Abstract
Lymphovascular invasion (LVI) is a fundamental step toward the spread of cancer. Extracellular vesicles (EVs) promote cellular communication by shuttling cargo, such as microRNAs (miRNAs). However, whether EV-associated miRNAs serve as biomarkers for LVI remains unclear. This study aimed to identify EV-associated miRNAs related to LVI and validate the miRNA levels from patients with early-stage lung adenocarcinoma (LADC). Blood samples were collected from patients undergoing pulmonary resection for stage I LADC before surgery. The patients were classified into three groups according to the presence of LVI and postoperative recurrence. Serum-derived EVs in the derivation cohort were used for small RNA sequencing, while the selected LVI miRNA candidates were validated via real-time quantitative polymerase chain reaction using 44 patient and 16 healthy donor samples as the validation cohorts. Five miRNAs (miR-99b-3p, miR-26a-5p, miR-93-5p, miR-30d-5p, and miR-365b-3p) were assessed, and miR-30d-5p (p = 0.036) levels were significantly downregulated in the LVI-positive group. miR-30d-5p levels in healthy donors were lower than those in LADC patients. Patients with high miR-30d-5p levels had favorable survival compared to those with low miR-30d-5p levels. miR-30d-5p level in EVs may serve as a promising biomarker for detecting LVI in patients with early-stage LADC.
Similar content being viewed by others
Introduction
Pathological lymphovascular invasion (LVI), which is the presence of vascular invasion and/or lymphatic permeation, is a strong prognostic factor in many types of cancer, including lung cancer1,2,3,4,5,6,7. The presence of LVI, which reflects tumor aggressiveness, is an essential step toward locoregional and systemic tumor spread. Invasion and metastasis are the primary causes of death in patients with lung cancer. Therefore, understanding the regulatory mechanisms of LVI may help in overcoming lung cancer and developing new therapeutic targets.
MicroRNAs (miRNAs) are non-coding RNAs that regulate gene expression at the post-transcriptional level, and have emerged as promising biomarkers for cancer diagnosis and therapy8,9. miRNAs are transported via body fluids within extracellular vesicles (EVs), including exosomes10,11,12. EVs are bioactive vesicles that promote cell–cell communications by shuttling cargo, such as mRNAs, proteins, miRNAs, and lipids13,14,15. EVs have been widely studied, and cancer-derived EVs have been found to be involved in metastatic cascades, such as tumorigenesis, migration, priming of metastatic niches, and LVI16,17,18,19,20,21,22,23. Several studies have demonstrated the correlation between LVI and aberrant expression of specific miRNAs24,25. However, only a few studies have performed EV-associated miRNA profiling with small RNA sequencing. It remains unclear whether EVs containing unique miRNAs can serve as non-invasive tools for the early diagnosis and in-depth understanding of the mechanism of LVI in patients with early-stage lung cancer, especially lung adenocarcinoma, which is the most common type of non-small cell lung cancer (NSCLC).
This study aimed to identify specific EV-associated miRNAs related to LVI via small RNA sequencing and quantify their miRNA levels in serum EVs from patients with early-stage lung adenocarcinoma for validation analysis.
Materials and methods
Prognostic analysis according to LVI status using a large database
We used a clinical database from three institutions (Tokyo Medical University Hospital, Hiroshima University Hospital, and Kanagawa Cancer Center), including 4676 patients who underwent surgical resection for lung cancer between January, 2010 and December, 2020. Among them, 2044 patients with pathological stage I NSCLC undergoing radical anatomical resection (segmentectomy or lobectomy) between January, 2010 and December, 2016 were selected to assess postoperative survival outcomes according to LVI status. The TNM stage was determined according to the seventh edition of the TNM classification of malignant tumors.
Research subjects
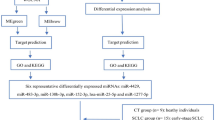
Blood samples were collected from 68 patients before surgery in the operating room. All patients underwent complete surgical resection for pathological stage I lung adenocarcinoma at the Tokyo Medical University Hospital between January, 2015 and December, 2019. The tumor specimens were fixed in neutral buffered formalin, processed routinely and embedded in paraffin and serially sectioned at 4-μm thickness and stained with hematoxylin/eosin and Elastica van Gieson to evaluate the extent of blood vessel invasion and pleural invasion. The sections were also stained with D2-40 to evaluate the extent of lymphatic permeation. The patients were classified into three groups based on the presence of LVI and postoperative recurrence. The derivation set included serum-derived EVs from 24 blood samples used for small RNA sequencing, while 44 blood samples were used for the validation study. Selected LVI and recurrence-related miRNA candidates from small RNA sequencing were subsequently validated using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in the validation cohort. A schematic overview of the study is shown in Fig. 1. Serum samples from 16 healthy donors were also collected as controls for RT-qPCR. Each blood sample was centrifuged at 3000 rpm for 5 min to separate the serum, which was stored at – 80 °C until RNA extraction. This study was approved by the institutional review board of Tokyo Medical University (study approval no. T2021-0140). Written informed consent for the use and analysis of clinical data was obtained preoperatively from each patient and healthy donor. All experiment protocols were approved by the Institutional Review Board of Tokyo Medical University, and all methods were carried out in accordance with relevant guidelines and regulations.
Schematic overview of the strategy used to identify extracellular vesicle-associated microRNA (miRNA) signature associated with vascular invasion and postoperative recurrence. NGS nest generation sequencing, LVI vascular invasion, EV extracellular vesicle, miRNAs microRNAs, RT-qPCR reverse transcription-quantitative polymerase chain reaction.
EV isolation
A sequential centrifugation procedure was used to recover EVs. Venous blood from each patient was separated into serum and cellular fractions. Cells were pelleted via centrifugation at 300×g for 5 min, followed by centrifugation at 1200×g for 20 min. To eliminate other cellular debris, the supernatant was centrifuged at 10,000×g for 30 min. For EV preparation, the sample was ultracentrifuged at 210,000×g for 35 min at 4 °C. Pellets were washed with phosphate-buffered saline.
Small RNA library preparation and sequencing
Total EV-associated miRNA of 24 samples in the derivation set was isolated using the miRNeasy Mini Kit (QIAGEN, The Netherlands) and sent to DNA Chip Research Inc., where small RNA library preparation and sequencing were performed. Small RNA libraries were prepared using the QIAseq miRNA library kit and QIAseq miRNA NGS 96 Index IL for Illumina, and yields were evaluated using the Agilent 2100 BioAnalyzer. Small RNA sequencing was performed using the Illumina NexSeq 500 system.
Identification of LVI and recurrence-related miRNAs
EV-associated miRNA selection criteria from small RNA sequencing data included known miRNAs and differentially expressed genes (DEGs) that were obtained based on the criteria of |log2 fold changes|> 1.5, and corrected p-value by Benjamini–Hochberg false discovery rate controlling procedure of less than 1. The miRNA databases, miRBase (https://www.mirbase.org) and HMDD (https://www.cuilab.cn/hmdd) were used to identify the LVI and recurrence-related miRNAs associated with cancer.
RT-qPCR
We performed RT-qPCR analysis of selected EV-associated miRNAs related to LVI in the validation cohort to validate the small RNA sequencing data. EV-associated miRNAs were isolated using the miRNeasy Mini Kit and cDNAs were generated using the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, Massachusetts). Gene-specific TaqMan MicroRNA Probes (Thermo Fisher Scientific) were used for quantitative analyses of the miRNA transcript levels of miR-99b-3p, miR-26a-5p, miR-93-5p, miR-30d-5p, miR-365b-3p, miR-29c-3p, miR-486-3p, miR-486-5p, and miR-548ae-5p. To normalize miRNA expression, let7f was selected as an internal control in our experiment because the gene as an endogenous control miRNA has been reported to be stable during senescence and aging26.
Statistical analyses
Statistical analyses were performed using the Statistical Analysis Software Package R (R Project for Statistical Computing; http://www.r-project.org) and the SPSS statistical software package (version 28.0; DDR3 RDIMM; SPSS Inc., Chicago, IL, USA). Overall survival (OS) was measured from the day of surgery to the day of death from any cause or the day on which the patient was last known to be alive. Recurrence-free survival (RFS) was measured as the interval between the date of surgery and the date of recurrence, the date of death from any cause, or the date on which the patient was last known to be alive. OS and RFS curves were plotted using the Kaplan–Meier method, and differences in variables were determined using the log-rank test. For survival analysis in the validation cohort, we excluded those with a follow-up period of less than 1 year and those who died of adverse events or non-cancer-related death within 1 year after surgery. Univariate and multivariate logistic regression analyses were performed to identify the factors associated with OS and RFS using a Cox proportional hazards model. A backward stepwise selection method was used to build the logistic regression models, and variables with a threshold of p < 0.15 were adopted for the stepwise model selection procedure to prevent overlooking relevant factors27. Student’s t test for continuous data was used to compare the two groups. All tests were two-sided, and p-values < 0.05 were considered to be statistically significant.
Results
Significance of LVI for survival in early-stage NSCLC
Among 2044 patients with pathological stage I NSCLC in our database, 559 (27.3%) were found to be LVI-positive. Characteristics of the patients from our database was shown in Supplementary Table 1. OS and RFS were significantly worse in patients with LVI than in those without LVI (5-year OS rates of 93.4 vs. 79.8%, p < 0.001, Fig. 2A; 5-year RFS rates of 91.4 vs. 69.6%, p < 0.001, Fig. 2B). Multivariate analysis showed that maximum standardized uptake value (SUVmax) of tumor (hazard ratio [HR], 1.043; 95% confidence interval CI 1.008–1.079; p = 0.017), pathological tumor size, (HR, 1.357; 95% CI 1.060–1.733; p = 0.015), pathological stage (HR, 2.427; 95% CI 1.428–4.124; p = 0.001), and LVI (HR, 4.134; 95% CI 2.431–7.031; p < 0.001) were independent risk factors for OS (Supplementary Table 2), while age (HR, 1.048; 95% CI 1.032–1063; p < 0.001), smoking history (HR, 1.657; 95% CI 1.140–2.408; p = 0.008), SUVmax of tumor (HR, 1.024; 95% CI, 1.004–1.044; p = 0.020), surgical procedure (HR, 1.563; 95% CI 1.195–2.045; p = 0.001), pathological stage (HR, 1.771; 95% CI 1.350–2.323; p < 0.001), and LVI (HR, 2.664; 95% CI 2.000–3.548; p < 0.001) were the significant risk factors for RFS (Supplementary Table 3).
Characteristics of the research population
In the research population, there were 24 patients with LVI and postoperative recurrence in group 1, 22 patients with only LVI in group 2, and 22 patients without LVI and recurrence in group 3. The general patient characteristics are shown in Table 1. The numbers of patients in the derivation and validation sets were 24 and 44, respectively. A significant difference was observed between patients with LVI (G1 + 2) and those without LVI (G3) in terms of smoking history (p = 0.006), pathological stage (p = 0.002), presence of pleural invasion (p < 0.001), and tumor differentiation (p < 0.001). In contrast, there was no significant difference between G1 and G2, except for the pathological tumor size (p = 0.037).
Identification of LVI and recurrence-related EV-associated miRNAs in the two cohorts
Expression levels of 2555 miRNAs were determined via small RNA sequencing. The miRNA levels of DEGs between patients with LVI (G1 + 2) and those without LVI (G3) are shown as a heatmap in Fig. 3A. As shown in the volcano plot, miRNAs were differentially expressed between the two cohorts (Fig. 3B). The criteria of |log2 fold changes|> 1.5, corrected p-value < 1, and being known miRNAs were used to identify the LVI-associated miRNAs, and 56 miRNAs were selected (Fig. 3C). Because let7f was selected as the internal control for RT-qPCR, let7 family miRNAs were excluded from the 56 miRNAs. We then used the miRNA databases, miRbase and HMDD, searchable databases for published miRNA sequences and annotation data, to narrow down the cancer-related candidate miRNAs, and 17 miRNAs were finally selected. Likewise, the miRNA levels of DEGs between patients with LVI but no recurrence (G2) and those with both factors (G1) are shown in Supplementary Fig. 1A. The volcano plot shows the differentially expressed miRNAs between the two cohorts (Supplementary Fig. 1B). We extracted 79 miRNAs using the same criteria as those for the LVI marker candidates (Supplementary Fig. 1C), and 14 miRNAs were ultimately selected.
The exploration of miRNA associated with LVI and recurrence
Of the 17 miRNAs related to LVI, we excluded 12 genes (miR-519a-3p, miR-518d-5p, miR-520c-5p, miR-526a, miR548o-3p, miR-518f-5p, miR-191-5p, miR-486-5p, miR-223-3p, miR-16-5p, miR-146a-5p, miR-142-3p) due to undetermined gene expression levels via RT-qPCR. Thus, we selected the remaining five miRNAs (miR-99b-3p, miR-26a-5p, miR-93-5p, miR-30d-5p, and miR-365b-3p) for RT-qPCR confirmation in the validation cohort. Patients with LVI had significantly lower miR-30d-5p levels (p = 0.036) than those without LVI (Fig. 4A). In the other validation study with healthy control groups, the miR-30d-5p levels in the healthy control group were significantly lower than those in patients with G2 (p = 0.022; Fig. 4B) and G3 (p = 0.047; Fig. 4B), as well as those with lung adenocarcinoma (p < 0.001; Fig. 4C). To test the prognostic significance of serum EV miR-30d-5p level dichotomized at its median value, Kaplan–Meier curves of 27 patients with stage I lung adenocarcinoma showed that those with high miR-30d-5p levels had significantly favorable RFS compared to those with low miR-30d-5p levels (Fig. 4D).
(A) Quantification of five miRNAs obtained from the serum extracellular vesicles of 44 patients for a validation study. G1 + 2 (LVI +) cohort has significantly lower miR-30d-5p levels (p = 0.036) than the G3 (LVI–) cohort. (B) miR-30d-5p levels in the healthy control group were significantly lower than those in the G2 (p = 0.022) and G3 (p = 0.047) cohorts. (C) miR-30d-5p levels in the healthy control group were significantly lower than those in the patients with lung adenocarcinoma (p < 0.001). (D) Recurrence-free survival curves of 27 patients with stage I lung adenocarcinoma according to their miR-30d-5p levels. EV extracellular vesicle.
Of the 14 miRNAs related to recurrence, we excluded 10 miRNAs (miR-519a-3p, miR-520c-3p, miR-523-5p, miR-548ah-3p, miR-642b-3p, miR-548 h-3p, miR-520c-5p, miR-92a-3p, miR-519e-3p, miR-517c-3p) due to undetermined gene expression levels via RT-qPCR. Therefore, we selected four miRNAs (miR-29c-3p, miR-486-3p, miR-486-5p, and miR-548ae-5p) for RT-qPCR confirmation in the validation cohort. Patients who experienced postoperative recurrence had significantly lower miR-29c-3p (p = 0.011) and miR-486-3p (p = 0.040) levels than those without recurrence (Supplementary Fig. 2). However, the expression level of these two miRNA in healthy donors was higher than that in patients with lung adenocarcinoma (miR-29c-3p, p = 0.013; and miR-486-3p, p = 0.121, data not shown).
Enrichment and pathway analyses of EV-associated miRNAs related to LVI and recurrence
To fully determine the roles of miR-30d-5p in LVI and miR-29c-3p and miR-486-3p in recurrence, we collected statistically significant target genes from three databases, PITA, TargetScan, and microRNA.org., and performed Gene Ontology (GO) term and pathway annotation using WikiPathways and PathVisionRPC v1.2. Among the 69 significant targeted genes for miR-30d-5p, a list of the top 20 genes with strong correlations based on p-values is shown in Fig. 5A. GO enrichment analysis showed that two enriched GO terms, protein kinase binding and kinase binding in molecular functions, were correlated with the miR-30d-5p target genes (Fig. 5B). A chord diagram illustrates the interaction between GO terms and relevant gene symbols (Fig. 5C). The pathway analysis results showed that the target genes for miR-30d-5p were significantly related to leptin–insulin signaling overlap and alpha 6 beta 4 signaling (Fig. 5D). The Z-score of the most enriched pathways was greater than two, indicating that most of the pathways were enhanced.
(A) List of the top 20 genes with strong correlations with miR-30d-5p target genes based on the p-value. (B) Two enriched Gene Ontology (GO) terms in molecular functions were correlated with the miR-30d-5p target genes. (C) Chord diagram illustrates the interaction between GO terms and the relevant gene symbols. (D) Top 20 pathways for target genes associated with miR-30d-5p based on the Z-score.
There were 67 and 369 significant target genes for miR-29c-3p and miR-486-3p, respectively, and a list of the top 20 target genes is shown in Supplementary Fig. 3A. Six enriched GO terms correlated with miR-29c-3p target genes and the top 10 GO terms associated with miR-486-3p targets in three main categories (biological process, molecular function, and cellular component) are shown in Supplementary Fig. 3B. Chord diagrams illustrate the interaction between GO terms and relevant gene symbols in miR-29c-3p and miR-486-3p target genes (Supplementary Fig. 3C). Top 20 pathways for the target genes associated with the two recurrence-related miRNAs, based on the Z-score, are shown in Supplementary Fig. 3D.
Discussion
The present study found that LVI is a prominent prognostic factor in a large number of patients with stage I NSCLC who underwent complete surgical resection. EV-associated miRNA profiling via small RNA sequencing identified miR-30d-5p to be associated with LVI using serum EVs derived from patients with stage I lung adenocarcinoma. Validation analysis using RT-qPCR demonstrated significant downregulation of EV-associated miR-30-5p levels in the LVI cohort, and gene expression was confirmed exclusively in tumors. Kaplan–Meier curves for RFS showed that miR-30d-5p was a prognostic marker in patients with early-stage adenocarcinoma. Finally, enrichment and pathway analyses of miR-30d-5p identified the correlated targeted genes, relevant enriched GO terms, and potential pathways involved in the occurrence of LVI in lung cancer.
LVI has prognostic significance in patients with resected lung cancer, especially adenocarcinoma, and reflects the tumor aggressiveness1,2,3,4,5,6,7. Local tumor invasion and entry of tumor cells into the vasculature occur at the early-stage of the metastatic cascade. Therefore, this phenomenon is often observed in patients with early-stage lung cancer without lymph node metastasis, and a better understanding of the regulatory mechanism of LVI may lead to the development of novel prognostic markers and effective therapeutic strategies to prevent the spread of metastasis. Complex cancer tissue components, such as immune cells, cancer-associated fibroblasts, and endothelial cells, contribute to the formation of a tumor microenvironment, which is involved in tumor cell intravasation and angiogenesis16,18,21,28,29,30. However, the mechanism by which cancer cells modulate the surrounding cells to facilitate LVI and the specific genetic determinants involved in cancer cell intravasation remain poorly understood.
Cancer-derived EVs contribute to the modulation of a tumor microenvironment favorable to cancer cells via cell–cell communication21,22,31. They play various roles in promoting tumor progression, invasion, migration, angiogenesis, and establishment of the pre-metastatic niche15,18,19. Cancer-derived EVs modulate endothelial cells, which play roles in intravasation, angiogenesis, loss of the endothelial vascular barrier, and extravasation, for vascular-related functions24,25,29,32. EVs contain a large number of miRNAs, and EV-associated miR-140-3p, miR-30d-5p, miR-29b-3p, miR-130-3p, miR-330-5p, and miR-296-3p have been reported to be associated with the migration ability of hepatocellular cells via comparative analysis of EV miRNA profiles33. Mao et al. reported that tumor-derived miR-494 promoted angiogenesis, and miR-21 transferred via EVs was taken up by the endothelial cells in their lung cancer study25. However, there is a lack of reports that patients’ serum derived EVs containing unique miRNAs that can serve as non-invasive biomarkers for LVI in early-stage lung adenocarcinoma.
Here, the validation dataset demonstrated that miR-30d-5p levels in serum EVs from LVI-negative patients were significantly higher than those from LVI-positive patients and healthy donors. miR-30d-5p has been reported to act as a tumor suppressor in various types of cancer, but only a limited number of studies have reported its role as an oncogene27,34,35. Gao et al. reported that miR-30d-5p levels were decreased in NSCLC tissues, and patients with lower miR-30d-5p levels tended to show an advanced clinical progression36. Chen et al. demonstrated that miR-30-5p levels were downregulated in NSCLC tissues, and that the gene and its direct target, cyclin E2 axis, may contribute to NSCLC proliferation and motility35. Zheng et al. showed that miR-30d-5p was a significant diagnostic biomarker for cervical cancer and its precursors34. Hu et al. demonstrated that the upregulation of four miRNAs, including miR-30d and miR-486, was significantly associated with poor OS using a large number of NSCLC samples37. The opposite results between their study and ours may be explained by differences in sample size, patients backgrounds, the lack of standardization for normalization, miRNA processing, the inability to discriminate among closely related miRNAs, and the source of miRNAs. Although the source of circulating miRNA is reported to be diverse, it can be inferred that exosomal miRNAs can act as a better source for biomarker studies because of its advantages in terms of quantity, quality, and stability by a systemic review38. In the current study, the level of exosomal miR-30d-5p in the healthy control group was significantly lower than that in samples from lung adenocarcinoma, suggesting that the biomarker can also aid in the diagnosis of early-stage lung adenocarcinoma on the purpose of a non-invasive early detection screening.
EV-associated miR-29c-3p and miR-486-3p levels from recurrent patients were significantly lower than those from non-recurrent patients. There are a small number of published studies related to the association between miR-29c-3p expression and cancer biology. The levels of miR-29c-3p were reported to be lower in colorectal cancer tissues than in non-cancerous tissues39. Ji et al. found that the miR-486-3p gene expression levels were downregulated in tumor tissues than in normal tissues in patients with hepatocellular carcinoma, and that it mediated sorafenib resistance by targeting the fibroblast growth factor receptor 4 and epidermal growth factor receptor40. Overall, these results suggest that patients with LVI-positive or recurrent lung adenocarcinoma may show significantly lower expression levels of these tumor suppressive genes than LVI-negative or non-recurrent patients and healthy donors.
Disrupting the intratumor vasculature and anti-angiogenic targeted therapy are key treatments for various types of tumors, including lung adenocarcinoma41. These therapies help starve cancer cells by halting angiogenesis and destroying pre-existing tumor-related vascular networks. Although several anti-vascular endothelial growth factor therapies have been well established, their effects are transient. Leptin-insulin signaling overlap and alpha 6 beta 4 signaling are the most significantly activated canonical pathways relating to miR-30d-5p in the current study. Leptin and insulin upregulate miR-4443 in colorectal cancer and decrease the invasiveness of colon cancer cells42. Alpha 6 beta 4 integrin signaling is reported to stimulate the invasion and migration of endothelial cells and promote angiogenesis43. Although there is no study to investigate miR-30d-5p and these cancer-related signaling, more in-depth gene expression profiling, and functional analysis may allow for understanding prognostic significance in malignancies including lung adenocarcinoma. Further research is required to investigate the roles of EV-associated miR-30d-5p in intravasation and angiogenesis in lung cancer, and to assess whether this liquid biopsy approach can be applied in daily clinical practice to predict postoperative survival and treatment efficacy.
Our study has several limitations. The small number of participants enrolled in this study could be a limitation; however, the inclusion of the validation cohort strengthened our results. The type of patients recruited may be a limitation; therefore, further research using larger and independent cohorts is warranted to validate the EV-associated miRNA signatures reported here. Finally, the methods of isolation and characterization of circulating EVs remain ambiguous. However, we have reported several other studies on EVs obtained using the same isolation methodology as serum-derived biomarkers.
In conclusion, our study found that EV-associated miRNA profiles associated with LVI target some critical pathways, suggesting the potential of an EV-associated miRNA, miR-30d-5p, as a non-invasive biomarker and therapeutic target to prevent cancer cell intravasation at the pivotal early step of the metastatic cascade.
Data availability
The analyzed data in this article will be shared on reasonable request to the corresponding author.
Abbreviations
- LVI:
-
Lymphovascular invasion
- miRNA:
-
MicroRNA
- NSCLC:
-
Non-small cell lung caner
- RT-qPCR:
-
Reverse transcription-quantitative polymerase chain reaction
- DEG:
-
Differentially expressed gene
- EV:
-
Extracellular vesicle
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Saijo, T. et al. Evaluation of extratumoral lymphatic permeation in non-small cell lung cancer as a means of predicting outcome. Lung Cancer 55(1), 61–66 (2007).
Shimada, Y. et al. Extratumoral vascular invasion is a significant prognostic indicator and a predicting factor of distant metastasis in non-small cell lung cancer. J. Thorac. Oncol. 5(7), 970–975 (2010).
Shimada, Y. et al. Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J. Thorac. Oncol. 7(8), 1263–1270 (2012).
Shimada, Y. et al. The frequency and prognostic impact of pathological microscopic vascular invasion according to tumor size in non-small cell lung cancer. Chest 149(3), 775–785 (2016).
Kato, T. et al. Angiolymphatic invasion exerts a strong impact on surgical outcomes for stage I lung adenocarcinoma, but not non-adenocarcinoma. Lung Cancer 77(2), 394–400 (2012).
Usui, S. et al. Differences in the prognostic implications of vascular invasion between lung adenocarcinoma and squamous cell carcinoma. Lung Cancer 82(3), 407–412 (2013).
Suaiti, L. et al. Vascular invasion predicts recurrence in stage IA2-IB lung adenocarcinoma but not squamous cell carcinoma. Clin. Lung Cancer https://doi.org/10.1016/j.cllc.2022.12.006 (2022).
Liu, C. G. et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc. Natl. Acad. Sci. U. S. A. 101(26), 9740–9744 (2004).
Peng, Y. & Croce, C. M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 1, 15004 (2016).
Zhang, J. et al. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 13(1), 17–24 (2015).
Ono, M. et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 7(332), ra63 (2014).
Kohama, I., Kosaka, N., Chikuda, H. & Ochiya, T. An insight into the roles of microRNAs and exosomes in sarcoma. Cancers (Basel) 11(3), 428 (2019).
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2(8), 569–579 (2002).
Kahlert, C. & Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. (Berl). 91(4), 431–437 (2013).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200(4), 373–383 (2013).
Al-Nedawi, K., Meehan, B., Kerbel, R. S., Allison, A. C. & Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. U. S. A. 106(10), 3794–3799 (2009).
Whiteside, T. L. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin. Exp. Immunol. 189(3), 259–267 (2017).
Paggetti, J. et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 126(9), 1106–1117 (2015).
Tickner, J. A., Urquhart, A. J., Stephenson, S. A., Richard, D. J. & O’Byrne, K. J. Functions and therapeutic roles of exosomes in cancer. Front. Oncol. 4, 127 (2014).
Rahman, M. A. et al. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget 7(34), 54852–54866 (2016).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18(6), 883–891 (2012).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17(6), 816–826 (2015).
Yokoi, A. & Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 74, 79–91 (2021).
Kikuchi, S., Yoshioka, Y., Prieto-Vila, M. & Ochiya, T. Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. Int. J. Mol. Sci. 20(10), 2584 (2019).
Mao, G. et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 18(3), 373–382 (2015).
Kaur, J. et al. Identification of a suitable endogenous control miRNA in bone aging and senescence. Gene 835, 146642 (2022).
Max Kuhn, K. J. Feature Engineering and Selection: A Practical Approach for Predictive Models (Chapman and Hall/CRC, 2019).
Qiu, J. J. et al. Exosomal metastasis associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. Sci. 14(14), 1960–1973 (2018).
Marciel, M. P. & Hoffmann, P. R. Selenoproteins and metastasis. Adv. Cancer Res. 136, 85–108 (2017).
Muturi, H. T. et al. Tumor and endothelial cell-derived microvesicles carry distinct CEACAMs and influence T-cell behavior. PLoS One 8(9), e74654 (2013).
Vlassov, A. V., Magdaleno, S., Setterquist, R. & Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 1820(7), 940–948 (2012).
Kuriyama, N., Yoshioka, Y., Kikuchi, S., Azuma, N. & Ochiya, T. Extracellular vesicles are key regulators of tumor neovasculature. Front. Cell Dev. Biol. 8, 611039 (2020).
Yu, L. X. et al. Exosomal microRNAs as potential biomarkers for cancer cell migration and prognosis in hepatocellular carcinoma patient-derived cell models. Oncol. Rep. 41(1), 257–269 (2019).
Zheng, M. et al. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 18(1), 76 (2019).
Chen, D. et al. MicroRNA-30d-5p inhibits tumour cell proliferation and motility by directly targeting CCNE2 in non-small cell lung cancer. Cancer Lett. 362(2), 208–217 (2015).
Gao, L. et al. Expression signature and role of miR-30d-5p in non-small cell lung cancer: A comprehensive study based on in silico analysis of public databases and in vitro experiments. Cell. Physiol. Biochem. 50(5), 1964–1987 (2018).
Hu, Z. et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J. Clin. Oncol. 28(10), 1721–1726 (2010).
Nik Mohamed Kamal, N. & Shahidan, W. N. S. Non-exosomal and exosomal circulatory microRNAs: Which are more valid as biomarkers?. Front. Pharmacol. 10, 1500 (2019).
Zhang, S., Jin, J., Tian, X. & Wu, L. hsa-miR-29c-3p regulates biological function of colorectal cancer by targeting SPARC. Oncotarget 8(61), 104508–104524 (2017).
Ji, L. et al. miR-486-3p mediates hepatocellular carcinoma sorafenib resistance by targeting FGFR4 and EGFR. Cell Death Dis. 11(4), 250 (2020).
Reck, M. et al. Antiangiogenic therapy for patients with aggressive or refractory advanced non-small cell lung cancer in the second-line setting. Lung Cancer 120, 62–69 (2018).
Meerson, A. & Yehuda, H. Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer 16(1), 882 (2016).
Stewart, R. L. & O’Connor, K. L. Clinical significance of the integrin alpha6beta4 in human malignancies. Lab. Investig. 95(9), 976–986 (2015).
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. We thank Kumiko Nagase for her technical support. We also thank Mami Murakami for helping with statistical analyses of this work.
Funding
This study was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Education, Culture, Sports and Technology, Japan (#18K08799), the Project for Cancer Research and Therapeutic Evolution (P-CREATE) grant number JP20cm0106402 (to T.O.) and Project for Cancer Research and Therapeutic Evolution (P-PROMOTE) grant number 22ama221405h0001 (to Y.Y.) from the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
Y.S., Y.Y.: Conception and design. Y.S., Y.Y., J.M.: Development of methodology. Y.S.: Acquisition of data. Y.S., Y.Y., T.M., Y.M., H.A., Y.K., T.O., M.O., H.I.: Interpretation of data; writing, review, and revision of the manuscript. N.I. and T.O.: Administrative, technical, or material support; study supervision.
Corresponding author
Ethics declarations
Competing interests
Y. Shimada, Y. Yoshioka, Y. Kudo, T. Mimae, Y. Miyata, H. Adachi, H. Ito, M. Okada, T. Ohira, J. Matsubayashi, and T. Ochiya have no conflicts to disclose. N. Ikeda reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chugai Pharma, grants and personal fees from Eli Lilly, grants and personal fees from MSD, grants and personal fees from Nihon Medi-Physics, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Pfizer, grants and personal fees from Taiho Pharmaceutical, grants and personal fees from Teijin, and personal fees from Bristol-Meyers Squibb Japan outside the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimada, Y., Yoshioka, Y., Kudo, Y. et al. Extracellular vesicle-associated microRNA signatures related to lymphovascular invasion in early-stage lung adenocarcinoma. Sci Rep 13, 4823 (2023). https://doi.org/10.1038/s41598-023-32041-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32041-5
- Springer Nature Limited
This article is cited by
-
Comparing preprocessing strategies for 3D-Gene microarray data of extracellular vesicle-derived miRNAs
BMC Bioinformatics (2024)