Abstract
In a survey conducted during the period of March–May 2019 in nurseries, warehouses, and shops at three governorates (Alexandria, El-Behera, and Giza governorates, Egypt), symptoms of root rot, basal stem rot, and wilt disease complex were observed in the lucky bamboo (Dracaena sanderiana hort. ex. Mast.). The highest disease infection percentage was found in lucky bamboo collected from Alexandria City (47.67%), while the highest disease severity was in lucky bamboo collected from El-Behera Governorate (35.19%). Rhizoctonia solani, Fusarium oxysporum, F. solani, Aspergillus niger, and Alternaria alternate were isolated and identified in the infected lucky bamboo samples. R. solani isolates were the most dominant among the recovered fungal species with a percentage of 80.89% of the total isolates (246). Pathogenicity tests showed that R. solani was the most pathogen with 100% disease infection and 76.67% disease severity. Molecular identification characterized R. solani isolate as R. solani AUMC 15120, MZ723906. Meanwhile, four biological control agents (bioagents) were isolated from the healthy lucky bamboo samples and identified based on cultural, morphological, microscopic characteristics, and the molecular phylogenetic analysis as Clonostachys rosea AUMC 15121, OL461708; Bacillus circulans TAG1, MW441316; B. siamensis TAP1, MW441318 and Ochrobactrum anthropi TAM1, MW441317. The four bioagents showed potential inhibition of R. solani in vitro as well as in vivo on lucky bamboo plants in vase treatments compared to the untreated inoculated control as well as certain fungicides and biocides used (Moncut, Rizolex-T, Topsin-M, Bio-Zeid, and Bio-Arc). The bioagent O. anthropi showed the highest inhibition growth (85.11%) of the in vitro R. solani colony, which was not significantly different from the biocide Bio-Arc (83.78%). However, C. rosea, B. siamensis and B. circulans showed inhibition values of 65.33, 64.44, and 60.44%, respectively. On the other hand, the biocide Bio-Zeid showed less inhibitory effect (43.11%), while the lowest growth inhibition was recorded by Rizolex-T (34.22%) and Topsin-M (28.67%). Furthermore, the in vivo experiment supported the in vitro results for the most effective treatments, where all the treatments significantly decreased the percentage of infection and disease severity compared to the inoculated untreated control. Additionally, the bioagent O. anthropi showed the highest effect, i.e., the lowest disease incidence and disease severity being 13.33% and 10%, compared to 100% and 75%, respectively, in the untreated inoculated control. This was not significantly different from the fungicide Moncut (13.33% and 21%) and from the bioagent C. rosea (20% and 15%) treatments for both parameters, respectively. In conclusion, the bioagents O. anthropi MW441317 at 1 × 108 CFU/ml as well as C. rosea AUMC15121 at 1 × 107/ml proved to be efficient to control R. solani causing root rot, and basal stem rot on lucky bamboo, compared to fungicide Moncut and can be used for disease management without the negative impact of the chemical control. Furthermore, this is the first report of the isolation and identification of Rhizoctonia solani, a pathogenic fungus, and four biocontrol agents (Bacillus circulans, B. siamensis, Ochrobactrum anthropi and Clonostachys rosea) associated with the healthy lucky bamboo plants.
Similar content being viewed by others
Introduction
Dracaena sanderiana hort. ex. Mast., belongs to the Asparagaceae family, and is commonly known as "lucky bamboo"1. It is not bamboo (Poaceae family), but the stems resemble real bamboo stalks. D. sanderiana is one of the most vase plants and is widely used in indoor decorations and good-luck houseplants popular in Egypt and all over the world in public spaces like malls, offices, and schools2. Its importance lies in the easily grown just in water with minimum requirements of care. Low light tolerance attracts positive energy with the beauty of its coordination in vases. It contributes to overcoming air and environmental pollution3.
However, lucky bamboo is affected by several fungal diseases, where the root rot, basal stem rot, and wilt disease complex are considered the main constraint for its industry3,4. Initial symptoms included a few yellowing rotted areas on the roots, a visible brown discoloration, and rotted areas on the base of the stems as the cortical tissues showed a distinct brown discoloration of water-soaked spots. These lesions became longer up and down and had-dark brown centers with a brown margin, and eventually severe wilting resulted in a significant loss of lucky bamboo plants5,6.
Rhizoctonia solani is one of the most destructive fungal pathogens that affect large numbers of ornamental crops causing root rot, basal stem rot, and wilt worldwide7,8,9. It can attack many ornamental plants, particularly during production and/or post-harvest storage latently, where it can lie dormant for many years7,10,11. Subsequently, fungicides such as Moncut, Rizolex-T, and Topsin-M are used to control the diseases12,13,14. Furthermore, increasing fungicide inputs has several negative consequences, i.e., the development of pathogen resistance to the fungicides, and the killing of the beneficial microorganisms15, besides the negative effects of environmental pollution16,17.
R. solani growth can be controlled by the application of biological controls (bioagents) including the use of microorganisms or their antibiotics offers an attractive alternative for the use of fungicides or management of plant diseases without the negative impact of chemical control. Such bioagents competitively can be colonized plant parts and stimulate the growth and/or reduce the incidence of the plant disease17,18. The bioagents Trichoderma viride, T. harzianum, Aspergillus niger, Penicillium spp., and Bacillus subtilis were observed in in vitro conditions against the tobacco sore shin pathogen, R. solani the percentage inhibition values of 70, 67, 57, 50 and 44%, respectively19. T. harzianum culture filtrates reduced root-rot incidence and damping-off caused by R. solani in eggplants, followed by T. viride, P. fluorescens and B. subtilis20. Some strains of P. fluorescens and B. subtilis observed a maximum inhibition of R. solani in terms of mycelial growth and sclerotial germination21. The bioagent T. harzianum reduced the natural incidence of wilt and wet root rot of chickpea in a field plot, as well as increased the seed yield22. In dual culture studies, the highest growth inhibition of R.solani that causes sheath blight disease of rice was recorded by T. harzianum followed by T. viride, G. virens and Trichothecium sp., while disease severity was highest when crop treated with Trichothecium sp., G. roseum, G. virens, and T. viride23.
It was reported that the Bio-Arc and Bio-Zeid as active bioagents are classified as biocides12,15. Many studies evaluated the activity of Trichoderma spp. and Bacillus spp. in reducing the spread of R. solani and causing completely inhibited growth24. Trichoderma spp. and Bacillus spp. are antagonistic microorganisms used as the most effective pathogen antagonists among biological control agents for many soil-borne and foliar diseases25,26,27. Furthermore, they have been shown to have an antagonistic effect on a wide range of diseases and used to control R. solani17. The bioagents based on Bacillus spp. including B. circulans majorly prevent the growth of plant fungal pathogenic microorganisms including R. solani28. Bacillus siamensis as a biocontrol agent showed antagonistic activity against Aspergillus niger29.
Therefore, the objectives of the present study were to identify and characterize, morphologically and at the molecular level, the pathogen of root rot, basal stem rot, and wilt disease complex that cause damage and kill lucky bamboo plants, isolate and molecular identification of fungal and bacterial bioagents associated with healthy lucky bamboo, and estimate the potential and efficacy of some biocontrol agents to control the main pathogen, R. solani, in vitro, and in vivo under laboratory conditions compared to certain fungicides and biocides.
Materials and methods
This study has complied with relevant institutional, national, and international guidelines and legislation. This study does not contain any studies with human participants or animals performed by any of the authors. This study was conducted with the permission of the Plant Pathology Research Institute, Ornamental, Medicinal, and Aromatic Plant Diseases Research Department, El-Sabihia Agricultural Plant Protection Research Station, Alexandria, and Faculty of Agriculture, Saba Basha, Alexandria University” during the 2019–2021 period.
Survey for root rot, basal stem rot, and wilt disease complex on lucky bamboo
During the period of March–May 2019, 226 samples of naturally infected lucky bamboo showing characteristic symptoms of severe root rot, basal stem rot, and wilt disease complex were randomly collected from 630 lucky bamboo plants surveyed at various locations at retail stores, shops, and nurseries in Alexandria, El-Behera, and Giza governorates, Egypt. In all the mentioned sites, the percentages of disease infection were recorded and calculated30,31 using the ratings shown in Table 1.
The percentages of disease infection and disease severity were calculated by the following equations:
Isolation and identification of the associated fungal species
Seventy-five lucky bamboo plant samples showing symptoms of root rot, stem basal rot and wilt disease complex were collected in the survey, labeled separately in paper bags, and transferred to the laboratory to isolate the associated fungal species on the day following collection. Each sample was thoroughly washed in running tap water and cut into small pieces (4 mm), with half of the tissue being healthy and half being diseased tissue. The surface of the pieces was sterilized by soaking in sodium hypochlorite (5%) for 3 min after being rinsed with sterile distilled water (DW).
The pieces were washed thrice with sterile DW, then dried between two layers of sterilized filter papers, and subsequently placed in Petri dishes with potato dextrose agar (PDA) medium supplemented with 250 µL/mL of streptomycin (Egypt Masters Co. (EMC), Dakahlia, Egypt). The plates were then incubated in darkness at 26 ± 1 °C for 7 days32, and the developed fungal colonies were purified by a single spore or hyphal tip technique. The developed fungal species were identified based on morphological and microscopic characteristics33,34,35. The frequency of each fungal isolate was calculated according to the following equation.
Pathogenicity tests and molecular identification of the isolated fungal species
Pathogenicity tests
Ostensibly healthy-looking lucky bamboo plants, with uniform stem lengths averaging 70 cm, were purchased from a famous private commercial nursery in Cairo. In the laboratory, plants were well-washed several times with running tap water, surface disinfected in 1% sodium hypochlorite for 2 min, then washed several times with running tap water and rinsed with sterilized DW. To ensure that the plants are healthy and free of any pathogens, they were sown in glassware containing 300 ml of sterile DW for 60 days under the laboratory conditions (12 h photoperiod at 26 ± 2 °C with an average relative humidity of 65–70%) before conducting any experiments on them. Furthermore, to reduce bacteria entry from the surrounding air and prevent water evaporation, all glassware is wrapped with a sterilized cotton stopper around the bamboo stem. Before sown, each glassware was cleaned and sterilized in a hot air oven for 2 h at 180 °C and then left to cool3,36.
Thirty healthy bamboo plants were placed in 1-L sterile glassware (1 plant/glassware) by dipping 5 cm of basal stems with 300 ml of sterile DW. Fifteen plants were inoculated separately by adding directly an excerpt of Rhizoctonia solani mycelial agar plug (0.5 cm diameter) cut from a 7-day-old culture disc at 26 ± 2 °C of the active margins of the fungal culture recovered in the survey. The culture was inserted into a cut in the basal stem segment with a sterile Cork borer. A similar plug of sterile PDA served as the negative control of the remaining 15 bamboo plants. The inoculated areas were then covered with Parafilm strips and to provide wet conditions, the plants were covered with polyethylene plastic bags for 24 h. All bamboo plants were kept for 4 weeks under laboratory conditions. At the end of the test, the percentages of infection and severity of disease were calculated as described above30,37.
To ensure that the pathogen was associated with the symptoms, it was re-isolated from the symptoms in artificially infected plant tissues. Subsequently, the developed fungal cultures were purified as described above, then they were identified based on morphological and microscopic characteristics and molecular identification.
Molecular identification of the recovered Rhizoctonia solani isolates
The most dominant fungal species, i.e., R. solani, was further identified and molecularly characterized by polymerase chain reaction (PCR) amplification and 18S sequencing. The cultures were sent to the “Molecular Biology Research Unit, Assiut University for DNA extraction using a Patho Gene-spin DNA/RNA extraction Kit provided by Intron Biotechnology Company, Korea. Samples of fungal DNA were then sent to SolGent Company, Daejeon, South Korea for PCR and 18S sequencing. PCR was performed using ITS1 (forward) and ITS4 (reverse) primers, which were incorporated into the reaction mixture38. Primers have areas with universal primer pairs including ITS1 (5'-TCC GTA GGT GAA CCT GCG G-3'), and ITS4 (5'-TCC TCC GCT TAT TGA TAT GC-3'). The purified PCR products (amplicons) were sequenced using the same primers, but with ddNTPs added to the reaction mixture39. Identification of isolate was confirmed by the obtained sequences of the amplified regions and analyzed using “the Basic Local Alignment Search Tool (BLAST) from the National Center of Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov). Molecular Evolutionary Genetics Analysis version 5.05 of MegAlign (DNA Star) software was used to perform the alignments. The identified phylogenetic tree based on ITS sequences of rDNA of the isolated fungal strain aligned with closely related sequences was accessed from the GenBank.
Control studies
Isolation of the associated biocontrol agents of the healthy lucky bamboo
Isolation of the antagonistic micro-organisms on healthy lucky bamboo plants was conducted after the incubation period of bamboo plants, which exceeded 60 days under the laboratory conditions, to ensure that they are healthy plants free of any apparent infestations3. Plant parts of 2 cm long were cut from the basal stem with roots of 20 bamboo plants, then 1 g of each sample was taken and processed as mentioned above for the isolation and microscopic and molecular identification of the fungi.
The isolation of the associated bacteria was conducted according to Abdel–Rahman40, with minor modifications. The crushed plant parts were sterilized with sodium hypochlorite solution (10%) for 2 min, immersed in sterilized DW for 2 min, and washed thoroughly several times with sterilized DW. Afterward, 1 g from each sample tissue (parts of each basal stem and root) was mixed with 9.9 ml of sterile saline (sterile physiological water from NaCl, 9 g/l) individually and squashed into the sterilized mortar and pestle to homogenize. Then, the solution was diluted serially in the sterile saline individually for each sample up to 106 CFU/ml41. A loopful (1 ml) of the resulting suspension of each dilution was spread on nutrient agar (9 ml of NA) plates and incubated for 24 h at 30 ± 1 °C. In order to obtain new separate colonies, single colonies were selected and purified on fresh NA plates on the base of variance in morphology, e.g., color, size, and shape. After 24–48 h at the same temperature, pure bacterial colonies appeared, then identified according to their morphological and biochemical characteristics42,43, and performed using standard methods by Agricultural Laboratories Company (Agro Lab, Sadat City, Egypt).
Molecular identification of the associated biocontrol agents of the healthy lucky bamboo
DNA was extracted from the isolation of pure cultures of fungi and bacteria ABT DNA mini extraction Kit (Applied Biotechnology Co. Ltd, Egypt) for molecular characterization of the Internal Transcript Spacer (ITS) region using Polymerase Chain Reaction (PCR) amplification, "2X Red master Mix (Applied Biotechnology Co., Egypt), and Oligonucleotide (Alpha DNA Co, Canada)"44. The ITS DNA region of these isolates was amplified via PCR using universal primers. The following is the optimized thermal profile for PCR: Initial denaturation (95 °C for 3 min), Denaturation (95 °C for 30 s., Annealing (50 °C for 30 s.), Extension (72 °C for 90 s.), and Final extension (72 °C for 5 min.), Repeating for 35 cycles.
To confirm the targeted PCR amplification, five μl of the PCR product was electrophoresed along with 100 bp DNA molecular weight 1% agarose gel containing ethidium bromide (0.5 μg/ml) at constant 80 V for 30 min in 1X TAE buffer. The amplified product was visualized as a single compact band of expected size under UV light and documented by the Samsung Note 4 smartphone.
Sequencing of the PCR product for the amplified PCR products was submitted to Solgent Co Ltd (South Korea) for gel purification and sequencing. The resulted sequences were trimmed and assembled in Geneious software (Biomatters). Consequently, the trimmed sequences were identified by search in the basic local alignment search tool (BLAST) in GenBank.
Furthermore, phylogenetic analysis by nucleotide sequences was downloaded from GenBank and aligned with the identified sequences, using MAFFT alignment45. Phylogenetic trees were constructed using the neighbor-joining method, employing the Tamura–Nei Model46. The trees were assessed using 1000 bootstrap replicates.
Evaluation of the isolated bioagents against Rhizoctonia solani in vitro
The in vitro inhibition effects of the recovered bioagent isolates, i.e., the fungal isolate Clonostachys rosea, and the three recovered bacterial isolates Bacillus circulans, B. siamensis, and Ochrobactrum anthropi, were tested against the highly aggressive isolate of R. solani AUMC15120, which recovered in the conducted survey. This was done in comparison with the untreated inoculated control as a negative control as well as three fungicides and two biocides as positive controls (Table 2).
The tested fungal and bacterial bioagents as well as the tested fungal and bacterial biocides were grown under laboratory conditions. For biocides, a disc of filter paper 5 mm in diameter, impregnated with Bio-Zeid (Trichoderma album 25 × 106 spores/g) suspension, was inoculated at the recommended rate into the middle of a Petri dish, then incubated at 26 ± 2 °C for 7-days, while; bacterial isolates and/or Bio-Arc were grown individually in 250 ml flasks that each contained 50 ml of the NA medium. Then after the incubation for 72 h, they were used for the streaked method.
Antagonistic potential of the isolated bioagents against Rhizoctonia solani
Isolated bioagents were screened for their antagonistic potential against R. solani by double culture assay using solid PDA plates50. Each PDA plate was divided into two equal halves, and at a distance of 1 cm from the edge of every plate from opposite sides, plates were inoculated on one side with a mycelial disc (5 mm diameter) taken from the margins of the active growing R. solani of 7-day-old PDA cultures. Furthermore, on the other opposite side of each plate at 1 cm distance from the plate edge, a 5 mm of the tested fungal bioagent (C. rosea) or fungal biocide plug (taken from advanced margins of 7-day-old PDA cultures) was placed.
Likewise, a filter paper disc of 5 mm diameter, impregnated with each fungicide separately was placed51. Additionally, the bacterial bioagent isolates and/or Bio-Arc were treated by a streaking method3. Untreated check (control) plates were treated without bioagents and/or fungicides.
Five replicate plates were used for each treatment and incubated at 26 ± 2 °C until the untreated control mycelium (R. solani) totally colonized the plate. At that time, radial growth in plates for each treatment was measured to determine the percentage of the growth inhibition by calculating the percentage of radial growth reduction in diameter mycelia of R. solani3,50 as follows3,44:
In which (GDu) is the radial growth diameter of pathogen mycelia untreated (R. solani) in the control plate, apart from the bioagents (cm), and (GDT) is the radial growth diameter of the treated pathogen mycelia (R. solani) toward the bioagents and/or fungicides (cm).
The in vivo evaluation of the efficacy of the isolated bioagents to control Rhizoctonia solani on lucky bamboo in vase under laboratory conditions
The antagonistic effect of fungal isolate (C. rosea), bacterial isolates (B. circulans, B. siamensis, and O. anthropi), fungicides and biocides (Table 2) that showed maximum inhibitory activity in vitro against the highly aggressive R. solani AUMC15120 isolate. i.e., Clonostachys rosea AUMC15121″, Bacillus circulans MW441316, B. siamensis MW441318, Ochrobactrum anthropi MW441316, Moncut, Bio-Zeid and Bio-Arc were tested in vivo on lucky bamboo in the vase under laboratory conditions at the recommended dose (Table 2).
Inocula of the fungal bioagent C. rosea were prepared as follows; flasks of 100 ml of potato dextrose liquid (PDL) were inoculated by adding directly a sporulating mycelial agar plug (0.5 cm diameter) taken from a 7-day-old culture active margins of C. rosea and incubated at 22 ± 2 °C with photoperiods 12 h under white fluorescent lamps until sufficient mycelial growth was obtained. Subsequently, the collected mycelial growth was blended with 100 ml of sterile distilled water for 1 min. The conidia spore’s concentration was adjusted using the hemocytometer technique47, viz 107 conidia spores/ml inoculum concentrations.
The bacterial suspensions of bioagents and biocides were cultured on Luria–Bertani (LB) broth medium at flasks containing 100 ml of medium for 2 days at 30 ± 1 °C and shaken at 200 rpm to harvest bacteria. Then the bacterial concentrations in the solution were adjusted by serial dilution with sterile saline individually 1 × 107 CFU/ml48, and 1 × 108 CFU/ml49.
All treatment were conducted one day after inoculation with R. solani (as mentioned above in the pathogenicity testing) to 120 glassware vessels planted with healthy lucky bamboo separately. Sterile distilled water was used instead of the treatments as an untreated control. All treatments and control were kept for 4 weeks under laboratory conditions. At the end of the trial, the percentages of disease infection and disease severity (%) were recorded as mentioned above. Subsequently, the percentage decrease or increase from the untreated control was calculated52. In addition, the percentage of treatment efficiency was evaluated using the formulas proposed53, respectively:
Statistical analysis
The obtained data of the tested treatments (bioagents, fungicides, and biocides) against the growth of R. solani were statistically analyzed using Statistix program, by using the software analysis of variance with one-way ANOVA test54 and compared with the untreated control. All trials were carried out with a randomized complete block design (RCBD). Each treatment with five replicates, each replicate contains 3 plates and/or 3 glassware. Each glassware contains one bamboo plant. Comparisons among the means were evaluated using the least significance difference (LSD) at a 5% level of probability.
Results
Survey for root rot, basal stem rot and wilt disease complex on lucky bamboo
During the period March–May 2019, the conducted survey for root rot, basal stem rot and wilt disease complex on lucky bamboo plants, typical symptoms were identified (Fig. 1) in the surveyed retail stores, shops, and nurseries from different locations in Egypt (Alexandria, El-Behera, and Giza governorates). However, the surveyed locations and governorates showed considerable variations in the percentage of infection and disease severity % (Table 3).
Appearance healthy and naturally infected for lucky bamboo (Dracaena sanderiana hort. ex. Mast.), and Symptoms of basal stems and roots (A & D) complete plant form (B) healthy basal stem and -brown lesions) (F) few yellowing roots, (C) healthy leaves, (E) basal stem rot, root rot (infected basal stem have dry of brown to reddish leaves).
From the data in Table 3, the mean percentage of disease infection was the highest in plants collected from Alexandria (47.67%) followed by Giza (33.14%) and El-Behera (26.98%) Governorates. However, the disease severity for the three surveyed governorates ranged between 35.19and 31.53%, with no significant differences between the surveyed governorates (Table 3).
Fungi associated with root rot, basal stem rot, and wilt disease complex of lucky bamboo plants
Data in Table 4 show that the five fungal isolates R. solani, F. oxysporum, F. solani, Aspergillus niger, and Alternaria alternata were found to be associated with the surveyed lucky bamboo samples with symptoms of root rot, basal stem rot, and wilt disease complex. However, R. solani isolates were the most frequent among fungal species recovered and constituted 80.89% of the total isolates while the other fungal species showed frequencies lower than 10% (Fig. 2).
Pathogenicity test
It is evident in Table 5 that the tested fungal species recovered in the survey were able to incite root rot and basal stem rot to different degrees. However, data in Table 5 showed that R. solani isolates were the most pathogenic occurred on lucky bamboo and exhibited a 100% percentage of infection with 76.67% disease severity. Meanwhile, F. oxysporum and F. solani showed percentages of infection of 33.33% and 26.67% with 35.0% and 27.5% disease severity, respectively. The other two fungal species Aspergillus niger, and Alternaria alternata showed an infection percentage of 20% or less with a disease severity of 15% or less (Table 5). Moreover, Fig. 3 illustrated the symptoms of basal stem rot and root rot when artificial infestation by R. solani during the pathogenicity test and compared it with that of natural infestation of lucky bamboo plants.
Symptoms of basal stem rot, root rot at natural and artificial infestation caused by Rhizoctonia solani during pathogenicity test of lucky bamboo (D. sanderiana hort. ex. Mast.) in vitro, (A) naturally infected, and (B, C & D) symptoms of dieback of the rotting basal stem party after artificial infected (r) rotting brown discoloration at the middle of the basal stem, (E, F, and G) longitudinal sections of the basal stem, (d) internal brown discoloration of the basal stem's, and (H) uninfected control.
Molecular characterization of the recovered Rhizoctonia solani isolates
After the morphological and microscopic characteristics of R. solani isolates (Fig. 4), the molecular identification of R. solani isolates was conducted by PCR amplification and 18S sequencing. The tested strain showed 100% identity and 100% coverage with several strains of R. solani accessed from the GenBank (Fig. 5). The fungus was putatively identified as Rhizoctonia solani AUMC 15120 (GenBank accession No. MZ723906). Furthermore, the phylogenetic tree identified showed that R. solani (AUMC 15120) aligned with closely related sequences accessed from the GenBank, i.e., Thanatephorus cucumeris which is the teleomorph (sexual stage) of R. solani (Fig. 5).
Morphological and microscopic characteristics of Rhizoctonia solani fungus isolated from basal stems and roots of bamboo plants, (A, B): mycelial of R. solani on PDA medium after 10 days (A: top surface and A: lower surface) for the petri dish, and (C, D & E) microscopic traits of R. solani of the hyphal stage at a typical angle branching (ab) with numerous branching filaments of specialized hyphae composed of compact cells called monilioid cells (m), branching hyphae (bh) and septum (s).
Phylogenetic tree based on ITS sequences of rDNA of the fungal strain isolated in the present study (Rhizoctonia solani AUMC 15,120) aligned with closely related sequences accessed from the GenBank, (R. = Rhizoctonia, T. = Thanatephorus and B. = Bjerkandera), (https://www.ncbi.nlm.nih.gov/nuccore/gb:MZ723906).
Control studies
Isolation and identification of the associated biocontrol agents of the healthy lucky bamboo
Four biological control agents were isolated from healthy lucky bamboo plants (Table 6). Three of them were bacterial strains (Bacillus circulans, B. siamensis, and Ochrobactrum anthropi), and one fungal strain (Clonostachys rosea). The isolated fungus was morphologically investigated (Fig. 6) and all bioagents were characterized at the molecular level (Figs. 7 and 8).
The amplified product as a single compact band of expected size under UV light, and phylogenetic tree based on 16S sequences of rDNA of the bacterial strains isolated in the present study (Bacillus circulans TAG1, Bacillus siamensis TAP1, and Ochrobactrum anthropi TAM1, arrowed) aligned with closely related sequences accessed from the GenBank. Escherichia coli is included in the tree as an outgroup strain.
The molecular characterization showed that Clonostachys rosea AUMC 15,121 fungal isolate exhibited 99.63–99.63% identity and 100% coverage with several strains of C. rosea (Fig. 7). The three bacterial isolates (B. circulans, B. siamensis, and O. anthropi) amplified products were visualized as single compact bands of expected size under UV light (Fig. 8). B. circulans TAG1 showed 97.60–97.69% identity and 99% coverage with several strains of the same species recorded in the GenBank including the type material B. circulans strain NBRC 13626 while B. siamensis TAP1 showed 95.68–95.97% identity and 97% coverage with different strains of B. siamensis recorded in the GenBank.
However, O. anthropi TAM1 exhibited 96.92–97.11% identity and 99% coverage with other strains accessed from the GenBank encompassing the type strain Brucella anthropic ATCC49188 which is registered in the GenBank as the type strain of O. anthropi, Family Brucellaceae (Fig. 8).
The in vitro evaluation of the isolated bioagents against Rhizoctonia solani
The isolated bioagents from healthy lucky bamboo were evaluated for their antagonistic potential against R. solani recovered from lucky bamboo, in vitro and in vivo and compared with some other fungicides and biocides.
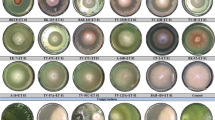
It is evident in Fig. 9 that all tested bioagents and the tested fungicides and biocides affected the tested R. solani isolate to different degrees. However, data in Table 7 showed that the highest R. solani colony growth inhibition was presented by bioagent O. anthropi isolate (85.11%), followed by biocide Bio-Arc (83.78%), while bioagent C. rosea showed 65.33%. The bioagents B. siamensis and B. circulans exhibited 64.44, and 60.44%, respectively, while the biocide Bio-Zeid showed an inhibition value of 43.11%. However, the least growth inhibition was recorded by Rizolex-T with 34.22% and Topsin-M with 28.67% of inhibition (Table 7).
Direct confrontation dual culture plate assays for determining the antagonistic potential of fungus and bacterial isolates, fungicide, and biocide against Rhizoctonia solani AUMC 15120 by inhibition of pathogen mycelial growth compared to the untreated control plates in vitro. The cultures were incubated at 26 ± 2 °C.
The in vivo evaluation of the efficacy of the isolated bioagents to control Rhizoctonia solani on lucky bamboo in vase under laboratory conditions
The most effective treatments revealed in vitro were further tested in vivo in vases under laboratory conditions. Data in Table 8 showed the disease incidence and disease severity caused by R. solani. A decrease or increase in infection with and treatment effectiveness against R. solani on bamboo artificially inoculated under laboratory conditions for 4 weeks after inoculation and treatments are shown in Fig. 10.
All the tested treatments (Table 8) significantly decreased the infection percentage and disease severity compared to the inoculated untreated control. However, the best effect was obtained by O. anthropi and the fungicide Moncut, with the lowest disease incidence value of 13.33%, followed by C. rosea (20%) compared to the control (100%). The lowest disease severity (10%) was obtained by O. anthropi followed by Clonostachys rosea (15%) and B. siamensis (15%) compared to the control (75%). However, B. circulans showed 40% and 27% while, Bio-Zeid (Trichoderma album) showed the least effect with values of 66.67% and 43.33%, for both disease parameters, respectively (Table 8).
Furthermore, all the tested treatments significantly decreased the disease incidence and gave positive increasing responses regarding the treatment efficiency in comparison to the untreated control (Fig. 10). O. anthropi afforded the highest disease reduction to other isolates, and it gave the highest efficiency at 86.67%. Bio-Zeid (T. album) yielded the lowest reduction of the pathogen and the lowest mean percentage of efficiency (viz 33.33 and 42.22%, respectively, both disease parameters) compared to the infected untreated control.
Discussion
Lucky bamboo (D. sanderiana hort. ex. Mast.) is well known in Egypt and other parts of the world as an important indoor ornamental plant. Meanwhile, it possesses importance viz the ease of care, low light tolerance, attract positive energy with its beauty and contribution to overcoming air and environmental pollution, etc.2,3,55,56. However, several reports indicated that lucky bamboo plants are seriously affected by root rot, basal stem rot and wilt disease complex3,8,57,58. Furthermore, several works indicated the presence of latent diseases in imported bamboo plants5,6,58.
The present study confirmed these reports in a survey conducted during the period of March–May 2019 in nurseries, warehouses, and shops in different locations(Alexandria, El-Behera, and Giza), in Egypt. The percentage of disease infection was the highest in Alexandria (47.67%) followed by Giza and El-Behera Governorates with 33.14% and 26.98%, respectively. In addition, the disease severity for the three surveyed governorates ranged between 35.19% (El-Behera Governorate) and 31.53% (Giza Governorate). These findings are not unexpected as disease infection and severity were of the highest values in such governorates with high humidity, Alexandria, and El-Behera Governorates. These results are in harmony with other investigators2,3,8.
In the present work, five fungal species were found to be associated with the surveyed lucky bamboo samples showing root rot, basal stem rot, and wilt disease complex. These fungal species were R. solani, Fusarium oxysporum, F. solani, Aspergillus niger, and Alternaria alternata. Most of these fungi were isolated from lucky bamboo by several researchers3,4,5,6. However, R. solani isolates were the most dominant among fungal species recovered with a percentage of 80.89%. These findings also are in harmony with other investigators10,11. R. solani is one of the most dangerous and highly destructive fungal pathogens affecting many ornamental crops and several treatments and trials using natural products and bioagents have been done to control its growth7,8,59,60,61,62,63,64.
Adoption of biological control is one of the crucial approaches currently at the forefront and is strongly desired for sustainable agriculture18. Several features of using biocontrol agents have been reported as one eco-friendly alternative or a supplemental way of reducing the use of toxic fungicides in ornamental plants65. Therefore, it is important to come up with new biological control products for eco-friendly and sustainable efficient benefits17. Furthermore, increasing levels of plant resistance using biological inducers isolated from the same plant is a new sustainable strategy for plant disease control. Identification of biocompatible isolates for managing lucky bamboo root rot, basal stem rot, and wilt caused by R. solani would be a beneficial contribution to disease management with low toxicity and minimal potential risk to the environment.
The present study supported this phenomenon, where four bioagents Clonostachys rosea, Bacillus circulans, B. siamensis, and Ochrobactrum anthropi were isolated from healthy lucky bamboo plants and were identified at the molecular level and significantly showed potential to inhibit R. solani in vitro as well as in vivo on lucky bamboo plants in vase treatments compared with the untreated inoculated control (negative control) as well as certain fungicides and biocides. All the tested bioagents (C. rosea, B. circulans, B. siamensis, and O. anthropi) as well as the tested fungicides and biocides, i.e., Moncut (2 g/l), Rizolex-T (2.0 g/l), Topsin-M (1 g/l), Bio-Zeid (2.5 g/l), and Bio-Arc (2.5 g/l), significantly inhibited the in vitro growth (colony diameter) of the tested R. solani isolate to different degrees.
Meanwhile, the in vivo experiment supported the in vitro results for the most effective treatments. All the tested treatments significantly decreased the percentage of infection and disease severity, showed significantly decreased disease incidence, and gave positive increasing responses regarding the treatment efficiency compared to the inoculated untreated control. The bioagent O. anthropi showed the highest effect, i.e., the lowest disease incidence and disease severity compared to the untreated inoculated control. This was not significantly different from the fungicide Moncut, and the bioagent C. rosea. These results are consistence with other investigators14,15,28,66,67,68,69.
The bioagent fungus Clonostachys rosea (Gliocladium roseum) is proven to be a promising and strong biocontrol agent for a variety of plant pathogens including fungal, nematodes, and insects. It has been recorded as an aggressive parasite against many fungi of excellent bioagent in plant diseases through techniques like nutrient competition and hyperparasitism47,70,71. Also, mechanisms of C. rosea biocontrol are represented in the production of a wide range of volatile organic compounds which are toxic to organism's pathogens, and it could be due to the induction of plant defense responses systems, e.g., excretes cell wall‐degrading enzymes, antifungal secondary metabolite production, or leading to induced systemic resistance (ISR)72,73.
O. anthropi, a siderophore-producing bacteria, is used as a potential biological control agent. It has shown a pretty antagonistic activity against many fungi such Botrytis cinerea, Colletotrichum orbiculare, and Fusarium oxysporum, etc.66,68,74.
Meanwhile, it has been indicated that Bacillus circulans emergence of chitinase enzyme activity against various plant pathogenic fungi, and also the B. spp. as a biocontrol has many benefits, including viz increasing mineral uptake, nitrogen fixation, and growing a strong and disease-resistant plant28,41,75,76. Additionally, B. siamensis succeeded as a biocontrol agent by producing antifungal compounds29 such as poly-γ-glutamic acid67, and it was proven to enhance plant growth and heighten plant growth-promoting qualities27.
The most important findings of this research are the isolation and identification of the pathogenic fungus R. solani from the symptoms of natural infection of bamboo plants. Furthermore, isolation and identification of four associated biocontrol agents of the healthy bamboo plants were done based on morphological and microscopic characteristics and molecular phylogenetic analysis and GenBank accession. To the best of our knowledge, this is the first report of the isolation of each R. solani AUMC 15120, Clonostachys rosea AUMC15121, OL461708 5.8S, Bacillus circulans TAG1, 16S MW441316, B. siamensis TAP1, 16S MW441318, and Ochrobactrum anthropi TAM1, 16S MW441317 of lucky bamboo (Dracaena sanderiana hort. ex. Mast.) in Egypt.
The present study supported the identification of biocompatible isolates for managing lucky bamboo root rot, and basal stem rot caused by R. solani that would be a beneficial contribution to disease management with low toxicity and minimal potential risk to the environment.
Conclusions
This is the first report of the isolation and identification of the pathogenic fungus Rhizoctonia solani from the symptoms of natural infection of bamboo, and, four associated biocontrol agents of the healthy lucky bamboo. They were identified based on morphological and microscopic characteristics, molecular phylogenetic analysis and GenBank accession. Consequently, the bioagents Ochrobactrum anthropi MW441317 at 1 × 108 CFU/ml, or Clonostachys rosea AUMC15121 at 1 × 107/ml proved to be efficient to control the growth of R. solani which causes root rot, and basal stem rot on lucky bamboo plants, which are as effective as the most efficient Moncut fungicide. This work recommends the use of biocides isolated from the same plant to combat R. solani, despite the efficiency of some fungicides in reducing infection rates and severity for sustainable protection of the environment.
Data availability
All data generated or analyzed during this study are included in this published article. The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Damen, T., van der Burg, W., Wiland-Szymańska, J. & Sosef, M. Taxonomic novelties in African Dracaena (Dracaenaceae). Blumea 63, 31–53. https://doi.org/10.3767/blumea.2018.63.01.05 (2018).
Morsy, A. A. & Elshahawy, I. E. Anthracnose of lucky bamboo Dracaena sanderiana caused by the fungus Colletotrichum dracaenophilum in Egypt. J. Adv. Res. 7, 327–335. https://doi.org/10.1016/j.jare.2016.01.002 (2016).
Abdel-Rahman, T. F. M., El-Morsy, S. A. & Halawa, A. E. A. Occurrence of stem and leaf spots on lucky bamboo (Dracaena sanderiana hort. ex. mast.) plants in vase and its cure with safe means. J Plant Prot. Pathol. 11, 705–713. https://doi.org/10.21608/jppp.2020.170648 (2020).
Sharma, K. et al. Isolation, characterization, and management of Colletotrichum spp. causing anthracnose on lucky bamboo (Dracaena sanderiana). HortScience Horts 49, 453–459. https://doi.org/10.21273/HORTSCI.49.4.453 (2014).
Abedi-Tizaki, M., Zafari, D. & Sadeghi, J. First report of Fusarium solani causing stem rot of Dracaena in Iran. J. Plant Prot. Res. 56, 100–103. https://doi.org/10.1515/jppr-2016-0013 (2016).
Kumar, N., Dubey, S. C., Kumar, P. & Khurana, S. M. P. Fusarium solani causing stem rot and wilt of lucky Bamboo (Dracaena sanderiana) in India-first record. Indian Phytopathol. 72, 367–371. https://doi.org/10.1007/s42360-019-00119-8 (2019).
Aiello, D., Guarnaccia, V., Formica, P. T., Hyakumachi, M. & Polizzi, G. Occurrence and characterisation of Rhizoctonia species causing diseases of ornamental plants in Italy. Eur. J. Plant Pathol. 148, 967–982. https://doi.org/10.1007/s10658-017-1150-8 (2017).
Jemai, N. et al. Rhizoctonia solani affecting micropropagated Garnem (Prunus amygdalus × Prunus persica) rootstock—Characterization and biocontrol with Rhizobia. J. Plant Pathol. 103, 207–215. https://doi.org/10.1007/s42161-020-00712-1 (2021).
Rashed, O. et al. Characterization of inter and intra anastomosis group of Rhizoctonia spp. isolated from different crops in Peninsular Malaysia. Trop. Plant Pathol. 46, 422–434. https://doi.org/10.1007/s40858-021-00433-5 (2021).
Gullino, M. L., Gilardi, G., Bertetti, D. & Garibaldi, A. In International Society for Horticultural Science (ISHS) 1270 edn, 9–22 (Leuven, Belgium).
Traversari, S. et al. Precision agriculture digital technologies for sustainable fungal disease management of ornamental plants. Sustainability 13, 3707. https://doi.org/10.3390/su13073707 (2021).
Mahmoud, N. A., Khalifa, N. A., Abbas, M., Sobhy, H. & Abou-Zeid, N. Efficacy of antagonistic fungal and bacterial bioagents against faba bean damping-off disease. Zagazig J. Agric. Res. 45, 917–929. https://doi.org/10.21608/zjar.2018.49131 (2018).
Mekapogu, M. et al. Recent progress in enhancing fungal disease resistance in ornamental plants. Int. J. Mol. Sci. 22, 7956. https://doi.org/10.3390/ijms22157956 (2021).
Al-Mansoury, B.A.-R. & Salih, Y. A. Evaluation of the efficiency of bio agents Trichoderma harzianum and T. longibrachiatum and some fungicides and a chemical compound against the fungus Rhizoctonia sp. that causes eggplant root rot disease in vitro. Euphrates J. Agric. Sci. 13, 210–231 (2022).
Khalifa, N. A., Saleh, R. A. & Mahmoud, N. A. Efficiency of some bio-control agents and plant extracts against beans (Phaseolus vulgaris L.) damping-off and root rot diseases under greenhouse and field conditions. Egypt. J. Phytopathol. 49, 152–167. https://doi.org/10.21608/ejp.2021.111016.1052 (2021).
Fritz, V. et al. Effect of inoculation with arbuscular mycorrhizal fungi and fungicide application on the secondary metabolism of Solanum tuberosum leaves. Plants 11, 278. https://doi.org/10.3390/plants11030278 (2022).
Lahlali, R. et al. Biological control of plant pathogens: A global perspective. Microorganisms 10, 596. https://doi.org/10.3390/microorganisms10030596 (2022).
Baysal-Gurel, F. & Kabir, N. Comparative performance of fungicides and biocontrol products in suppression of Rhizoctonia root rot in viburnum. J. Plant Pathol. Microbiol 9, 451–456. https://doi.org/10.4172/2157-7471.100045 (2018).
Seema, M. & Devaki, N. In vitro evaluation of biological control agents against Rhizoctonia solani. J. Agric. Technol. 8, 233–240 (2012).
El-Nagdi, W. & Abd-El-Khair, H. Biological control of Meloidogyne incognita and Rhizoctonia solani in eggplant. Nematol. Mediterr. 36, 85–92 (2008).
Nagendran, S., Kulanthaivelu, S. & Sundararajan, T. Assessment on antagonistic potential of bacterial bio agents Pseudomonas fluorescens and Bacillus subtilis against Rhizoctonia solani Kühn. An incitant of Sheath blight of rice. J. Entomol. Zool. Stud 7, 128–142 (2019).
Prasad, R., Rangeshwaran, R., Anuroop, C. & Rashmi, H. Biological control of wilt and root rot of chickpea under field conditions. Ann. Plant Prot. Sci. 10, 72–75 (2002).
Bhat, K., Anwar, A. & Wani, A. Evaluation of bio-control agents against Rhizoctonia solani Kuhn and sheath blight disease of rice under temperate ecology. Plant Dis. Res. 24, 15–18 (2009).
Singh, S., Balodi, R., Meena, P. N. & Singhal, S. Biocontrol activity of Trichoderma harzianum, Bacillus subtilis and Pseudomonas fluorescens against Meloidogyne incognita, Fusarium oxysporum and Rhizoctonia solani. Indian Phytopathol. 74, 703–714. https://doi.org/10.1007/s42360-021-00368-6 (2021).
Abbas, A. et al. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. C. R. Biol. 342, 124–135. https://doi.org/10.1016/j.crvi.2019.05.002 (2019).
Abbas, A., Jiang, D. & Fu, Y. Trichoderma spp. as antagonist of Rhizoctonia solani. J. Plant Pathol. Microbiol. 8, 1–9 (2017).
Manikandan, A. et al. Gamma-induced mutants of Bacillus and Streptomyces display enhanced antagonistic activities and suppression of the root rot and wilt diseases in pulses. Biomol. Concepts 13, 103–118. https://doi.org/10.1515/bmc-2022-0004 (2022).
Nowocień, K. & Sokołowska, B. Bacillus spp. as a new direction in biocontrol and deodorization of organic fertilizers. AIMS Environ. Sci. 9, 95–105. https://doi.org/10.3934/environsci.2022007 (2022).
Putri, B., Santoso, I. & Yasman, Y. In AIP Conference Proceedings 050017 (AIP Publishing LLC).
Abdel-Rahman, T. F. M. Evaluation of the efficiency of some bio-fertilizers and different silicon sources for controlling bulb rot of Lilium Spp. In Egypt. J. Adv. Agric. Res. 26, 454–465. https://doi.org/10.21608/jalexu.2022.115736.1041 (2021).
Sun, S. et al. Use of lentinan and fluopimomide to control cotton seedling damping-off disease caused by Rhizoctonia solani. Agriculture 12, 75. https://doi.org/10.3390/agriculture12010075 (2022).
El-Kazzaz, M. K. et al. Suppression of pepper root rot and wilt diseases caused by Rhizoctonia solani and Fusarium oxysporum. Life 12, 587. https://doi.org/10.3390/life12040587 (2022).
Booth, C. The genus Fusarium. Kew, UK, Commonwealth Mycological Institute, pp 237, 1971.
Barnett, H. & Hunter, B. Illustrated Genera of Imperfect Fungi 4th edn. (The American Phytopathological Society, 1998).
Singh, V., Kumar, S., Lal, M. & Hooda, K. Cultural and morphological variability among Rhizoctonia solani isolates from trans-gangetic plains of India. Res. Crops 15, 644–650. https://doi.org/10.5958/2348-7542.2014.01390.4 (2014).
Yadav, A. K., Kumari, A. & Anwar, A. Management of sheath blight of rice (Oryza sativa) under in-vitro condition with indigenous Trichoderma spp. J. Pharmacogn. Phytochem. 8, 1763–1771 (2019).
Lahuf, A. A. First report of causing stem and root rot on lucky bamboo (Dracaena braunii) in Iraq. Hell. Plant Prot. J. 12, 1–5. https://doi.org/10.2478/hppj-2019-0001 (2019).
Moore, D., Robson, G. & Trinci, A. 21st Century Guidebook to Fungi 640 (Cambridge University Press, 2011).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18, 315–322 (1990).
Abdel-Rahman, T. F. Evaluation activity of some antimicrobial agents in reduction microbial load and their impact on vase life of Asparagus aethiopicus L. Egypt. J. Phytopathol. 47, 157–178. https://doi.org/10.21608/ejp.2019.160902 (2019).
Hafez, Y. M. et al. Exogenous application of Bacillus subtilis and H2O2 mitigated fire pear blight bacterial dis-ease incidence in correltaed with yield and fruit quality improvement. Fresenius Environ. Bull. 07A, 6315–6327 (2020).
Garrity, G. M., Brenner, D. J., Krieg, N., Staley, J. & Bergey's Manual. Systematic bacteriology. In The Proteobacteria, Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria, Bergey’s Manual Trust, Department of Microbiology and Molecular Genetics, Volume Two: The Proteobacteria, Part A Introductory Essays, Springer New York, NY, pp. XXVI, 304, https://doi.org/10.1007/0-387-28021-9 (2005).
Benson, H. J. Microbiological Applications; A Laboratory Manual in General Microbiology 8th edn, 478 (McGraw Hill, 2002).
Shahid, I. et al. Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front. Sustain. Food Syst. 5, 605195. https://doi.org/10.3389/fsufs.2021.605195 (2021).
Mount, D. W. Maximum parsimony method for phylogenetic prediction. Cold Spring Harbor Protoc. 2008, pdb. top32. https://doi.org/10.1101/pdb.top43 (2008).
Molloy, D. In California Conference on Biological Control III, University of California at Davis, USA, 15–16 August, 2002 86–94 (Center for Biological Control, College of Natural Resources, University of …).
Morandi, M. A. B., Sutton, J. C. & Maffia, L. A. Effects of host and microbial factors on development of Clonostachys rosea and control of Botrytis cinerea in rose. Eur. J. Plant Pathol. 106, 439–448. https://doi.org/10.1023/A:1008738513748 (2000).
Shen, N., Li, S., Li, S., Zhang, H. & Jiang, M. The siderophore-producing bacterium, Bacillus siamensis Gxun-6, has an antifungal activity against Fusarium oxysporum and promotes the growth of banana. Egypt. J. Biol. Pest Control 32, 34. https://doi.org/10.1186/s41938-022-00533-7 (2022).
Sowndhararajan, K., Marimuthu, S. & Manian, S. Biocontrol potential of phylloplane bacterium Ochrobactrum anthropi BMO-111 against blister blight disease of tea. J. Appl. Microbiol. 114, 209–218. https://doi.org/10.1111/jam.12026 (2013).
Rosli, N. M., Ashari, K. I. A. H. & Azmi, N. S. A. Isolation and preliminary screening of endophytic fungi from Ficus carica for biocontrol and phosphate solubilization. Environ. Ecosyst. Sci. 4, 77–84. https://doi.org/10.26480/ees.02.2020.77.84 (2020).
Rios Velasco, C. et al. Identification and antagonistic activity in vitro of Bacillus spp. and Trichoderma spp. isolates against common phytopathogenic fungi. Rev. Mex. Fitopatol. 34, 85–99. https://doi.org/10.18781/R.MEX.FIT.1507-1 (2016).
Zian, A. H., El-Gendy, H. M. & Shehata, H. S. Enhancing biocontrol agents by hydroquinone and salicylic acid for controlling root-rot and wilt diseases of lupine. Egypt. J. Phytopathol. 47, 97–120. https://doi.org/10.21608/ejp.2019.120017 (2019).
Khalil, M. & Ashmawy, E. Induction of systemic disease resistance in cucumber plants treated by the spray of some biotic and abiotic agents to control downy mildew disease. Egypt. J. Agric. Res. 97, 19–37. https://doi.org/10.21608/ejar.2019.68547 (2019).
Ridgman, W. J. Statistical methods, 8th edn, by G. W. Snedecor & W. G. Cochran. xx + 503 pp. Ames: Iowa State University Press (1989). ISBN 0 8138 1561 6. J. Agric. Sci. 115, 153–153. https://doi.org/10.1017/S0021859600074104 (1990).
Aslam, J., Mujib, A. & Sharma, M. P. In vitro micropropagation of Dracaena sanderiana Sander ex Mast: An important indoor ornamental plant. Saudi J. Biol. Sci. 20, 63–68. https://doi.org/10.1016/j.sjbs.2012.11.005 (2013).
Kakuei, F. & Salehi, H. Factors affecting in vitro propagation of Dracaena sanderiana Sander ex Mast. cultivars. II. MS salt strengths, subculturing times, rooting and acclimatization. Adv. Hortic. Sci. 29, 165–170 (2015).
Abdel-Wahed, G. A. Management of root rot, damping-off and wilt diseases of verbascum (Verbascum thapsus L.) using nanometal particles and fungicides. Egypt. J. Phytopathol. 46, 157–178. https://doi.org/10.21608/ejp.2018.115754 (2018).
Kesimci, T., Durak, E. & Demirci, E. Rhizoctonia species from strawberry plants in Erzincan, Turkey: Anastomosis groups and pathogenicity. J. Anim. Plant Sci. 32, 721–728. https://doi.org/10.36899/japs.2022.3.0473 (2022).
Salem, M. Z. M., Behiry, S. I. & El-Hefny, M. Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-hexane extracts of three plant species as a wood-treated oil fungicide. J. Appl. Microbiol. 126, 1683–1699. https://doi.org/10.1111/jam.14256 (2019).
Behiry, S. I. et al. Antifungal and antibacterial activities of Musa paradisiaca L. peel extract: HPLC analysis of phenolic and flavonoid contents. Processes 7, 215. https://doi.org/10.3390/pr7040215 (2019).
El-Hefny, M., Salem, M. Z., Behiry, S. I. & Ali, H. M. The potential antibacterial and antifungal activities of wood treated with Withania somnifera fruit extract, and the phenolic, caffeine, and flavonoid composition of the extract according to HPLC. Processes 8, 113. https://doi.org/10.3390/pr8010113 (2020).
Elkahoui, S. et al. Antifungal activity of volatile compounds-producing Pseudomonas P2 strain against Rhizoctonia solani. World J. Microbiol. Biotechnol. 31, 175–185. https://doi.org/10.1007/s11274-014-1772-3 (2015).
Salem, M. Z. M., Ali, H. M. & Akrami, M. Moringa oleifera seeds-removed ripened pods as alternative for papersheet production: Antimicrobial activity and their phytoconstituents profile using HPLC. Sci. Rep. 11, 19027. https://doi.org/10.1038/s41598-021-98415-9 (2021).
Boccardo, N. A. et al. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 9, 2791. https://doi.org/10.1038/s41598-019-39568-6 (2019).
Souza, B. & Marucci, R. C. Biological control in ornamental plants: from basic to applied knowledge. Ornam. Hortic. 27, 255–267. https://doi.org/10.1590/2447-536X.v27i2.2365 (2021).
Sipahutar, M. K. & Vangnai, A. S. Role of plant growth-promoting Ochrobactrum sp. MC22 on triclocarban degradation and toxicity mitigation to legume plants. J. Hazard. Mater. 329, 38–48. https://doi.org/10.1016/j.jhazmat.2017.01.020 (2017).
Wang, D. et al. A newly isolated Bacillus siamensis SB1001 for mass production of poly-γ-glutamic acid. Process Biochem. 92, 164–173. https://doi.org/10.1016/j.procbio.2019.11.034 (2020).
Zang, C. et al. The biological control of the grapevine downy mildew disease using Ochrobactrum sp. Plant Prot. Sci. 56, 52–61. https://doi.org/10.17221/87/2019-PPS (2020).
Xie, Z. et al. Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biol. Control 154, 104508. https://doi.org/10.1016/j.biocontrol.2020.104508 (2021).
Fatema, U., Broberg, A., Jensen, D. F., Karlsson, M. & Dubey, M. Functional analysis of polyketide synthase genes in the biocontrol fungus Clonostachys rosea. Sci. Rep. 8, 15009. https://doi.org/10.1038/s41598-018-33391-1 (2018).
Sun, Z. B. et al. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 129, 486–495. https://doi.org/10.1111/jam.14625 (2020).
Kamou, N. N., Cazorla, F., Kandylas, G. & Lagopodi, A. L. Induction of defense-related genes in tomato plants after treatments with the biocontrol agents Pseudomonas chlororaphis ToZa7 and Clonostachys rosea IK726. Arch. Microbiol. 202, 257–267. https://doi.org/10.1007/s00203-019-01739-4 (2020).
Köhl, J. & Ravensberg, W. Clonostachys rosea to Control Plant Diseases. Microbial Bioprotectants for Plant Disease Management (Burleigh Dodds Science Publishing, 2021).
Chaiharn, M., Chunhaleuchanon, S. & Lumyong, S. Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J. Microbiol. Biotechnol. 25, 1919–1928. https://doi.org/10.1007/s11274-009-0090-7 (2009).
Abada, E. A., El-Hendawy, H. H., Osman, M. E. & Hafez, M. A. Antimicrobial activity of Bacillus circulans isolated from rhizosphere of Medicago sativa. Life Sci. J. 8, 711–719 (2014).
Miljaković, D., Marinković, J. & Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8, 1037. https://doi.org/10.3390/microorganisms8071037 (2020).
Acknowledgements
We would like our appreciation to the scientific cooperation among Ornamental, Medicinal & Aromatic Plants Dis. Res. Dept., Plant Pathology Research Institute, Agricultural Research Center, Giza, Egypt; Department of Plant Protection, Faculty of Agriculture, (Saba Basha), Alexandria University, Egypt, and Forestry and Wood Technology Department, Faculty of Agriculture (El-Shatby), Alexandria University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
T.F.M.A.-R. wrote the main manuscript text, T.F.M.A.-R., A.A.-M., and M.Z.M.S. prepared figures, T.F.M.A.-R. carried out the methodology, T.F.M.A.-R., A.A.-M., and M.Z.M.S. investigated the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Rahman, T.F.M., Abdel-Megeed, A. & Salem, M.Z.M. Characterization and control of Rhizoctonia solani affecting lucky bamboo (Dracaena sanderiana hort. ex. Mast.) using some bioagents. Sci Rep 13, 6691 (2023). https://doi.org/10.1038/s41598-023-33628-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33628-8
- Springer Nature Limited