Abstract
The worldwide inequitable access to vaccination claims for a re-assessment of policies that could minimize the COVID-19 burden in low-income countries. Nine months after the launch of the national vaccination program in March 2021, only 3.4% of the Ethiopian population received two doses of COVID-19 vaccine. We used a SARS-CoV-2 transmission model to estimate the level of immunity accrued before the launch of vaccination in the Southwest Shewa Zone (SWSZ) and to evaluate the impact of alternative age priority vaccination targets in a context of limited vaccine supply. The model was informed with available epidemiological evidence and detailed contact data collected across different geographical settings (urban, rural, or remote). We found that, during the first year of the pandemic, the mean proportion of critical cases occurred in SWSZ attributable to infectors under 30 years of age would range between 24.9 and 48.0%, depending on the geographical setting. During the Delta wave, the contribution of this age group in causing critical cases was estimated to increase on average to 66.7–70.6%. Our findings suggest that, when considering the vaccine product available at the time (ChAdOx1 nCoV-19; 65% efficacy against infection after 2 doses), prioritizing the elderly for vaccination remained the best strategy to minimize the disease burden caused by Delta, irrespectively of the number of available doses. Vaccination of all individuals aged ≥ 50 years would have averted 40 (95%PI: 18–60), 90 (95%PI: 61–111), and 62 (95%PI: 21–108) critical cases per 100,000 residents in urban, rural, and remote areas, respectively. Vaccination of all individuals aged ≥ 30 years would have averted an average of 86–152 critical cases per 100,000 individuals, depending on the setting considered. Despite infections among children and young adults likely caused 70% of critical cases during the Delta wave in SWSZ, most vulnerable ages should remain a key priority target for vaccination against COVID-19.
Similar content being viewed by others
Introduction
Two years into the pandemic, the reported burden of the coronavirus disease 2019 (COVID-19) has been relatively low throughout Africa as compared to high-income countries1,2. In Africa, approximately 40% of people are aged less than 15 years, compared to a global mean of 25%3, and severe outcomes of COVID-19 are strongly associated with age4,5,6. However, the impact of COVID-19 in low-income countries may have been vastly underestimated due to lacking testing capacity7,8,9. For instance, a recent post-mortem study in Zambia revealed that, contrary to expectations, deaths possibly ascribable to COVID-19 were common among patients of a referral hospital, with about 20% deceased individuals resulting infected with SARS-CoV-2 compared to less than 10% tested before death10.
The identification of appropriate strategies to minimize COVID-19 burden in sub-Saharan settings remains an open challenge. Unprecedented social distancing measures have been applied worldwide to mitigate the COVID-19 pandemic11,12,13,14,15. However, the implementation of drastic restrictions for long time periods would have disproportionate effects on the already vulnerable economies of low-income countries11,13,14. Mass immunization programs still represent the main public health strategy to reduce COVID-19 burden. While high-income countries have rapidly progressed in the deployment of multiple vaccine doses, at the end of 2021, only 15% of the total African population was vaccinated with at least one dose2.
Ethiopia represents an illustrative case study for the limited access to vaccination experienced by sub-Saharan countries during 2021. In this country, the national vaccination campaign was launched on March 13, 202116, prioritizing healthcare workers at first, and then the elderly and patients with chronic diseases17. On November 16, 2021, the vaccination campaign was expanded to all individuals aged 12 years or more. By the end of 2021, Ethiopia had received a total of 14.6 million doses18, a vaccine supply that would suffice for covering at most 6% of the country’s population with the recommended two-doses schedule. However, only 3.4% of the citizens were fully vaccinated by the end of 202119 and even at the end of 2022, only 34% of the population had completed a primary vaccination course20. Besides the low availability of vaccines, the high vaccine hesitancy found among both healthcare workers21,22,23,24 and the general community25,26,27, in addition to logistic difficulties, likely contributed to the slow deployment of COVID-19 vaccination in Ethiopia.
In this study, we assess the potential impact of different vaccination policies in reducing the burden caused by the Delta variant of SARS-CoV-2 across different geographical settings of the Southwest Shewa Zone (SWSZ) of Ethiopia in the context of limited vaccine supply. Alternative priority targets for vaccination are evaluated by considering different scenarios regarding the available number of vaccine doses and by taking into account the immunity acquired by natural infection before the launch of the national vaccination campaign. To do this, we developed and simulated a transmission model for SARS-CoV-2 informed with data on age-specific mixing patterns recently collected across different areas of the SWSZ, characterized by heterogeneous population density, age structure, and access to primary care12. The effect of different immunization strategies is evaluated in terms of the number of infections and critical cases that could have been averted after the rollout of vaccination based on ChAdOx1 nCoV-19, representing the vaccine predominately adopted during 2021 in Ethiopia. Obtained results could be instrumental to identify the optimal strategies for the deployment of vaccines in geographical contexts characterized by an initially limited vaccine supply.
Methods
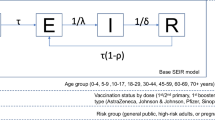
The SARS-CoV-2 transmission dynamics is simulated by using a deterministic age-structured SIR (Susceptible-Infectious-Recovered) model. Susceptibility to SARS-CoV-2 infection is assumed to vary with age according to estimates made available by Hu et al.28. Specifically, taking the age group of 20–59 years as the reference, the authors estimated the relative susceptibility for individuals aged 0–19 years at 0.59 (95%CI: 0.35–0.92) and at 1.75 (95%CI: 1.07–2.81) for the individuals aged 60 years or more. A homogeneous susceptibility across ages is explored for sensitivity analysis. An average generation time of 6.6 days and homogenous infectiousness across different ages are assumed29,30.
The adopted approach leverages on contact data collected in four districts of the SWSZ of the Oromia Region (Ethiopia), representing the main geographical area served by the St. Luke Hospital of Woliso Town, the referral hospital of the Zone12. These districts count 449,460 inhabitants, corresponding to 40.8% of the total population of the SWSZ. Age-specific contact matrices were recently estimated for three types of geographical contexts: rural villages, dispersed subsistence farming settlements, and urban neighborhoods of Woliso Town12. The model is run separately for each geographical context, assuming a constant population size over time, and accounting for the age structure characterizing the settings under study (Table 1)12.
The developed model keeps track of the contribution of infectors of different ages in causing secondary infections and critical cases across the different geographical contexts. Critical disease cases are defined as positive individuals who would either require intensive care or result in a fatal outcome. Age-specific risks of developing critical disease after SARS-CoV-2 infection are considered5.
The contribution of different ages in causing secondary infections and critical cases is explored by considering two pandemic phases. As for the first phase, lasting until the launch of the national vaccination program in March 2021, we consider the emergence of SARS-CoV-2 in a fully naïve population of individuals and analyze the epidemic dynamics under the dominance of the ancestral strain of SARS-CoV-2 and in the absence of vaccination. A school closure mandate is also assumed for the entire period as this represented a persistent restriction adopted by the government to counter the spread of COVID-19 during the first pandemic wave12,31. The spread of infection is simulated by considering an initial reproduction number R of 1.62 (95%CI: 1.55–1.70), as estimated from the exponential growth of cases reported in Ethiopia from May to mid-June 202012. This corresponds to assuming for the ancestral strain a basic reproduction number (R0) around 3, which is in line with the estimate of R0 = 2.55 provided for Ethiopia by Iyaniwura et al.32 as well as with estimates available from other countries33,34,35,36. We carried out a sensitivity analysis where we considered an R0 = 2.5532, corresponding to an R of 1.40 in the presence of school closure. The transmission dynamics during this pandemic phase is simulated until a given setting-specific proportion of the population gets infected. Such proportion is defined according to the levels of serological prevalence estimated for March 2021 in the Jimma Zone of Ethiopia: 31% in rural and remote sites and 45% in urban areas37. Different seroprevalence values are considered for sensitivity analysis to account for the uncertainty surrounding the circulation of the infection before March 2021 and the potential waning of naturally acquired immunity. Lower levels of protection may also reflect the potential ability of the circulating SARS-CoV-2 variants to escape natural immunity. The model ability in capturing the observed epidemiological patterns is assessed by comparing the age distribution of the cumulative number of simulated infections with the one associated with SARS-CoV-2 positive individuals ascertained with real-time reverse transcription polymerase chain reaction (RT-PCR) between March and September 2020 in the Oromia Region38. To assess the robustness of the estimated age distribution with respect to the assumed immunity levels, we investigate how the model performances would change considering the levels of serological prevalence estimated for the Jimma Zone in December 2020 (18% in rural and remote sites and 32% in urban areas)37.
The second pandemic phase that we consider mirrors the SARS-CoV-2 transmission dynamics after the launch of the national vaccination program in March 2021, when students were regularly receiving in-person education31. To account for the replacement of the ancestral lineages by the Delta variant of SARS-CoV-2 likely occurred in mid 202139, we calibrate the transmission rate parameter in such a way to obtain an R0 = 6 in absence of interventions and population immunity, based on published estimates40,41,42,43,44; alternative values of R0 are explored for sensitivity analysis. Model estimates of the natural immunity acquired by different age groups during the first pandemic phase are used to initialize the immunological status of the population in this second phase. We set the maximum duration of the simulations at 2 years to guarantee the modeling of the entire course of the Delta epidemics.
The impact of different vaccination strategies on the burden of COVID-19 is assessed in terms of the potential attack rate of infection and the cumulative incidence of critical cases expected after March 2021, in the absence of restrictions on the individuals’ contacts. The comparison of alternative vaccination priority groups is carried out by assuming that the considered vaccination target is achieved before the upsurge of cases caused by the emergence of the Delta variant, neglecting the transient dynamic characterizing the rollout of the vaccination.
Five illustrative scenarios are analyzed. First, we consider a scenario where the number of administered vaccines is negligible, and we evaluate the impact of pre-existing immunity levels in shaping the contribution of different ages to the disease spread. Given the low vaccine uptake recorded in Ethiopia, this scenario may reflect what might have occurred in the months following the launch of vaccination because of Delta expansion in the population. Second, we investigate the potential benefit of the vaccination campaign conducted in Ethiopia until the end of 2021, when only 3.4% of Ethiopian citizens were fully vaccinated19. Specifically, we assume that the administered doses were distributed to individuals aged 50 years or older (thereby achieving a coverage of 33% in this age group), since they represent the main initial priority target (together with healthcare workers) defined by the Ethiopian vaccination program17. In the third scenario, we still consider that a limited number of doses is available, and we compare a vaccination program targeting 100% of individuals aged 50 years or older, with an alternative scenario where the same number of vaccine doses is offered to all ages eligible for vaccination (≥ 10 years of age). Fourth, we assume that all individuals aged 50 years or more are fully vaccinated and we project the potential impact of expanding vaccination to other age groups. In this case, we compare the impact of administering the vaccine only to individuals aged 30–49 years with an alternative scenario where the same number of doses is uniformly distributed to all eligible ages (10–49 years). Different coverage levels (from 0 to 100%) among individuals aged 30–49 years are considered. Finally, to provide a comprehensive view of the potential benefits of vaccination, we consider different combinations of coverage levels attained among subjects aged 50 years or more and individuals aged between 10 and 49 years, irrespectively of the number of doses and logistic efforts required to achieve the considered targets.
In the model, vaccinated individuals are assumed to receive two doses of vaccine which significantly reduce their risk of infection and of developing severe outcomes45,46,47,48,49,50,51. Since ChAdOx1 nCoV-19 was the dominant vaccine employed in Ethiopia during 202152, the vaccine efficacy against infection and critical diseases is set at 65% and 71.5%, respectively45,48,49,50,51,53. In a sensitivity analysis, different values for the vaccine efficacy are considered to reflect the use of alternative vaccine products, the administration of only one dose of the vaccine, and a lower vaccine effectiveness against the Delta variant caused by the progressive waning of vaccine-induced protection54. The infectiousness of SARS-CoV-2 breakthrough infections (i.e., infections occurring among vaccinees) is assumed to be reduced by 50%46; equal infectiousness is considered as sensitivity analysis.
Epidemiological transitions are modeled by the following system of ordinary differential equations:
where \(a\) defines the age of the individuals, \({S}_{a}\) represents susceptible individuals of age \(a\) who have never been vaccinated, \({S}_{a}^{v}\) represents vaccinated individuals of age \(a\) who experienced a reduced force of infection, \({VE}^{inf}\) is the vaccine efficacy against the infection, \({I}_{a,\widetilde{a}}\) and \({I}_{a,\widetilde{a}}^{v}\) represent the unvaccinated and vaccinated individuals of age \(a\) infected by subjects of age \(\widetilde{a}\), \({R}_{a,\widetilde{a}}\) and \({R}_{a,\widetilde{a}}^{v}\) represent the corresponding number of individuals who recovered from these two classes, \({r}_{a}\) is the relative susceptibility in the age class \(a\), \(1/\gamma\) is the average duration of the infectivity period. Finally, \({\lambda }_{a,\widetilde{a}}\) represents the contribution of age \(\widetilde{a}\) to the force of infection experienced by susceptible individuals of age \(a\), which is defined as follows:
where \({M}_{a,\widetilde{a}}\) represents the average number of daily contacts that an individual of age class \(a\) has with persons of age group \(\widetilde{a}\), \(\beta\) is a scaling factor shaping the SARS-CoV-2 transmission rate, \({N}_{\widetilde{a}}\) is the total population in the age class \(\widetilde{a}\), and \(\delta\) is the relative infectiousness of vaccinated cases, hereafter assumed to be 0.5.
The number of critical cases \({C}_{a,\widetilde{a}}\) among infectees of age \(a\) attributable to the age group of infectors \(\widetilde{a}\) is computed by applying the estimated risk of developing critical disease for age \(a\), \({\rho }_{a}\)5 to the simulated cumulative number of infections caused by infectors of age \(\widetilde{a}\) in age group \(a\), \({i}_{a,\widetilde{a}}\), and accounting for the reduction of critical disease risk, \(V{E}^{crit}\), in breakthrough infections \({i}_{a,\widetilde{a}}^{v}\):
Results are presented in terms of mean values and 95% Prediction Intervals (PI) computed over 1000 model realizations using different samples of the model input distributions. For the sake of brevity, some results are provided as the range between the minimum and maximum values of the mean estimates obtained across the different geographical contexts. Model simulations were implemented in C programming language and all subsequent analyses and graphics were obtained with the statistical software R (version 4.1.2).
Ethics approval and consent to participate
The analysis relies only on secondary data published in12,37,38. Human participants were not involved in this study.
Results
SARS-CoV-2 transmission in the pre-vaccination period
The age distribution of the infections estimated with the model under the assumption of a fully susceptible population and by considering the school closure mandate well compares with the one associated with SARS-CoV-2 infections ascertained via PCR in the Oromia Region between March and September 202038 (Fig. 1A). Similar results are also obtained with a model mimicking the achievement of immunity levels estimated for the Jimma Zone in December 202038 (see Supplementary Fig. S3). Results obtained on the spread of SARS-CoV-2 before the start of COVID-19 vaccination (March 2021) suggest a marked variability across the different geographical contexts in the expected proportion of individuals over 50 years of age who acquired natural immunity: from 47.6% (95%PI: 37.5–59.9%) in rural areas to 64.6% (95%PI: 48.4–78.9%) in the remote settlements (Fig. 1B). Our estimates of serological profiles also show a relatively higher immunity among individuals under 50 years of age in urban neighborhoods compared to other settings.
(A) Comparison between the age distribution of all confirmed cases reported between March and September 2020 in the Oromia Region38 and the age distribution of the cumulative infections as obtained with a model mimicking the school closure and the achievement of immunity levels estimated for the Jimma Zone in March 202137. Aggregated model estimates for the entire SWSZ are obtained by considering the proportion of population living in remote settlements, rural villages, and urban neighborhoods of the SWSZ, their age structure, and the age-specific infection attack rate expected across the different social contexts before March 202112. (B) Model estimates of the age-specific percentage of the population immune to SARS-CoV-2 after natural infection at the beginning of the vaccination campaign (March 2021) in urban, rural, and remote areas of the SWSZ. Colored bars represent average estimates; solid lines represent the 95% PI of model estimates.
According to our simulations, the highest fraction of SARS-CoV-2 infections during the first pandemic year was caused by infectors aged less than 30 years, with the mean estimates ranging from 46.1 to 58.7% across all the considered geographical contexts (Fig. 2C). The mean fraction of critical cases attributable to infectors younger than 30 years was in the range of 24.9–48.0% depending on the geographical context considered. However, a non-negligible fraction of transmission was found to be assortative, i.e., characterized by a similar age between the infectors and their secondary cases (Fig. 2A). Specifically, we estimate that, in remote settlements, 48.7% of infections over 60 years of age might have occurred because of social interactions occurred within this age group. In this setting, individuals aged 50 years or more might have caused half of all critical cases (50.9% in all ages vs 15.9–18.9% in the urban and rural areas, see Fig. 2C). This may be explained by the older population structure characterizing less urbanized populations (see Fig. 2C and Table 1), and the higher number of community contacts reported by the elderly with individuals of similar age (see Supplementary Figs. S1 and S2).
(A,B) Percentage of SARS-CoV-2 infections caused by contacts between susceptible individuals in the age group \(a\) (x axis) and infected individuals in the age group \(\widetilde{a}\) (y axis), as estimated by the model before and after March 2021 in urban neighborhoods, rural villages, and remote settlements. (C) Age distribution of the population residing in the three geographical contexts and bar plots of the overall proportion of infections and critical cases caused by infectors aged 0–29, 30–49, 50+ years.
SARS-CoV-2 transmission at vaccination launch
To mimic the COVID-19 epidemiology during the emergence of the Delta variant, we simulate the SARS-CoV-2 transmission under the assumption that the vaccine uptake achieved in the entire population was negligible. However, pre-existing levels of natural immunity as estimated for March 2021 are considered and an increased viral transmissibility is assumed to reflect the transmission advantage of the Delta variant compared to pre-circulating strains41. We estimate that at the launch of the vaccination campaign, the effective reproduction number was 2.96 (95%PI: 1.84–4.42), 3.91 (95%PI: 3.51–4.37), and 3.80 (95%PI: 2.48–5.88) in urban, rural, and remote settings, respectively (see Supplementary Fig. S4). Our results suggest that the natural immunity acquired in the first pandemic phase and the reopening of teaching activities would have reshaped the contribution of different ages in the spread of COVID-19 (Fig. 2B,C). Specifically, we find that, after March 2021, the mean contribution of individuals under 30 years of age in causing new infections and critical cases might have increased to 84.5–87.3% and 66.7–70.6%, respectively. Accordingly, we estimate a mean decrease in the contribution of the elderly in generating SARS-CoV-2 secondary infections in the range of 2.0–3.5% and critical cases in the range of 7.2–13.5% depending on the geographical setting.
Our estimates suggest that, as the fraction of vaccinated individuals has remained negligible until December 2021, the cumulative incidence of critical cases expected during the Delta wave might have reached 134 (95%PI: 91–174), 223 (95%PI: 180–259), 173 (95%PI: 118–234) per 100,000 residents in the urban, rural, and remote settings, respectively.
Epidemiological outcome considering different vaccine uptake and priority targets
We evaluate the potential benefit of the low vaccination uptake achieved in Ethiopia at the end of 2021, when only 3.4% of Ethiopian citizens were fully vaccinated19, by assuming that all the administered doses were distributed throughout the population over 50 years (thereby achieving a coverage of 33% in this age group). We estimate that the number of averted critical cases would be 14 (95%PI: 6–21), 30 (95%PI: 20–37), and 20 (95%PI: 7–36) per 100,000 residents in urban, rural, and remote areas, respectively, corresponding to 10.0–13.5% of expected critical cases in absence of vaccination. These estimates are based on the assumption that all individuals were vaccinated before being infected with SARS-CoV-2 and therefore correspond to an upper limit of the efficacy of the vaccination program by the end of 2021.
Moreover, we compare the impact of two alternative vaccination strategies in a context of limited vaccine supply: prioritizing individuals older than 50 years or distributing the available vaccines throughout the population over 10 years. Our findings suggest that the best strategy to reduce the potential burden of critical disease is to prioritize vaccination of older individuals (Fig. 3). Specifically, we find that the vaccination of 100% of individuals aged 50 years or more has the potential of averting 40 (95%PI: 18–60), 90 (95%PI: 61–111), and 62 (95%PI: 21–108) critical cases per 100,000 residents in urban, rural, and remote areas, respectively (Fig. 3D). If the same number of vaccine doses would be uniformly administered to individuals over 10 years, the mean number of averted critical cases is expected to be in the range of 11–22 per 100,000 residents, depending on the geographical context considered. As concerns the reduction in the number of infections, the two alternative vaccination strategies are substantially equivalent, with differences in the expected mean attack rates ranging from 0.5 to 1.1% across the three geographical contexts (Fig. 3B).
(A) Population age structure in urban, rural, and remote settings of the SWSZ. The shaded area highlights the age segments of the population who are not yet eligible for COVID-19 vaccination. (B–D) Infection attack rate, cumulative incidence of critical cases, and averted critical cases per 100,000 residents as estimated for different geographical contexts (urban, rural, and remote) under the assumption that either all the individuals aged 50 years or older are vaccinated or that the same number of vaccine doses is uniformly distributed throughout the population over 10 years. Therefore, an equal number of people is assumed to be vaccinated in the two scenarios. Bars represent average estimates, stratified by the age group of infected individuals (0–9, 10–29, 30–49, 50+ years); solid lines represent the 95% PI of model estimates.
We then explore the scenario where vaccination is expanded to younger age groups after all individuals over 50 years of age are fully vaccinated. We find that the best vaccination policy to further reduce the burden of critical cases remains prioritizing the older segments of the population (i.e., people aged between 30 and 49 years, see Fig. 4B). Compared to a scenario with no vaccination, administering the vaccine to all individuals aged 30 years or more would avert 86 (95%PI: 56–113), 152 (95%PI: 120–181), 114 (95%PI: 68–164) critical cases per 100,000 residents in urban, rural, and remote areas, respectively. This policy is estimated to halve the cumulative incidence of critical disease otherwise expected if only individuals older than 50 years get the vaccine (range of mean estimates: 48–71 vs 93–133 per 100,000 residents). Our estimates suggest that the most effective strategy to reduce the infection attack rate is to uniformly distribute the available vaccines among individuals aged 10–49 years. However, the percentage of infections averted under this policy is limited to less than 10% across all considered contexts (Fig. 4A).
Estimated infection attack rate (A) and cumulative incidence of critical cases (B) in urban, rural, and remote areas, as obtained under the assumption that all individuals above 50 years are vaccinated with two doses and by considering different scenarios for the number of additional doses that would be available. In each panel, two strategies are compared: in the first, a further vaccination effort is simulated to reach a specific coverage level in subjects aged 30–49 years (orange); in the second, the same number of doses is used to uniformly vaccinate individuals aged 10–49 years (blue). Solid lines represent the mean model estimates; shaded areas represent the 95% PI.
To illustrate the full potential of COVID-19 vaccination, we finally estimate the infection attack rate and the cumulative incidence of critical cases under different combinations of vaccination coverage in the elderly (≥ 50 years of age) and in individuals aged 10–49 years, irrespectively of possible limits in the vaccine supply and logistic constraints (Fig. 5). Obtained results confirm that the most effective strategy to reduce the number of SARS-CoV-2 infections is the vaccination of younger subjects. We find that the vaccination of the entire population over 10 years with two doses of ChAdOx1 nCoV-19 would reduce the reproduction number to 2.17–2.81 (see Supplementary Fig. S4), therefore suggesting that further efforts would have been required to interrupt the SARS-CoV-2 circulation in Ethiopia. This may be due to several factors, including the low effectiveness of 2 doses of ChAdOx1 nCoV-19 against infection with the Delta variant, the high viral transmissibility of Delta, and the high fraction of individuals younger than 10 years, which represent between one fourth and one third of the total population residing in the three geographical contexts (Table 1).
When assuming that all individuals aged 50 years or more are vaccinated, the lowest cumulative incidence of critical cases is estimated to occur in urban neighborhoods, where 93 (95%PI: 66–118) subjects per 100,000 residents are estimated to be exposed to COVID-19 critical disease (Fig. 5B). To reduce the number of critical cases in rural areas under such an incidence level, the strategy minimizing the number of administered doses requires the vaccination of all individuals aged 50 years or more and the vaccination of at least 30% of younger individuals. In remote settlements, the same achievement would require the vaccination of at least 90% individuals over 50 years of age and a vaccination coverage of 20% in younger ages.
To reduce the cumulative incidence of critical disease under 50 cases per 100,000 individuals in less urbanized areas, a 90% vaccination coverage over 50 years of age should be complemented with more than 70–80% coverage among younger eligible subjects. In urban neighborhoods, the same result would require 90% coverage among the elderly and 50% coverage in younger individuals. If a maximum uptake level of 80% would be achieved in the elderly, to obtain similar results the vaccination of at least 60%, 90%, and 80% of the population under 50 years of age is needed in urban, rural, and remote areas, respectively.
The ranking of different vaccination strategies highlighted under our baseline assumptions is confirmed in a wide spectrum of sensitivity analyses accounting for (i) a different efficacy of the vaccine (see Supplementary Fig. S5), (ii) the uncertainty in the immunity levels acquired during the first pandemic phase (see Supplementary Fig. S6), (iii) the uncertainty in the reproduction number due to possible changes in the transmission determined by social distancing measures and in the increased transmissibility estimated for Delta compared to pre-circulating lineages (see Supplementary Fig. S7), (iv) equal infectiousness of breakthrough infections and infections among unvaccinated individuals (see Supplementary Fig. S8), (v) a homogeneous susceptibility by age (see Supplementary Fig. S9), and (vi) the estimate of the basic reproduction number of the ancestral lineages provided for Ethiopia by Iyaniwura et al.32 (see Supplementary Fig. S10).
Discussion
A limited vaccine supply should be considered when exploring the impact of vaccination strategies against COVID-19 in low-income countries2,19. In this study, we evaluated different age priority targets for vaccination in Ethiopia, considering changes in the disease spread determined by natural immunity acquired during the first year of the pandemic. To this aim, we simulated SARS-COV-2 spread before the launch of the national immunization campaign and assessed the potential disease burden caused by the Delta variant under different vaccination scenarios across urban, rural, and remote areas of the Southwest Shewa Zone.
Obtained results suggest that, before March 2021, infected individuals aged 50 years or more might have been responsible on average for 50.9%, 18.9%, and 15.9% of all critical cases occurred in remote, rural, and urban settings, respectively. Nonetheless, we found that a pivotal role in the spread of SARS-CoV-2 was played by subjects under 30 years, who might have been responsible for about half of the infections in all the considered areas.
Vaccination coverage against COVID-19 has remained extremely low in Ethiopia throughout 20212,19. As COVID-19 deaths ascertained in this country until December 2021 suggest a mortality rate around 5.9 per 100,000 residents55, our estimates of the incidence of critical cases in the absence of vaccination highlight that COVID-19 deaths may have been poorly detected in sub-Saharan settings. This is in line with a post-mortem surveillance suggesting 91.4% underreporting of COVID-19 deaths in Zambia10. We found that less urbanized areas might have been exposed to a higher burden of COVID-19 cases during the Delta epidemic wave due to older populations or a lower circulation of the infection during the first pandemic year. Additionally, the natural immunity acquired in the first pandemic phase and the reopening of schools significantly increased the proportion of critical cases caused by younger infectors. Nonetheless, our estimates highlight that prioritizing older age segments of the population for vaccination remains the most effective strategy to minimize the burden of critical illness in the Southwest Shewa Zone of Ethiopia. This conclusion emerges irrespectively of the overall number of available doses and despite the high infection rates experienced by the elderly during the first year of the pandemic and the large contribution played by young individuals in the spread of the disease afterwards. Our findings therefore confirm the results obtained across different countries in early 202146,56,57.
Presented results should be interpreted considering the following limitations. The comparison of alternative vaccination priority groups was carried out by assuming that the vaccine is instantaneously administered to all individuals in the target ages, therefore neglecting the time required for the rollout of the vaccination. To better highlight the overall potential of vaccination, we simulated its impact from March 2021, when the national vaccination program was officially launched. Due to the circulation of SARS-CoV-2 after this date and the waning of immunity acquired from natural infection, initial conditions considered to compare the different vaccination strategies do not reflect the current epidemiological conditions in the Southwest Shewa Zone. Nonetheless, the resulting priority ages were found to be robust under alternative modeling assumptions on the immunity level acquired in the first pandemic year and on the vaccine efficacy. Another limitation of this study is that we did not consider the waning of immunity. This model assumption may result in an underestimation of the disease burden expected after the launch of the COVID-19 vaccination. No data specific for the Southwest Shewa Zone were available that could allow the estimation of region-specific reproduction numbers; therefore, we used estimates from nationally aggregated data, which may be biased by overrepresentation of cases in Addis Ababa, where infection dynamics may be different from the rest of the country. It is also worth mentioning that school closure was the only intervention we considered when estimating the age-specific immunity profile before the vaccination launch. This means that variations in the social distancing measures adopted during the first pandemic year were not considered. These include an initial suspension of nonessential productive activities in early 202012 and the progressive re-opening of schools from November 202031,58. However, the carried-out analysis shows that our model was sufficiently robust to reproduce the age distribution of SARS-CoV-2 infections identified in the considered region during the first wave of COVID-19. Moreover, the impact of different COVID-19 prioritization strategies was simulated under the hypothetical scenario of an unmitigated COVID-19 epidemic, without considering any restriction or intervention. Therefore, our estimates of the expected number of infections and critical cases after March 2021 should be considered as illustrative worst-case scenarios to compare the performance of alternative vaccination strategies. The lack of available estimates on the infection-fatality ratio and infection-hospitalization ratio for the Delta variant in African countries did not allow us to quantify the reduction in the number of hospitalizations and years of life lost to COVID-19 determined by vaccination. We did not consider possible heterogeneities in infectiousness by age and symptomatic status. The spatial spread of COVID-19 was not considered in this work. Data on mixing patterns show that more than 97% of contacts occurred within the neighborhood of residence12. The low interconnectivity may suggest a slow spatial spread of the infection, especially in remote areas; however, this should not affect the total burden of disease if SARS-CoV-2 reached almost all populated areas (as suggested by the high number of infections reported in all regions of Ethiopia19). We therefore expect our conclusions to be robust with respect to the lack of spatial structure in the model. Finally, because of the lack of direct data from Africa, the relative susceptibility, the age-specific risks of developing critical disease, and the potential increased transmissibility and immune escape associated with the Delta variant were assumed from evidence gathered in other countries5,28,41.
Conclusions
Despite infections among children and young adults likely caused 70% of critical cases during the Delta wave in SWSZ, most vulnerable ages should remain a key priority target for vaccination against COVID-19. Considering the potential emergence of novel variants of SARS-CoV-2 in the future, our estimates suggest that in Ethiopia older individuals residing in less urbanized settlements should be prioritized for vaccination. Future non-pharmaceutical interventions should focus on reducing potential infectious interactions between the elderly and individuals under 30 years of age, representing their most likely infectors.
References
World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Internet]. https://covid19.who.int. Accessed 7 Apr 2022 (2022).
Our World in Data. COVID-19 Data Explorer [Internet]. https://ourworldindata.org/explorers/coronavirus-data-explorer. Accessed 7 Jan 2022 (2022).
United Nations Department of Economic and Social Affairs. World Population Prospects [Internet]. https://population.un.org/wpp/. Accessed 1 Feb 2022 (2022).
Poletti, P. et al. Age-specific SARS-CoV-2 infection fatality ratio and associated risk factors Italy, February to April 2020. Euro Surveill. 25, 2001383 (2020).
Zardini, A. et al. A quantitative assessment of epidemiological parameters required to investigate COVID-19 burden. Epidemics 37, 100530 (2021).
Poletti, P. et al. Association of age with likelihood of developing symptoms and critical disease among close contacts exposed to patients with confirmed SARS-CoV-2 infection in Italy. JAMA Netw. Open 4, e211085 (2021).
Cabore, J. W. et al. COVID-19 in the 47 countries of the WHO African region: A modelling analysis of past trends and future patterns. Lancet Glob. Health 10, e1099–e1114 (2022).
Ofotokun, I. & Sheth, A. N. Africa’s COVID-19 experience—A window of opportunity to act. JAMA Netw. Open 4, e2124556 (2021).
Burki, T. K. Undetected COVID-19 cases in Africa. Lancet Respir. Med. 9, e121 (2021).
Mwananyanda, L. et al. Covid-19 deaths in Africa: Prospective systematic postmortem surveillance study. BMJ 372, n334 (2021).
Massinga Loembé, M. et al. COVID-19 in Africa: The spread and response. Nat. Med. 26, 999–1003 (2020).
Trentini, F. et al. Modeling the interplay between demography, social contact patterns, and SARS-CoV-2 transmission in the South West Shewa Zone of Oromia Region, Ethiopia. BMC Med. 19, 89 (2021).
van Zandvoort, K. et al. Response strategies for COVID-19 epidemics in African settings: A mathematical modelling study. BMC Med. 18, 324 (2020).
Quaife, M. et al. The impact of COVID-19 control measures on social contacts and transmission in Kenyan informal settlements. BMC Med. 18, 316 (2020).
Walker, P. G. T. et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 369, 413–422 (2020).
World Health Organization. Ethiopia Introduces COVID-19 Vaccine in a National Launching Ceremony [Internet]. https://www.afro.who.int/news/ethiopia-introduces-covid-19-vaccine-national-launching-ceremony. Accessed 11 Feb 2022 (2021).
The World Bank. Ethiopia—Additional Financing for the Ethiopia COVID-19 Emergency Response Project [Internet]. https://documents1.worldbank.org/curated/en/721611617069718773/pdf/Ethiopia-COVID-19-Emergency-Response-Project-Additional-Financing.pdf. Accessed 7 Jan 2022 (2021).
World Health Organization Africa. Africa COVID-19 Vaccination Dashboard [Internet]. https://app.powerbi.com/view?r=eyJrIjoiOTI0ZDlhZWEtMjUxMC00ZDhhLWFjOTYtYjZlMGYzOWI4NGIwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9. Accessed 16 Feb 2023 (2023).
Ethiopian Public Health Institute. COVID-19 Pandemic Preparedness and Response in Ethiopia, Weekly Bulletin Epi-Week- 51 (December 20–26, 2021). [Internet]. https://ephi.gov.et/wp-content/uploads/2021/02/EPHI_PHEOC_COVID-19_Weekly_Bulletin_87_English_01032021.pdf. Accessed 6 Apr 2022 (2022).
World Health Organization Africa. COVID-19 Vaccination in the WHO African Region—Monthly Bulletin November 2022 [Internet]. https://apps.who.int/iris/bitstream/handle/10665/365353/CV-20221210-eng.pdf (2022).
Tolossa, T. et al. Attitude of health professionals towards COVID-19 vaccination and associated factors among health professionals, Western Ethiopia: A cross-sectional survey. PLoS ONE 17, e0265061 (2022).
Adane, M., Ademas, A. & Kloos, H. Knowledge, attitudes, and perceptions of COVID-19 vaccine and refusal to receive COVID-19 vaccine among healthcare workers in northeastern Ethiopia. BMC Public Health 22, 128 (2022).
Mohammed, R., Nguse, T. M., Habte, B. M., Fentie, A. M. & Gebretekle, G. B. COVID-19 vaccine hesitancy among Ethiopian healthcare workers. PLoS ONE 16, e0261125 (2021).
Aemro, A., Amare, N. S., Shetie, B., Chekol, B. & Wassie, M. Determinants of COVID-19 vaccine hesitancy among health care workers in Amhara region referral hospitals, Northwest Ethiopia: A cross-sectional study. Epidemiol. Infect. 149, e225 (2021).
Mekonnen, B. D. & Mengistu, B. A. COVID-19 vaccine acceptance and its associated factors in Ethiopia: A systematic review and meta-analysis. Clin. Epidemiol. Glob. Health 14, 101001 (2022).
Emire, M. S. & Shiferaw, B. Z. Attitudes towards receiving COVID-19 vaccine and its associated factors among Southwest Ethiopian adults, 2021. PLoS ONE 18, e0280633 (2023).
Mesele, M. COVID-19 vaccination acceptance and its associated factors in Sodo Town, Wolaita Zone, Southern Ethiopia: Cross-sectional study. Infect. Drug Resist. 14, 2361–2367 (2021).
Hu, S. et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat. Commun. 12, 1533 (2021).
Manica, M. et al. Estimation of the incubation period and generation time of SARS-CoV-2 Alpha and Delta variants from contact tracing data. Epidemiol. Infect. 151, e5 (2022).
Cereda, D. et al. The early phase of the COVID-19 epidemic in Lombardy, Italy. Epidemics 37, 100528 (2021).
Handebo, S., Adugna, A., Kassie, A. & Shitu, K. Determinants of COVID-19-related knowledge and preventive behaviours among students in reopened secondary schools: Cross-sectional study. BMJ Open 11, e050189 (2021).
Iyaniwura, S. A., Rabiu, M., David, J. F. & Kong, J. D. The basic reproduction number of COVID-19 across Africa. PLoS ONE 17, e0264455 (2022).
Muniz-Rodriguez, K. et al. Severe acute respiratory syndrome coronavirus 2 transmission potential, Iran, 2020. Emerg. Infect. Dis. 26, 1915–1917 (2020).
Park, M., Cook, A. R., Lim, J. T., Sun, Y. & Dickens, B. L. A systematic review of COVID-19 epidemiology based on current evidence. J. Clin. Med. 9, 967 (2020).
Riccardo, F. et al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill. 25, 2000790 (2020).
Munayco, C. V. et al. Early transmission dynamics of COVID-19 in a southern hemisphere setting: Lima-Peru: February 29th–March 30th, 2020. Infect. Dis. Model. 5, 338–345 (2020).
Gudina, E. K. et al. Seroepidemiology and model-based prediction of SARS-CoV-2 in Ethiopia: Longitudinal cohort study among front-line hospital workers and communities. Lancet Glob. Health 9, e1517–e1527 (2021).
Gudina, E. K. et al. COVID-19 in Oromia Region of Ethiopia: A review of the first 6 months’ surveillance data. BMJ Open 11, e046764 (2021).
Nextstrain Team. Genomic Epidemiology of Novel Coronavirus—Africa-Focused Subsampling [Internet]. https://nextstrain.org/ncov/gisaid/africa/6m. Accessed 7 Jan 2023 (2023).
Ofori, S. K., Schwind, J. S., Sullivan, K. L., Chowell, G., Cowling, B. J., & Fung, I. C. H. Modeling infections and deaths averted due to COVID-19 vaccination strategies in Ghana. MedRxiv [Internet]. https://www.medrxiv.org/content/10.1101/2022.07.09.22277458v1. Accessed 16 Feb 2023 (2022).
Liu, H. et al. Investigating vaccine-induced immunity and its effect in mitigating SARS-CoV-2 epidemics in China. BMC Med. 20, 37 (2022).
Burki, T. K. Omicron variant and booster COVID-19 vaccines. Lancet Respir. Med. 10, e17 (2022).
Chavda, V. P. & Apostolopoulos, V. Global impact of delta plus variant and vaccination. Expert Rev. Vaccines 21, 597–600 (2022).
Ye, Y. et al. Equitable access to COVID-19 vaccines makes a life-saving difference to all countries. Nat. Hum. Behav. 6, 207–216 (2022).
Falsey, A. R. et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 385, 2348–2360 (2021).
Marziano, V. et al. The effect of COVID-19 vaccination in Italy and perspectives for living with the virus. Nat. Commun. 12, 7272 (2021).
Harris, R. J. et al. Effect of vaccination on household transmission of SARS-CoV-2 in England. N. Engl. J. Med. 385, 759–760 (2021).
Subbarao, S., Copas, A., Andrews, N., Gower, C., Bernal, J.L., Ramsay, M.E. et al. Vaccine Effectiveness Against Infection and Death Due to SARS-CoV-2, Following One and Two Doses of the BNT162b2 and ChADox-1 in Residents of Long-Term Care Facilities in England, Using a Time-Varying Proportional Hazards Model [Internet]. https://papers.ssrn.com/abstract=3922678. Accessed 16 Feb 2023 (2021).
Sheikh, A., McMenamin, J., Taylor, B., Robertson, C., Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 397, 2461–2462 (2021).
Thiruvengadam, R. et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect. Dis. 22, 473–482 (2022).
Pouwels, K. B. et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 27, 2127–2135 (2021).
World Health Organization. Dashboard of the Officially Reported COVID-19 Vaccination Data [Internet]. https://app.powerbi.com/view?r=eyJrIjoiMWNjNzZkNjctZTNiNy00YmMzLTkxZjQtNmJiZDM2MTYxNzEwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9. Accessed 10 Feb 2022 (2022).
Lopez Bernal, J. et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 385, 585–594 (2021).
Fabiani, M. et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ 376, e069052 (2022).
Our World in Data. Cumulative Confirmed COVID-19 Deaths Per Million People [Internet]. https://ourworldindata.org/explorers/coronavirus-data-explorer?facet=none&Metric=Confirmed+deaths&Interval=Cumulative&Relative+to+Population=true&Color+by+test+positivity=false&country=~ETH. Accessed 4 Mar 2022 (2022).
Yang, J. et al. Despite vaccination, China needs non-pharmaceutical interventions to prevent widespread outbreaks of COVID-19 in 2021. Nat. Hum. Behav. 5, 1009–1020 (2021).
Bubar, K. M. et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 371, 916–921 (2021).
Tadese, M. & Mihretie, A. Attitude, preparedness, and perceived self-efficacy in controlling COVID-19 pandemics and associated factors among university students during school reopening. PLoS ONE 16, e0255121 (2021).
Funding
This work was supported by the Italian Ministry of Foreign Affairs and International Cooperation within the project entitled “Rafforzamento del sistema di sorveglianza e controllo delle malattie infettive in Etiopia”—AID 011330. The funders had no role in the study design, data collection and analysis, interpretation, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
P.P., M.A., and S.M. conceived the study. A.Z. and M.G. wrote the code and performed the analysis. A.Z., M.G., and P.P. wrote the first draft of the manuscript. P.P., M.A., and S.M. supervised the study. All authors contributed to interpret the results, read, reviewed, and approved the final version and the submission of the manuscript. The corresponding author had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
M.A. has received research funding from Seqirus. The funding is not related to COVID-19. All other authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galli, M., Zardini, A., Gamshie, W.N. et al. Priority age targets for COVID-19 vaccination in Ethiopia under limited vaccine supply. Sci Rep 13, 5586 (2023). https://doi.org/10.1038/s41598-023-32501-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32501-y
- Springer Nature Limited