Abstract
To establish the impact of COVID-19 on the pre-test probability for VTE in patients with suspected VTE. This was a retrospective, observational, cross-sectional study of patients 18 years and older undergoing diagnostic tests for VTE in an integrated healthcare system covering a population of 465,000 during the calendar year of 2020. We adjusted for risk factors such as age, sex, previous VTE, ongoing anticoagulant treatment, malignancy, Charlson score, ward care, ICU care and wave of COVID-19. In total, 303 of 5041 patients had a positive diagnosis of COVID-19 around the time of investigation. The prevalence of VTE in COVID-positive patients was 10.2% (36/354), 14.7% (473/3219) in COVID-19 negative patients, and 15.6% (399/2589) in patients without a COVID-19 test. A COVID-positive status was not associated with an increased risk for VTE (crude odds ratio 0.64, 95% CI 0.45–0.91, adjusted odds ratio 0.46, 95%CI 0.19–1.16). We found no increased VTE risk in COVID-positive patients. This indicates that COVID-19 status should not influence VTE workup.
The study was pre-registered on May 26, 2020 at ClinicalTrials.gov with identifier NCT04400877.
Similar content being viewed by others
Introduction
Background
In the ongoing COVID-19 pandemic reports have shown an increased risk of VTE, including both pulmonary embolism (PE) and deep venous thrombosis (DVT)1,2,3,4,5 and international guidelines recommend prophylactic anticoagulation for all hospitalized patients with COVID-196. The majority of the initial reports on VTE in COVID-19 have been carried out in the intensive care unit (ICU) and show a prevalence of VTE of 20–30%1,2,3. This is higher compared to cohorts of non-selected ICU patients, in whom the prevalence of VTE is closer to 10%7,8,9,10. However, studies on ICU patients with severe sepsis and viral infections like H1N1 influenza have shown a prevalence of VTE of 37% and 44%, respectively11,12. The prevalence of VTE in hospitalized non-ICU patients with COVID-19 is 3–4%13,14,15, similar to studies on internal medicine patients with prophylactic anticoagulation16. However the VTE risk and prevalence in outpatients with known or suspected COVID-19, is less studied17.
In a recent large nationwide cohort study from Sweden, Katsoularis et al.18 found a relative risk increase for VTE in patients with COVID-19. However the study only adjusted for chronic medical conditions and not for other concomitant acute illnesses. Since the inclusion was any type of contact with the Swedish health care system it is likely that the COVID-positive was a select group with an acute upper respiratory illness which is a known risk factor for VTE16,19.
In isolated outpatient cohorts, no increase in the risk of VTE has been found for patients with COVID-19. Freund et al., studied PE prevalence in patients undergoing computed tomography pulmonary angiography (CTPA) in 6 Emergency Departments (ED) in Europe and found no increased prevalence, or risk, of VTE in patients with COVID-19. Similarly, Thoppil et al.20 found no increased risk of VTE for COVID-positive patients in a retrospective observational trial of 27,051 patients in the United States. We have previously investigated the prevalence and risk of VTE in patients with and without COVID-19 in a regional healthcare system in Sweden during the first wave of the pandemic (March through May 2020). In that period, we found no significant increase in prevalence or risk for VTE but the number of COVID-positive patients was low and a larger cohort is needed to validate the results21.
Due to the alarming reports of high prevalence of VTE during the beginning of the pandemic, most patients with COVID-19 will be considered for potential VTE as outpatients in the ED, regardless whether they are admitted to hospital or discharged home. Hence an informed risk assessment is imperative to perform a reasonable workup while limiting the risk of overuse of health care resources on a system level.
Goals of this investigation
To investigate if COVID-19 was associated with an increased risk of VTE in patients undergoing testing for VTE in a regional healthcare system in Sweden during the calendar year of 2020.
Methods
Study design and setting
In this retrospective observational study we have evaluated the risk and prevalence of VTE during the calendar year of 2020 in the county of Östergötland (Region Östergötland, Sweden). The county has a population of 465,000 (December 31, 2019) and healthcare is provided by a central, publicly funded healthcare system. There is one rural community hospital, one urban community hospital and one academic tertiary care hospital. All hospitals, outpatient clinics and primary care centers in the county use the same electronic health records (EHRs) and all diagnostic studies of VTE are performed within the healthcare system.
Selection of participants
Adult patients (≥ 18 years of age) who underwent a diagnostic test for suspected VTE during the calendar year 2020 were included. Follow up investigations, tests performed on referred patients from another healthcare system, and planned but non-performed tests were excluded.
Exposures
The exposure was COVID-19 infection, defined as a positive PCR test up to 14 days prior to or 7 days after the diagnostic test for VTE. This timeframe was chosen a priori to account for the delay from symptom onset to deterioration22 and delay to PCR test in the beginning of the pandemic. All patients with at least one diagnostic test for VTE during 2020 were matched with the regional SARS-CoV-2 database of real time PCR results. PCR was the diagnostic criteria for COVID-19 used in our system and patients with high probability for COVID-19 despite a negative PCR were tested repeatedly. PCR data was extracted from the healthcare system’s central diagnostic laboratory, the only authorized SARS-CoV-2 laboratory during this period.
Measurements
Additional known risk factors for VTE were extracted from the EHR. Subgroup analysis of outpatients and patients in the ward or ICU based on date of investigation was performed to distinguish prevalence of VTE from COVID-19 infection, and from severe disease7,11. In-hospital care on a ward or in the ICU was defined as a minimum of 24 h of care, as tests related to the ED presentation may be deferred up to 24 h. Anticoagulant treatment was defined as treatment with any B01A class drug23 more than seven days prior to the diagnostic test. Risk factors for VTE were; age (continuous), sex (male/female), previous VTE (yes/no), malignancy (yes/no), ward care (yes/no), intensive care (yes/no) and Charlson score (continuous) were extracted from the EHR. The Charlson score was calculated from previous diagnosis registered in the EHR, which has partial coverage of diagnoses prior to 2008 and full coverage thereafter. Mortality at 30 days was defined as all-cause mortality based on the Swedish national civil registration registry.
Outcomes
The outcome was a diagnosis of VTE by CTPA or ultrasound. Written study reports were extracted from the picture archiving and communication system for all CTPA and ultrasound for deep venous thrombosis (DVT). Findings of PE were coded as positive for any contrast defect in a subsegmental, or more central pulmonary artery. Any additional finding classified as definitive or probable PE by the attending radiologist was coded as PE positive. DVT was diagnosed with complete compression ultrasound or 3-point compression ultrasound of the leg24. Isolated muscle vein thrombosis and thrombophlebitis were classified as negative examinations. Patients with multiple tests of the same modality, on the same day were classified as duplicates and combined to a single test. The reports were classified as positive or negative independently by JW, JA and MJ. Ambiguous reports and differences in classification were reviewed by JW, MJ and PBN and solved through full consensus. A subset of diagnostic tests were done in the department of clinical physiology and were already classified as positive or negative.
Analysis
Descriptive data was reported as percentage, mean with standard deviation (SD) or median with interquartile ranges (IQR). Prevalence was reported by separate diagnoses, e.g. the prevalence of PE was CTPA positive tests compared to all performed CTPA. Prevalence was compared with the chi-squared test with pre-defined subgroup analysis for PE and non-PE VTE, mainly DVT or Fisher’s exact test for small groups. Patients with no RT-PCR test results in 2020 were treated as a separate subgroup to avoid bias based on test availability. Differences in distributions of PE were assessed with the Kruskal–Wallis test. Logistic regression was used to analyze the crude and adjusted odd ratios (OR) for a VTE by COVID-19 status and to account for the different waves of COVID-19 during 2020.
A sample size calculation with the Chi-square goodness of fit test for two categorical variables with a conservative effect size of 0.1 (alpha 0.05, power 0.8) required 785 samples. Based on an expected test rate of 500 diagnostic studies per month, three months of data was included in our previous study21. No additional power calculation was made for this study. Data was imported into Pandas (v 0.23)25 and analyzed with Python using the Scipy library (v 1.17)26 and Statsmodels library (v. 0.12)27.
Ethical considerations
This study was carried out in accordance with The Declaration of Helsinki28. Ethical approval was granted by the Swedish Ethical Review Authority with permit reference 2020-02701. Informed consent was waived by the review authority. The study has been conducted according to the STROBE guidelines for reporting observational trials.
Results
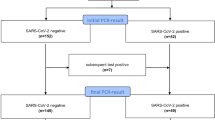
During the calendar year of 2020, 5401 patients were investigated for possible VTE on 6169 occasions in the Region Östergötland health care system of which 303 had a positive diagnosis of COVID-19 based on laboratory PCR data on 354 occasions. The COVID-positive patients were more often male, admitted to hospital or ICU and were more likely to receive invasive ventilation or die (Table 1). The prevalence of previous malignancy and treatment with anticoagulants were lower in the COVID-positive group while a diagnosis of previous VTE was higher. There were more investigations done for PE in 2020 compared to the five previous years (3425 vs mean 2662).
The prevalence of PE was lower in COVID-positive patients compared to negative or untested patients (p = 0.02), and lower but not statistically significant (p = 0.32) in patients with DVT (Table 2). There was no difference in distributions of thrombosis within the pulmonary arteries between negative and untested patients, and COVID-positive patients (p = 0.1) (Table 2).
The prevalence of VTE was higher in patients with COVID-19 treated in the ICU compared to COVID-negative or untested patients but not statistically significant (p < 0.14). In contrast, it was lower in both the ward and outpatient cohort although the association was only statistically significant for the outpatient group (p = 0.02). Further dividing the outpatient group to patients being admitted to the ICU or ward within 24 h from outpatient VTE testing, the prevalence of VTE was lower in the COVID-19 group compared to the negative and untested (Table 3).
The unadjusted OR for a positive diagnostic test of VTE when positive for COVID-19 was 0.64 (95%CI 0.45–0.91). When adjusting for potential confounders the OR was further decreased to 0.46 (95%CI 0.19–1.16). Previous VTE was the only factor which significantly increased the risk for VTE (OR 4.58, 95%CI 2.76–7.62) while age (OR 1.01, 95%CI 0.99–1.03) and ICU (OR 1.10, 95%CI 0.39–3.09) had a non-significant association. Ongoing anticoagulation treatment significantly reduced the risk for VTE with an OR of 0.55 (95%CI 0.43–0.71).
When adjusting for COVID-19 wave as adjudicated by the Swedish Ministry for Health and Welfare, the first wave (2020-03-01 to 2020-09-30) and second wave (2020-10-01 to 2020-12-31) of the pandemic had non-significant increases of risk for VTE with ORs of 1.02 (95%CI, 0.5–2.1) and 1.09 (95%CI, 0.5–2.4) respectively. The number of investigations for VTE had a nadir in the beginning of the pandemic and increased to similar pre-pandemic levels during the first wave and was higher during the second wave (Fig. 1).
Limitations
This was a retrospective observational study and the results are limited to the variables we were able to control for. We used commonly accepted risk factors for PE and DVT defined by Wells et al.29,30 but were not able to obtain physiologic data or referring physician’s assessment at the time of the diagnostic test to adjust for all criteria in the conventional diagnostic tools30,31. However, we believe that we have been able to adjust for the majority of confounders for VTE when evaluating infection with SARS-CoV-2 as a possible risk.
Only patients who had a diagnostic test for possible VTE were included in this study while we did not include patients ruled out by other means, like d-dimer, hence we cannot calculate the prevalence for all patients considered for VTE. However there was no increased prevalence of VTE in the untested cohort except for patients in intensive care. If there was a true increase of VTE from COVID-19, irrespective of other risk factors, we would expect to see an increase in VTE as the incidence of COVID-19 was rising in the community.
SARS-CoV-2 status was missing from a large proportion of patients undergoing testing for VTE in 2020 which limits the generalisability of the results. We only had the ability to adjudicate the patients’ COVID-19 status based on PCR testing, which was the accepted method in our healthcare system. The incidence of investigations with COVID-19 status unknown was fairly constant during the pandemic (Fig. 1) which likely limits systematic bias of the results.
We may have missed a few PEs by not including chest CT or scintigraphy. However, most findings associated with PE on chest CT would likely warrant a definitive workup including CTPA in our health care system as CT chest is not considered diagnostic for VTE. Scintigraphy is rarely performed in our system and we believe that this would not influence the results. We based the classification of PE on the radiology report and not an independent read of the image data. While this may have introduced a subjective interpretation, it reflects the actual practice in Sweden where treating physicians rely on the radiology report for the diagnosis of PE.
Discussion
An infection with SARS-CoV-2 virus confirmed on RT-PCR was not associated with an increased risk, or prevalence of VTE in patients undergoing a diagnostic test for VTE in a large integrated healthcare system in Sweden. This study confirms our findings from the first three months of the SARS-CoV-2 pandemic21 and concurs with the results from a large retrospective observational study from the US by Thoppil et al.20 which compared the prevalence and risk of VTE in ambulatory ED patients with and without COVID-19. Additionally Freund et al.32 found no association between confirmed diagnosis of COVID-19 and a PE in 3253 patients undergoing CTPA for suspected PE in the ED. However there was a high prevalence of VTE in patients with confirmed COVID-19 in the ICU in our study, similar to previous reports1,2,3, and similar to previously established levels of VTE in ICU patients with severe infections caused by other pathogens11,12.
Studies have attributed the reportedly high VTE rates in COVID-19 to conventional VTE, as well as in-situ immunothrombosis33. If the VTE rate in COVID-19 were driven by immunothrombosis in other than the most severe cases, however, we would expect an increased proportion of patients with peripheral clots in segmental or subsegmental pulmonary arteries34. This was not the case in our study, where the distribution of pulmonary embolisms was similar in both groups (Table 3). This further underlines the fact that, rather than focusing on COVID-19 as an independent risk factor for VTE, we should instead consider disease severity which has a well-established, positive correlation to pre-test probability of a VTE.
In a large nationwide study in Sweden, Katsoularis et al.18 found an increased relative and absolute risk of VTE in a cohort of patients with SARS-CoV-2 positive PCR tests compared to an undifferentiated cohort of patients with any type of contact with the Swedish healthcare system. It is reasonable that an upper respiratory virus with the potential to cause severe pneumonia will cause a small increase in baseline risk compared with any, or potentially no acute illness, as both acute illness and severity of acute illness is associated with increased prevalence of VTE7,8,9,11. However, this does not necessarily need to increase the predictive value of an airway infection per se in a more select population of patients, like patients deemed at risk for VTE. Hence the results by Katsoularis et al., do not contradict the results of this study or previous studies performed in the ED20,32.
There was an increase in the number of investigations of pulmonary embolism compared to the previous years in our healthcare system (3425 vs mean 2662). Together with the decreased OR for COVID-19 this may indicate overtesting for pulmonary embolism in patients with COVID-19. Computer tomography has been suggested as a modality to risk stratify patients with COVID-19 and providers may have deemed a CTPA convenient to risk-stratify and workup the patients for potential PE. With scarce but concerning data regarding VTE in COVID-19 during the beginning of the pandemic it was arguably reasonable to have a high degree of suspicion and a low threshold for VTE workup, which is likely reflected in high numbers of investigation. However, with accumulating data failing to show an increased risk of VTE in patients with COVID-19 investigated for VTE20,21,32, we question whether the increase in radiation and the added risk of contrast use is justified onwards.
In summary, testing positive for COVID-19 did not increase the OR for VTE compared to a negative test when adjusting for known risk factors and ongoing treatment with anticoagulation. This indicates that patients with COVID-19 being investigated for VTE may be risk-stratified using conventional tools with consideration for disease severity1,2,3. On a system-level, our results indicate a clear need for continuous feedback on the prevalence of certain conditions, such as VTEs, so that the treating physician can make informed decisions when planning the workup of patients. Although a few additional radiological examinations may not seem like much to the individual practitioner, widespread increases in certain diagnostic modalities put significant strain on a healthcare system and potentially cause displacement effects for other patient groups, as well as substantial cost increases.
Data availability
Abstracted data is available from the corresponding author on reasonable request.
References
Klok, F. A. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. https://doi.org/10.1016/j.thromres.2020.04.013 (2020).
Cui, S., Chen, S., Li, X., Liu, S. & Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. https://doi.org/10.1111/jth.14830 (2020).
Helms, J. et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. https://doi.org/10.1007/s00134-020-06062-x (2020).
Leonard-Lorant, I. et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020, 201561. https://doi.org/10.1148/radiol.2020201561 (2020).
Poissy, J. et al. Pulmonary embolism in COVID-19 patients: Awareness of an increased prevalence. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.047430 (2020).
Thachil, J. et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 18, 1023–1026 (2020).
Muscedere, J. G., Heyland, D. K. & Cook, D. Venous thromboembolism in critical illness in a community intensive care unit. J. Crit. Care 22, 285–289 (2007).
Cook, D. et al. Deep venous thrombosis in medical-surgical critically ill patients: Prevalence, incidence, and risk factors. Crit. Care Med. 33, 1565–1571 (2005).
Cook, D. et al. Venous thromboembolic disease: An observational study in medical-surgical intensive care unit patients. J. Crit. Care 15, 127–132 (2000).
PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group et al. Dalteparin versus unfractionated heparin in critically ill patients. N. Engl. J. Med. 364, 1305–1314 (2011).
Kaplan, D. et al. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest 148, 1224–1230 (2015).
Obi, A. T. et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J. Vasc. Surg. Venous Lymphat. Disord. 7, 317–324 (2019).
Lodigiani, C. et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy. Thromb. Res. 191, 9–14 (2020).
Middeldorp, S. et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. https://doi.org/10.1111/jth.14888 (2020).
Criel, M. et al. Venous thromboembolism in SARS-CoV-2 patients: Only a problem in ventilated ICU patients, or is there more to it?. Eur. Respir. J. https://doi.org/10.1183/13993003.01201-2020 (2020).
Samama, M. M. et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin Study Group. N. Engl. J. Med. 341, 793–800 (1999).
Jiménez, D. et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Chest https://doi.org/10.1016/j.chest.2020.11.005 (2020).
Katsoularis, I. et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. BMJ 377, e069590 (2022).
Clayton, T. C., Gaskin, M. & Meade, T. W. Recent respiratory infection and risk of venous thromboembolism: Case-control study through a general practice database. Int. J. Epidemiol. 40, 819–827 (2011).
Thoppil, J. J. et al. SARS-CoV-2 positivity in ambulatory symptomatic patients is not associated with increased venous or arterial thrombotic events in the subsequent 30 days. J. Emerg. Med. https://doi.org/10.1016/j.jemermed.2021.12.020 (2022).
Wretborn, J., Jörg, M., Benjaminsson Nyberg, P. & Wilhelms, D. B. Risk of venous thromboembolism in a Swedish healthcare system during the COVID-19 pandemic: A retrospective cross-sectional study. J. Am. Coll. Emerg. Phys. Open 2, e12530 (2021).
Chen, J. et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 80, e1–e6 (2020).
Norwegian Institute of Public Health. WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment 2023. 2022; Available from: https://www.whocc.no/filearchive/publications/2023_guidelines_web.pdf.
Laurence, N. et al. Ultrasound for lower extremity deep venous thrombosis. Circulation 137, 1505–1515 (2018).
McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference (eds van der Walt, S. & Millman, J.) vol. 445 51–56 (2010).
Millman, K. J. & Aivazis, M. Python for scientists and engineers. Comput. Sci. Eng. 13, 9–12 (2011).
Seabold, S. & Perktold, J. Statsmodels: Econometric and statistical modeling with python. In 9th Python in Science Conference (eds van der Walt, S. & Millman, J.) 92–96 (SciPy.org, 2010).
World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310, 2191–2194 (2013).
Wells, P. S. et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 349, 1227–1235 (2003).
Wolf, S. J., McCubbin, T. R., Feldhaus, K. M., Faragher, J. P. & Adcock, D. M. Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism. Ann. Emerg. Med. 44, 503–510 (2004).
Kline, J. A. et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J. Thromb. Haemost. 6, 772–780 (2008).
Freund, Y. et al. Association between pulmonary embolism and COVID-19 in ED patients undergoing CTPA: The PEPCOV international retrospective study. Acad. Emerg. Med. https://doi.org/10.1111/acem.14096 (2020).
Loo, J., Spittle, D. A. & Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 76, 412–420 (2021).
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18, 666–682 (2021).
Acknowledgements
The authors would also like to acknowledge Charlotte Brage, Department of Radiology, and Christina Svensson, Department of Clinical physiology, both at Region Östergötland, for their help in extracting diagnostic data. We would also like to acknowledge Johan Andreasson for help with data abstraction.
Funding
Open access funding provided by Linköping University. This work was supported by two public grants from Region Östergötland to author DBW (LIO-532001 and LIO-700271). The funding body had no role in the design, data collection, analysis or writing of this study.
Author information
Authors and Affiliations
Contributions
J. W., M. J., P. B. J. and D. B. W. conceived and designed the study. D. B. W. obtained funding and supervised the study. J. W. and M. J. collected the data. J. W., M. J. and P. B. J. abstracted study reports. J. W. analysed the data and performed statistical analysis. J. W. drafted the manuscript and all authors contributed substantially to its revision. J. W. takes responsibility for the paper as a whole.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wretborn, J., Jörg, M., Benjaminsson Nyberg, P. et al. Risk of venous thromboembolism in patients with COVID-19 during 2020; a retrospective cross-sectional study in a Swedish health care system. Sci Rep 13, 5469 (2023). https://doi.org/10.1038/s41598-023-32637-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32637-x
- Springer Nature Limited