective stress is further reduced, penetration increased. The overall trend showed a first decline and then an increase.
2. The permeability of shale increases significantly after SC-CO2 treated at pressures of 7.5–11.5 MPa, and the permeability growth is a linear function of SC-CO2 pressures, the porosity growth increases lineraly with the increase of SC-CO2 pressures.
3. The α value of shale specimens treated with different pressures of SC-CO2 are different, the lower the SC-CO2 pressure, the larger the α value. A larger α corresponds to a higher sensitivity of the permeability to the porosity, it shows that with the increase of SC-CO2 treatment pressure, the internal pore connectivity of shale specimens is better, the sensitivity to porosity is reduced, and the seepage path is more uniform.
4. Interaction between SC-CO2 and shale causes physical and chemical changes. The XRD results showed that, significant changes in mineral composition of shale samples treated with SC-CO2, with the increase of SC-CO2 treatment pressure, the percentage of quartz and some carbonate minerals increased, and the content of dolomite and clay minerals decreased sharply, description SC-CO2 can dissolve mineral components in shale well.
5. The SEM results showed that, after SC-CO2 treatment, the microstructure of shale changes obviously, and the primary pores and fissures in shale are corroded, Therefore, a new pore structure with enhanced connectivity is formed, which is beneficial to the seepage of gas in shale, which corresponds to the results of conclusion 3. The EDS results showed that, significant effect of SC-CO2 treatment pressure on dissolution, dissolution leads to the reduction in proportions of Ca and Mg as well as the slight increase in the ratio of C. The change trend of O, Ca, Mg content with SC-CO2 pressure is consistent with the change trend of SC-CO2 solubility with pressure. Therefore, supercritical carbon dioxide injection can effectively increase the permeability of shale.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
De Silva, P. N. K., Ranjith, P. G. & Choi, S. K. A study of methodologies for CO2 storage capacity estimation of coal. Fuel 91(1), 1–15 (2012).
Fathi, E. & Akkutlu, I. Y. Multi-component gas transport and adsorption effects during CO2 injection and enhanced shale gas recovery. Int. J. Coal Geol. 123, 52–61 (2014).
Ghanizadeh, A. et al. Experimental study of fluid transport processes in the matrix system of the European organic-rich shales: I. Scandinavian Alum Shale. Mar. Petrol. Geol. 51, 79–99 (2014).
Ghanizadeh, A. et al. Experimental study of fluid transport processes in the matrix system of the European organic-rich shales: II. Posidonia Shale (Lower Toarcian, northern Germany). Int. J. Coal Geol. 123, 20–33 (2014).
Estrada, J. M. & Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 182, 292–303 (2016).
Howarth, R. W., Ingraffea, A. & Engelder, T. Should fracking stop?. Nature 477, 271 (2011).
Zhang, D. & Yang, T. Environmental impacts of hydraulic fracturing in shale gas development in the USA. Pet. Explor. Dev. 42(6), 876–883 (2015).
Ellsworth, W. L. Injection-induced earthquakes. Science 341(6142), 1225942 (2013).
Langenbruch, C. & Zoback, M. D. How will induced seismicity in Oklahoma respond to decreased saltwater injection rates?. Sci. Adv. 2(11), e1601542 (2016).
Vidic, R. D. et al. Impact of shale gas development on regional water quality. Science 340(6134), 1235009 (2013).
Zhang, J. et al. Width design for gobs and isolated coal pillars based on overall burst-instability prevention in coal mines. J. Rock Mech. Geotech. Eng. 8(4), 551–558 (2016).
Levine, J. S. et al. U.S. DOE NETL methodology for estimating the prospective CO2 storage resource of shales at the national and regional scale. Int. J. Greenhouse Gas Control 51, 81–94 (2016).
Zou, C. et al. Shale gas formation and occurrence in China: An overview of the current status and future potential. Acta Geol. Sin. 90(4), 1249–1283 (2016).
Li, Q. et al. Affecting analysis of the rheological characteristic and reservoir damage of CO2 fracturing fluid in low permeability shale reservoir. Environ. Sci. Pollut Res 29(25), 37815–37826 (2022).
Lu, Y. et al. Swelling of shale in supercritical carbon dioxide. J. Nat. Gas Sci. Eng. 30, 268–275 (2016).
Liu, S. et al. The effects of CO2 on organic groups in bituminous coal and high-rank coal via Fourier transform infrared spectroscopy. Energy Explor. Exploit. 36(6), 1566–1592 (2018).
Liu, G. et al. Dynamic permeability change of supercritical CO2 fractured shale and its influencing factors. J. China Coal Soc. 42(10), 2670–2678 (2017).
Yin, H. et al. Experimental study of the effects of sub-and super-critical CO2 saturation on the mechanical characteristics of organic-rich shales. Energy 132, 84–95 (2017).
Qu, H. Y. et al. Impact of thermal processes on CO2 injectivity into a coal seam. IOP Confer. Ser. Mater. Sci. Eng. 10, 012090 (2010).
Gathitu, B. B., Chen, W. & McClure, M. Effects of coal interaction with supercritical CO2: Physical structure. Ind. Eng. Chem. Res. 48(10), 5024–5034 (2009).
Pan, Y. et al. Influences of subcritical and supercritical CO2 treatment on the pore structure characteristics of marine and terrestrial shales. J. Utiliz. 28, 152–167 (2018).
Yin, H. et al. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 184, 289–303 (2016).
Hui, D. et al. Effect of supercritical CO2 exposure on the high-pressure CO2 adsorption performance of shales. Fuel 247, 57–66 (2019).
Dahaghi A. K. Numerical simulation and modeling of enhanced gas recovery and CO2 sequestration in shale gas reservoirs: A feasibility study. In SPE international conference on CO2 capture, storage, and utilization. J. Society of Petroleum Engineers. (2010).
Godec, M. et al. Potential for enhanced gas recovery and 2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 118, 95–104 (2013).
Liu, F. et al. Assessing the feasibility of CO2 storage in the New Albany Shale (Devonian–Mississippian) with potential enhanced gas recovery using reservoir simulation. Int. J. Greenhouse Gas Control 17, 111–126 (2013).
Zhao, Y. et al. Experimental study of adsorption effects on shale permeability. Nat. Resour. Res. 28(4), 1575–1586 (2019).
Dicker, A.I., Smits, R.M. A practical approach for determining permeability from laboratory pressure-pulse decay measurements. In: International Meeting on Petroleum Engineering. (1988).
Jones, S. C. A technique for faster pulse-decay permeability measurements in tight rocks. SPE Form. Eval. 12, 19–25 (1997).
Ranathunga, A. S. et al. Super-critical carbon dioxide flow behaviour in low rank coal: A meso-scale experimental study. J. Utiliz. 20, 1–13 (2017).
Peng, D. Y. & Robinson, D. B. A new two-constant equation of state. Ind. Eng. Chem. Fund. 15(1), 59–64 (1976).
Ma, Y. et al. Organic matter/clay mineral intergranular pores in the Lower Cambrian Lujiaping Shale in the north-eastern part of the upper Yangtze area, China: A possible microscopic mechanism for gas preservation. Int. J. Coal Geol. 137, 38–54 (2015).
Ozdemir, E. & Schroeder, K. Effect of moisture on adsorption isotherms and adsorption capacities of CO2 on coals. Energy Fuels 23(5), 2821–2831 (2009).
Ross, D. J. K. & Marc, B. R. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 26(6), 916–927 (2009).
Darrell, L. S. Hernandez Rafael, AntonioEstévez L. Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci. 63(4), 292–301 (2008).
Ikuo, O., Xiaochun, Li. & Takashi, O. Effect of supercritical CO2 as the organic solvent on cap rock sealing performance for underground storage. Energy 30(23), 44–51 (2005).
Xiaoqiang, B., Zhimin, D. & Tang, Y. An improved density-based model for the solubility of some compounds insupercritical carbon dioxide. Thermochim Acta. 519, 16–21 (2011).
Pan, Z. & Connell, L. D. Modelling permeability for coal reservoirs: A review of analytical models and testing data. Int. Coal Geol. 92, 1–44 (2012).
Reiss, L. H. The Reservoir Engineering Aspects of Fractured Formations Vol. 3 (Editions Technip, 1980).
Barton, N., Bandis, S. & Bakhtar, K. Strength, deformation and conductivity coupling of rock joints. Int. Rock Mech. Min. Sci. 22(3), 121–140 (1985).
Xiao, K. et al. Anisotropy of the effective porosity and stress sensitivity of coal permeability considering natural fractures. Energy Rep. 7, 3898–3910 (2021).
Valle, L. M., Grima, C., Rodríguez, R. & Llopis, C. Effect of SC-CO2-brine mixture on injectivity and storage capacity in rock samples of naturally fractured carbonate formations. Nat. Gas Sci. Eng. 81, 103452 (2020).
Busch, A. et al. Carbon dioxide storage potential of shales. Int. J. Greenhouse Gas Control 2(3), 297–308 (2008).
Kweon, H. & Deo, M. The impact of reactive surface area on brine-rock-carbon dioxide reactions in CO2 sequestration. J. Fuel 188, 39–49 (2017).
Rathnaweera, T. D., Ranjith, P. G. & Perera, M. S. A. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci. Rep. 6, 19362 (2016).
Ao, X. et al. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. Utiliz. 20, 274–281 (2017).
Lyu, Q., Ranjith, P. G., Long, X. P. & Ji, B. Experimental investigation of mechanical properties of black shales after CO2-Water-Rock interaction. Materials 9(8), 663 (2016).
Niu, Y., Yue, C. T., Li, S. Y., Ma, Y. & Xu, X. Y. Influencing factors and selection of CH4 and CO2 adsorption on Silurian shale in Yibin, Sichuan Province of China. Energy Fuels 32, 3202–3210 (2018).
Cheng, Y. G. et al. Effects of supercritical CO2 treatment temperatures on mineral composition, pore structure and functional groups of shale: implications for CO2 sequestration. Sustainability 12, 3927 (2020).
Yiyu, Lu. et al. Mechanisms of shale water wettability alteration with chemical groups after CO2 injection: Implication for shale gas recovery and CO2 geo-storage. J. Nat. Gas Sci. Eng. 90, 103922 (2021).
Jiang, Y., Luo, Y., Yiyu, Lu., Qin, C. & Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 97, 173–181 (2016).
Thommes, M. et al. Physisorption ofgases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87(9–10), 1051–1069 (2015).
Wu, K. et al. Model for surface diffusion of adsorbed gas in manopores of shale gas reservoirs. Ind. Eng. Chem. Res. 54(12), 3225–3236 (2015).
Wu, K. et al. Flow behavior of gas confined in nanoporous shale at high pressure: Real gas effect. Fuel 205, 173–183 (2017).
Zou, Y. et al. Effects of CO2-brine-rock interaction on porosity/permeability and mechanical properties during supercritical-CO2 fracturing in shale reservoirs. J. Nat. Gas Sci. Eng. 2018(49), 157–168 (2018).
Jian C, S W, L Y. Natural Product of Supercritical CO2 Extraction. Chemical Industry Press (2005)
Kutchko, B. G. et al. Characterization of coal before and after supercritical CO2 exposure via feature relocation using field-emission scanning electron microscopy. Fuel 107, 777–786 (2013).
Jiang, Y. et al. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 97, 173–181 (2016).
Wu, D. et al. Experiments on displacing methane in coal by injecting supercritical carbon dioxide. Energy Fuels 32(12), 12766–12771 (2018).
Feng, G. et al. Effects of Supercritical CO2 adsorption on the mechanical characteristics and failure mechanisms of shale. Energy 173(15), 870–882 (2019).
Acknowledgements
This study was supported by National Natural Science Foundation of China (51974147, 51974186), and the Discipline Innovation Team of Liaoning Technical University (No. LNTU20TD-11, LNTU20TD-17).
Author information
Authors and Affiliations
Contributions
Wenbo Zhai: Data curation, Writing- Original draft preparation. Di Wu: Conceptualization, Methodology. Xueying Liu: Writing- Reviewing and Editing. Xiaochun Xiao: Project administration, Supervision. Feng Miao: Investigation. Jun Xu: Writing- Reviewing and Editing. Nan Jia: Investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, D., Zhai, W., Liu, X. et al. The permeability of shale exposed to supercritical carbon dioxide. Sci Rep 13, 6734 (2023). https://doi.org/10.1038/s41598-023-33702-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33702-1
- Springer Nature Limited