Abstract
High antibiotic resistance of gastric pathogen Helicobacter pylori (Hp) and the ability to escape the host immune response prompt searching for therapeutic immunomodulators. Bacillus Calmette–Guerin (BCG) vaccine with Mycobacterium bovis (Mb) is a candidate for modulation the activity of immunocompetent cells, and onco-BCG formulation was successfully used in immunotherapy of bladder cancer. We determined the influence of onco-BCG on the phagocytic capacity of human THP-1 monocyte/macrophage cells, using the model of Escherichia coli bioparticles and Hp fluorescently labeled. Deposition of cell integrins CD11b, CD11d, CD18, membrane/soluble lipopolysaccharide (LPS) receptors, CD14 and sCD14, respectively, and the production of macrophage chemotactic protein (MCP)-1 were determined. Furthermore, a global DNA methylation, was also assessed. Human THP-1 monocytes/macrophages (TIB 202) primed or primed and restimulated with onco-BCG or Hp, were used for assessment of phagocytosis towards E. coli or Hp, surface (immunostaining) or soluble activity determinants, and global DNA methylation (ELISA). THP-1 monocytes/macrophages primed/restimulated with BCG showed increased phagocytosis capacity towards E. coli fluorescent particles, elevated expression of CD11b, CD11d, CD18, CD14, sCD14, increased MCP-1 secretion and DNA methylation. Preliminary results indicate that BCG mycobacteria may also induce the phagocytosis of H. pylori by THP-1 monocytes. Priming or priming and restimulation of monocytes/macrophages with BCG resulted in an increased activity of these cells, which was negatively modulated by Hp.
Similar content being viewed by others
Introduction
Helicobacter pylori, spiral Gram-negative rods, colonize the gastric mucosa in humans (on average 50% population), and induce the development of chronic inflammatory response, gastric or duodenal ulcers, and gastric cancer1,2,3. The role of H. pylori in the development of extragastric diseases has been also suggested4,5,6. Locally in the stomach, H. pylori disintegrate gastric epithelial barrier due to elevated oxidative stress and an increased cell apoptosis7,8. World Health Organization (WHO) reported that resistance of H. pylori to antibiotics, including amoxicillin, clarithromycin, metronidazole, levofloxacin, used for treatment of infection is growing9,10,11,12. High antibiotic resistance together with different strategies of H. pylori to avoid the host immune responses may influence the effectiveness of eradication. Components of H. pylori diminish phagocytic activity of phagocytes13,14,15,16, and expansion as well as cytotoxic activity of natural killer cells (NK)17. Moreover, proliferation of T lymphocytes is affected in response to secreted or cell bound H. pylori compounds, while those which share common sequences with the host proteins may induce antibodies, potentially autoreactive6,8,18,19,20. The idea of using immunomodulating formulations for supporting the treatment of H. pylori is taken into account. Immunostimulants modulate the activity of innate immune cells, including monocytes and NK cells. Potentially it may result in an increased activity against homologous or heterologous infectious agents20,21,22,23,24. Increased anti-microbial activity of “trained cells” might be achieved through epigenetic modifications in genes encoding the immune mediators or by metabolic reprogramming of cells by microbial components25,26. H. pylori influence the first line of defense, where monocytic-macrophage cells play a key role. This prompts the search for formulations restoring the activity of these cells. Macrophages eliminate infectious agents as well as apoptotic cells by phagocytosis, and recover local homeostasis. Mycobacterium bovis Bacillus Calmette–Guerin (BCG) vaccine strain has been considered as candidate immunomodulator for macrophages27,28. It has been suggested that BCG may induce non-specific immune memory in innate immune cells that may be cross-protective against various not related pathogens. Several studies revealed, that BCG vaccination may reduce mortality of infants infected with different infectious agents and induce a better innate immune response against microorganisms other than Mycobacterium tuberculosis, including Candida albicans, Staphylococcus aureus, respiratory syncytial virus, and potentially Sars-Cov-229,30,31.
Onco-BCG vaccine has developed into the most mature immunotherapy for bladder cancer32. The potential mechanisms involve binding of BCG to tumor cell fibronectin and internalization of mycobacteria by tumor cells. Through variety of intracellular signal transduction pathways, cell apoptosis, cell necrosis and an oxidative stress BCG induce tumor cell death. On the other hand, mycobacteria induce cytokines, which drive an immune cascade that facilitates the host’s immune system to kill tumor cells32.
The aim of this study was to determine whether M. bovis onco-BCG vaccine formulation is able to improve phagocytic capacity of human THP-1 monocytes, which was affected in cell cultures in vitro by H. pylori. Furthermore, whether the exposure of THP-1 derived macrophages to BCG mycobacteria will influence the expression of surface integrins CD11b, CD11d, CD18, membrane and soluble lipopolysaccharide (LPS) receptors, CD14 and sCD14, respectively, and the production of macrophage chemotactic protein (MCP)-1. The global DNA methylation will be assessed as preliminary marker of epigenetic modifications.
Results and discussion
Upregulation by M. bovis BCG of monocyte/macrophage phagocytic capacity diminished in response to H. pylori
H. pylori developed several mechanism of escaping the immune mechanisms of the host, including the effectiveness of phagocytosis13,14,15. It has been suggested that an enhanced infiltration of macrophages to the gastric mucosa colonized by H. pylori may increase the elimination of these bacteria directly due to phagocytosis or due to induction of specific immune responses in the gut lymphoid tissue. It might also help to eliminate cells undergoing apoptosis, the number of which is increased during infection with H. pylori7,33. In this study we used in vitro model of THP-1 monocyte cell line to assess whether M. bovis BCG may upregulate the global phagocytic capacity of these cells, which was diminished by H. pylori, by measurement the ability of phagocytes to engulf fluorescently labeled E. coli bioparticles included in Vybrant phagocytosis assay, which is a model system for quantitating the effects of different factors on phagocytic function. This technique takes advantage of the detectability of the intracellular fluorescence emitted by the engulfed particles, as well as the effective fluorescence quenching of the extracellular probe by trypan blue. Phagocytic capacity of monocytes was assessed using cells exposed for 15 or 30 min to H. pylori or cells exposed for 15 or 30 min to BCG alone, to compare whether phagocytes are able to ingest H. pylori or BCG onco. Furthermore, monocytes primed with BCG for 15 min and then exposed to H. pylori for 15 or 30 min were used to determine whether short exposure of phagocytes to BCG onco will influence the phagocytic capacity of monocytes. The exposure time was selected experimentally in the preliminary study. The monocytes, which were exposed simultaneously to H. pylori and BCG for 15 or 30 min consisted an additional experimental variant (Fig. 1). We estimated the phagocytic capacity of such variants of THP-1 monocytes towards fluorescently labeled E. coli by measurement the fluorescence of cells with engulfed bacteria (localized intracellularly) (Fig. 1A).
The influence of short exposure of THP-1 monocytes to M. bovis BCG on their phagocytic properties towards fluorescently labeled E. coli particles or H. pylori. The ingestion by THP-1 cells of fluorescently labeled E. coli (Vybrant phagocytosis assay kit) (A) was assessed in time 0 and after 15 min and 30 min using the following variants of cell cultures: cells exposed to H. pylori alone, cells exposed to BCG (15 min) and then to H. pylori, cells exposed simultaneously to BCG and H. pylori, or cells exposed to BCG alone or cells in RPMI-1640 medium alone (control). The fluorescence of intracellular bacteria was measured. The phagocytic capacity of THP-1 cells towards fluorescently labeled H. pylori (B) was assessed in time 0 and after 15 min and 30 min using the following variants of cell cultures: cells exposed to fluorescently labeled H. pylori alone, cells exposed to unlabeled BCG (15 min) and then to fluorescently labeled H. pylori, cells exposed simultaneously to unlabeled BCG and fluorescently labeled H. pylori, or cells exposed to fluorescently labeled BCG alone. The intensity of fluorescently labeled bacteria remaining in the supernatants was measured. Results (A, B) are presented as ratio of median fluorescence units (RFU) ± range of three independent experiments. The difference statistically significant when p < 0.05 in Mann–Whitney U test. *Cells stimulated versus unstimulated (according to the time of stimulation). Images of THP-1 cells exposed to fluorescently labeled H. pylori or M. bovis BCG alone, to unlabeled M. bovis BCG and then to fluorescently labeled H. pylori, or simultaneously to unlabeled M. bovis BCG and fluorescently labeled H. pylori, in the fluorescence microscope (C). BCG or H. pylori MOI 10:1.
Cells exposed to BCG showed an increased phagocytic capacity towards E. coli as compared to control cells in RPMI-1640 medium alone. By comparison, phagocytic capacity of THP-1 cells exposed to H. pylori did not increase vs control cells (Fig. 1A). However, priming of THP-1 cells for 15 min with BCG and then the exposure of them to H. pylori or simultaneously to BCG and H. pylori resulted in enhanced phagocytosis of fluorescently labeled E. coli as compared to cells treated with H. pylori alone (Fig. 1A). The effectiveness of phagocytosis of E. coli by cells primed with BCG and then with H. pylori was better than phagocytic activity of cells exposed to BCG and H. pylori mix. These results show that short priming of monocytes with BCG-onco improved their phagocytic capacity, which was diminished by H. pylori.
Furthermore we assessed the phagocytic capacity of THP-1 monocytes specifically towards H. pylori or BCG alone, labelled fluorescently using commercial LIVE/DEAD BacLight. In this experimental variant we assessed the fluorescence of bacteria remaining in the cell culture supernatants after co-incubation of bacteria with the phagocytes (Fig. 1B). We showed that BCG bacilli were effectively engulfed by phagocytes after 15 min or 30 min of exposure while phagocytosis of H. pylori was not enhanced. The lower RFU ratio in BCG alone at 0 min, which is different from the H. pylori variant alone, might be the effect of different sensitivity of H. pylori vs BCG bacteria for fluorescence staining procedure, but not MOI, which was the same (10:1). However, this difference did not affect the analyzed results, which showed that phagocytes were able to ingest BCG bacilli, while H. pylori were not ingested. Furthermore, we used THP-1 monocytes primed for 15 min with BCG, which were not labeled. Then monocytes were exposed for 15 min or 30 min to fluorescently labelled H. pylori. We showed that such cells were activated to phagocytize H. pylori as compared to cells exposed to H. pylori alone. Similarly, cells treated simultaneously with mix of unlabeled BCG and labeled H. pylori showed better phagocytic capacity towards H. pylori than cells not treated with BCG (Fig. 1B). These preliminary results indicate that the phagocytic activity of monocytes was induced by BCG onco and resulted in ingestion of H. pylori.
We also looked for the cell images under fluorescent microscope. We could see that monocytes exposed to H. pylori alone after 15 min and 30 min were mainly coated with the clumps of these bacteria. Only some patches with green fluorescence were observed in the nuclear regions, which may indicate the intracellular localization of some H. pylori bacteria (Fig. 1C). In the case of cells treated with BCG alone we could see an intracellular localization of mycobacteria. Furthermore, in the images of monocytes primed with BCG and then exposed to H. pylori the clumps of H. pylori were smaller than those surrounding the phagocytes primed with H. pylori alone. Similar, although weaker effect was showed for phagocytes simultaneously treated with BCG and H. pylori (Fig. 1C). These preliminary results indicate that the priming of THP-1 monocytes with BCG for 15 min or the presence of BCG in the mix suspension with H. pylori which was added to phagocytes resulted in an increased uptake of H. pylori. Potentially it might be due to very effective adhesive properties of mycobacteria, which use phagocytes as target cells34,35. The fluorescence of supernatants in these experimental variants was significantly decreased after 15 min and 30 min of phagocytosis procedure with H. pylori as compared to 0 min time point. However, the measurements of RFU ratio after 15 min and 30 min were comparable, which indicate that the intensity of phagocytosis in these two time points was similar and meaning that maximum of engulfment was reached between 15 and 30 min. In our model of H. pylori phagocytosis, which was examined using LIVE/DEAD BacLight staining procedure it is unclear why if H. pylori formed big clumps around the THP-1 cell surface, the fluorescence of bacteria remaining in the culture supernatants in 15 min or 30 min in this group was not decreased compared to the 0 min time in this group. It could be due to release of fluorescence from H. pylori bacterial clumps or possible from some bacteria undergoing degradation. It may be also possible that soluble components of H. pylori i.e. urease or mediators delivered by macrophages tightly coated with labeled bacteria may drive the increased fluorochrome releasing. These results indicate that H. pylori phagocytosis is a complex process, which might be influenced by different components in the experimental milieu and prompt deeper analysis of H. pylori interaction with macrophages. Further studies are needed to elucidate the exact mechanism induced by BCG mycobacteria in elevation of phagocytic capacity of monocytes. Particularly, this effect is interesting in terms of enhancement of phagocytosis of H. pylori, which can modulate negatively this process13,14,15,16.
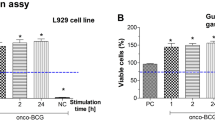
It was interesting to know whether the enhanced phagocytic activity of THP-1 monocytes using the reference green labeled E. coli particles will last in THP-1 derived macrophages after longer priming of phagocytes with BCG or after restimulation of cells with homologous or heterologous bacterial agent, BCG or H. pylori, respectively. Alternatively, whether downregulation of phagocytic activity of macrophages towards fluorescently labeled E. coli by H. pylori might be reversed by restimulation of cells with BCG. These experimental variants might reflect better the activity of immunocompetent cells exposed to immunomodulating factors during chronic H. pylori infection. In this part of the study we used THP-1 derived macrophages, which are long living cells, and evaluated their phagocytic capacity towards fluorescently labeled E.coli in Vybrant phagocytosis assay. The viability of control macrophages was compatible with the viability of cells, which were stimulated according to the following schedule: 24 h priming, 24 h priming and 5 days restimulation as well as 24 h priming, 5 days restimulation and 24 h of additional restimulation, was above 90% as showed by the reference MTT reduction assay (data not shown). Cells primed for 24 h with BCG showed significantly increased phagocytic activity towards E.coli than control cells in RPMI-1640 medium alone (Fig. 2). The elevated phagocytic activity of macrophages primed with onco-BCG was restored after 5 days restimulation of them with homologic BCG (Fig. 2Aa,b) while diminished after 5 days restimulation with H. pylori (Fig. 2A,c). Additional 24 h restimulation of cells with BCG did not result in re-enhancement of phagocytosis (Fig. 2A,d). However, the phagocytic activity of macrophages remained significantly higher than such activity of control cells. By comparison macrophages primed with H. pylori for 24 h and then restimulated for 5 days with the same bacteria did not show enhanced ability to engulf E. coli (Fig. 2Ba,b). However, the phagocytic activity of cells primed with H. pylori was significantly enhanced after restimulation of them for 5 days with BCG, and was restored even after additional 24 h restimulation of cells with H. pylori (Fig. 2Bc,d).
The role of priming/restimulation of THP-1 macrophages with M. bovis BCG in the enhancement and the maintenance of increased phagocytic activity of these cells in Vybrant phagocytosis assay using green-labeled E. coli. Phagocytic activity of THP-1 macrophages primed with M. bovis BCG (A) towards E. coli: cells primed for 24 h with M. bovis BCG (a); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with M. bovis BCG (b); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with H. pylori (c); cells primed for 24 h with M. bovis BCG then restimulated for 5 days with H. pylori and for an additional 24 h with M. bovis BCG (d). Phagocytic activity of THP-1 derived macrophages primed with H. pylori towards E. coli (B): cells primed for 24 h with H. pylori (a); cells primed for 24 h with H. pylori and restimulated for 5 days with H. pylori (b); cells primed for 24 h with H. pylori and restimulated for 5 days with M. bovis BCG (c); cells primed for 24 h with H. pylori, restimulated for 5 days with M. bovis BCG and an additional 24 h with H. pylori (d). Results are presented as median fluorescence units (RFU) ratio ± range of three independent experiments. The difference statistically significant when p < 0.05 in Mann–Whitney U test. *Cells stimulated versus unstimulated (according to the time of stimulation). BCG or H. pylori MOI 10:1.
The phenomenon of increased phagocytosis of E. coli bioparticles by THP-1 derived macrophages exposed to BCG mycobacteria (priming and restimulation) observed by us in vitro might be discussed in the context of cell exposure to H. pylori, which modulate negatively a macrophage phagocytic capacity. We showed that the ability of macrophages exposed to BCG-onco to engulf E.coli bioparticles was restored even after restimulation of cells with H. pylori. Furthermore, very low phagocytic activity of macrophages primed with H. pylori, was significantly enhanced after restimulation of cells with BCG. Further in vivo studies are necessary to see whether BCG mycobacteria can effectively activate phagocytes to prevent H. pylori infection or to stimulate phagocytes towards H. pylori during ongoing infection. In parallel study using a model of Caviae porcellus we showed that inoculation of animals with BCG prior to infection with H. pylori resulted in diminished amount of mucin receptors to H. pylori adhesins, and thus lower colonization of gastric mucosa with these bacteria was observed36. Mycobacteria are relatively intracellular pathogens that through numerous cell to cell interactions promote their own uptake by phagocytes37. On the other hand, some H. pylori adhesins through strong binding to phagocytes may inhibit their ability to engulf H. pylori13. It is possible that mycobacteria, thanks to their own broad interactions with phagocytes, may enhance phagocytic properties of these cells towards other microorganisms, including H. pylori. It has been shown that 19 kD antigen of M. tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytes and promotes phagocytosis of mycobacteria38. These lectin-like interaction of mycobacteria with monocytes/macrophages potentially may interfere with an interactions of H. pylori in driving phagocytosis.
Enhanced expression of cell surface integrins CD11b, CD11d, CD18 in response to priming and restimulation of macrophages with M. bovis BCG
We wanted to explain a potential background of increasing by BCG the phagocytic activity of THP-1 derived macrophages towards E. coli, which was showed in this study. The role of interactions between mycobacteria and macrophage receptors in increasing a phagocytic properties of these cells has been suggested34. In this study we assessed a deposition on phagocytes of selected surface molecules CD11b, CD11d, CD18 (Figs. 3, 4, 5) mediating an interaction of monocytes/macrophages with vascular endothelium in vivo and involved in pathogen recognition and phagocytosis, including complement dependent phagocytosis, as well as in cell survival and T cell tolerance39,40,41,42,43,44. In our experimental model, BCG mycobacteria induced an increased deposition of the above surface integrins on THP-1 macrophages (Figs. 3Aa,b, 4Aa,b, 5Aa,b). This deposition was diminished during restimulation of cells for 5 days with H. pylori (Figs. 3Ab,c, 4Ab,c, 5Ab,c), however, the exposure of cells to BCG for additional 24 h resulted in upregulation of studied surface molecules (Figs. 3Ad, 4Ad, 5Ad). These results suggest that monocytes/macrophages primed and restimulated with BCG are able to respond by an enhanced expression of CD11b, CD11d, CD18, despite its temporary inhibition due to cell exposure to H. pylori. The sensitivity of BCG treated cells to H. pylori modulation may indicate that BCG-driven effects might be not stable suggesting the lack of an innate memory phenomenon25. However, it is interesting, that BCG mycobacteria were able to upregulate the expression of CD11b, CD11d, and CD18 in THP-1 macrophages primed with H. pylori (Figs. 3Bc, 4Bc, 5Bc). H. pylori bacteria did not enhance the expression of studied integrins or even decreased their deposition on phagocytes (Figs. 3Ba,b, 4Ba,b, 5Ba,b). In the case of CD11d, an increased expression, was observed even on cells that were subjected to an additional 24 h restimulation with H. pylori, started after 5 day restimulation of cells with BCG (Fig. 4Bd), but not for CD11b, CD18 (Figs. 3Bd, 5Bd).
Positive modulation of CD11b expression in THP-1 macrophages by M. bovis BCG. Cells were primed with M. bovis BCG or H. pylori and then they underwent restimulation with homologous or heterologous microbial agent. THP-1 macrophages primed with M. bovis BCG (A): cells primed for 24 h with M. bovis BCG (a); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with M. bovis BCG (b); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with H. pylori (c); cells primed for 24 h with M. bovis BCG then restimulated for 5 days with H. pylori and for an additional 24 h with M. bovis BCG (d). THP-1 macrophages primed with H. pylori (B): cells primed for 24 h with H. pylori (a); cells primed for 24 h with H. pylori and restimulated for 5 days with H. pylori (b); cells primed for 24 h with H. pylori and restimulated for 5 days with M. bovis BCG (c); cells primed for 24 h with H. pylori, restimulated for 5 days with M. bovis BCG and an additional 24 h with H. pylori (d). The above variants of cells were stained using the fluorescently labeled specific anti-CD11b antibodies. Results are presented as median fluorescence units (RFU) ratio ± range of three independent experiments. The difference statistically significant when p < 0.05 in Mann–Whitney U. *Cells stimulated versus unstimulated (according to the time of stimulation). BCG or H. pylori MOI 10:1.
Positive modulation of CD11d expression in THP-1 macrophages by M. bovis BCG. Cells were primed with M. bovis BCG or H. pylori and then underwent restimulation with homologous or heterologous microbial agent. THP-1 macrophages primed with M. bovis BCG (A): cells primed for 24 h with M. bovis BCG (a); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with M. bovis BCG (b); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with H. pylori (c); cells primed for 24 h with M. bovis BCG then restimulated for 5 days with H. pylori and for an additional 24 h with M. bovis BCG (d). THP-1 macrophages primed with H. pylori (B): cells primed for 24 h with H. pylori (a); cells primed for 24 h with H. pylori and restimulated for 5 days with H. pylori (b); cells primed for 24 h with H. pylori and restimulated for 5 days with M. bovis BCG (c); cells primed for 24 h with H. pylori, restimulated for 5 days with M. bovis BCG and an additional 24 h with H. pylori (d). The above variants of cells were stained using the fluorescently labeled specific anti-CD11d antibodies. Results are presented as median fluorescence units (RFU) ratio ± range of three independent experiments. The difference statistically significant when p < 0.05 in Mann–Whitney U test. *Cells stimulated versus unstimulated (according to the time of stimulation). BCG or H. pylori MOI 10:1.
Positive modulation of CD18 expression in THP-1 macrophages by M. bovis BCG. Cells were primed with M. bovis BCG or H. pylori and then underwent restimulation with homologous or heterologous microbial agent. THP-1 cells primed with M. bovis BCG (A): cells primed for 24 h with M. bovis BCG (a); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with M. bovis BCG (b); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with H. pylori (c); cells primed for 24 h with M. bovis BCG then restimulated for 5 days with H. pylori and for an additional 24 h with M. bovis BCG (d). THP-1 macrophages primed with H. pylori (B): cells primed for 24 h with H. pylori (a); cells primed for 24 h with H. pylori and restimulated for 5 days with H. pylori (b); cells primed for 24 h with H. pylori and restimulated for 5 days with M. bovis BCG (c); cells primed for 24 h with H. pylori, restimulated for 5 days with M. bovis BCG and an additional 24 h with H. pylori (d). The above variants of cells were stained using the fluorescently labeled specific anti-CD18 antibodies. Results are presented as median fluorescence units (RFU) ratio ± range of three independent experiments. The difference statistically significant when p < 0.05 in Mann–Whitney U test. BCG or H. pylori MOI 10:1.
These observations suggest that BCG mycobacteria are able to induce the expression of the studied functional surface molecules in monocytes/macrophages even in cells exposed to negative modulator like H. pylori.
H. pylori and soluble components of these bacteria, including LPS, induce an elevated oxidative stress in the gastric mucosa in conjunction with an increased epithelial cell apoptosis7,33. Ganguly et al.44 showed that M. tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex may inhibit LPS-induced nuclear factor (NF)-kappa B transactivation by downregulation of reactive oxygen species production. It has been found that bacterial LPS specifically inhibited mouse macrophage uptake of apoptotic neutrophils through suppression of anti-inflammatory growth arrest-specific gene 6 (Gas6), and induction of tumor necrosis factor (TNF)-α45. The positively modulated expression of CD11b, CD11d, and CD18 on THP-1 macrophages by BCG-onco showed in this in vitro study may suggest that such upregulation is also possible in vivo. In the further study this hypothesis will be verified in vivo on an experimental model of H. pylori infection in Caviae porcellus, which was characterized by us in terms of inflammation and an immune responses46.
Upregulation by M. bovis BCG of LPS receptors CD14/sCD14 and sMCP
During infection, the immune cells mainly monocytes and macrophages respond to bacterial LPS, present in tissues or the bloodstream, via the Toll-like receptor 4 (TLR-4)/CD14 signaling in conjunction with soluble CD14 (sCD14) and lipopolysaccharide binding protein (LBP). These interactions trigger pro-inflammatory reactions facilitating eradication of the invading bacteria47. It has been shown that an experimental exclusion of TLR4-dependent cell signaling during low-grade polymicrobial sepsis resulted in an impaired bacterial clearance and thereby worsened organ injury leading to a higher mortality of mice48,49. On the other hand, an exaggerated and uncontrolled pro-inflammatory signaling triggered by TLR4/CD14 during infection can lead to sepsis50. As different forms of endotoxin are present in many micro-organisms, the response of the host to non-E. coli LPS may be weaker as compared to E. coli-derived endotoxin. Thus, other bacterial infections may require the full inflammatory potential of the endotoxin response. The phosphorylation and acylation of H. pylori lipid A, a part of LPS, is lower as compared to typical LPS of gut pathogens like E. coli51. In the previous study we showed that phagocytic activity of human granulocytes towards H. pylori was diminished in the milieu of LPS of these bacteria16, and other components of these bacteria like surface haemagglutinins and heparin binding proteins may be involved in an inhibition of H. pylori engulfment13. Also intracellular survival of these bacteria can be affected due to closing of H. pylori in cytoplasmic megasomes15. LPS H. pylori also induced apoptosis of macrophages diminishing their activity as antigen presenting cells52. In this study we asked whether M. bovis BCG may increase the expression of membrane CD14 and the secretion of sCD14 (Supplementary Figs. S1, S2).
The expression of CD14 on THP-1 macrophages exposed for 24 h to BCG was similar as in control cells sub-cultured in medium alone (Supplementary Fig. S1Aa). Restimulation of such cells for 5 days with homologous BCG, but not with heterologous agent as H. pylori, resulted in significantly increased expression of CD14 (Supplementary Fig. S1Ab,c). Furthermore, the priming of THP-1 derived macrophages for 24 h with BCG mycobacteria resulted in an enhancement CD14 membrane receptor expression on cells, which were then restimulated for 5 days with H. pylori (Supplementary Fig. S1Ac,d). Priming of phagocytes with H. pylori and then restimulation them with these homologous bacteria did not upregulate CD14 expression (Supplementary Fig. S1Ba,b). Deposition of CD14 was significantly increased only on cells primed with H. pylori and restimulated for 5 days with BCG (Fig. S1Bc). However, additional restimulation of such cells with H. pylori resulted in lower CD14 deposition (Supplementary Fig. S1Bc,d). These results again suggest that BCG mycobacteria and H. pylori drive different effects in THP-1 macrophages, increasing or diminishing a CD14 expression, respectively.
Also secretion of sCD14 was significantly increased in cell culture supernatants containingTHP-1 cells primed and restimulated with BCG, and even after restimulation of cells with H. pylori (Fig. S2). It remained higher than in control cells, which were sub-cultured in medium alone (Supplementary Fig. S2a–d). Priming and restimulation of macrophages with H. pylori alone did not stimulate these cells to sCD14 secretion (Supplementary Fig. S2Ba,b). However, BCG restimulation of cells primed with H. pylori, which did not release sCD14, resulted in an increased production of sCD14 (Supplementary Fig. S2Bc), which remained enhanced after an additional 24 h exposure of cells to H. pylori (Supplementary Fig. S2Bd). Increased secretion by macrophages stimulated with BCG a soluble CD14 receptor for bacterial LPS and an increased membrane CD14 expression, may reflect a higher potential of these cells to respond to infectious agents, via molecular pattern such as LPS.
In the inflammatory milieu, phagocytes are recruited via endogenous mediators such as soluble macrophage protein 1 (sMCP-1). In our experimental in vitro model only THP-1 cells primed or primed and restimulated with BCG delivered sMCP-1, and the effect of cells exposed to BCG twice was higher than the effect of cells exposed only once (Supplementary Fig. S3Aa,b). H. pylori downregulated sMCP-1 production by THP-1 macrophages exposed to BCG, however an additional 24 h stimulation of such cells with mycobacteria resulted in an increased sMCP-1 secretion (Supplementary Fig. S3Ac,d). By comparison, in cultures of THP-1 cells, which were primed or primed and restimulated with H. pylori the sMCP-1 concentration was on the level of control culture (cells in medium alone), and did not change after restimulation of cells with BCG (Supplementary Fig. S3). These results indicate that macrophages treated with BCG delivered sMCP-1, which in vivo potentially may increase the local population of monocytes/macrophages. On the contrary, cells primed or primed and restimulated with H. pylori did not respond by sMCP-1 production, even after restimulation with BCG. These may suggest that only in cells exposed to BCG, but not to H. pylori this activity can be induced, and restored.
Increased global monocyte DNA methylation induced by M. bovis BCG
An increased DNA methylation, is a proposed marker of an increased memory-like monocyte/macrophage activity without changing the DNA sequence53,54,55,56. In this study we asked whether in THP-1 derived macrophages primed or primed and restimulated with BCG-onco the global DNA methylation was increased (Fig. 6). We showed that priming of THP-1 cells and each variant of restimulation of these cells with M. bovis BCG resulted in an increased global DNA methylation (Fig. 6Aa–d, Ba–d).
Global DNA methylation. Cells were primed with M. bovis BCG or H. pylori and then underwent restimulation with homologous or heterologous microbial agent. THP-1 macrophages primed with M. bovis BCG (A): cells primed for 24 h with M. bovis BCG (a); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with M. bovis BCG (b); cells primed for 24 h with M. bovis BCG and restimulated for 5 days with H. pylori (c); cells primed for 24 h with M. bovis BCG then restimulated for 5 days with H. pylori and for an additional 24 h with M. bovis BCG (d). THP-1 macrophages primed with H. pylori (B): cells primed for 24 h with H. pylori (a); cells primed for 24 h with H. pylori and restimulated for 5 days with H. pylori (b); cells primed for 24 h with H. pylori and restimulated for 5 days with M. bovis BCG (c); cells primed for 24 h with H. pylori, restimulated for 5 days with M. bovis BCG and an additional 24 h with H. pylori (d). The global DNA methylation was determined by the ELISA with high DNA affinity strip wells, and capture as well as detection antibodies specific for 5 hmC were used. The quantity of hydroxymethylated DNA fragments was detected colorimetrically at OD = 450 nm. The percentage of 5hmC in DNA samples was calculated in reference to standard curve. Results are presented as median ratio ± range of three independent experiments. The difference statistically significant when p < 0.05 in Mann–Whitney U test. *Cells stimulated versus unstimulated (according to the time of stimulation). BCG or H. pylori MOI 10:1.
Conclusion
Using in vitro model of human THP-1 monocytes or THP-1 derived macrophages, we focused on searching whether M. bovis onco-BCG may increase the phagocytic activity of monocytes/macrophages towards the reference E.coli bioparticles. Alternatively, whether the activity of phagocytes diminished in response to H. pylori can be upregulated by BCG. The obtained results indicate that BCG-onco mycobacteria improved the phagocytic activity of THP-1 monocytes and THP-1 derived macrophages towards E.coli. Furthermore, the BCG priming and restimulation of THP-1 macrophages resulted in an increased expression of several functional cell surface molecules and secretion of macrophage chemotactic protein. In THP-1 cells primed or primed and restimulated with BCG the global DNA methylation was enhanced as compared to cells, which were not exposed to BCG. This may indicate that BCG induce in monocytes/macrophages epigenetic changes. Our preliminary results also indicate that THP-1 monocytes exposed to H. pylori were not able to engulf these bacteria while when primed with BCG mycobacteria showed such activity. These interesting result must be confirmed in further study using the quenching methodology of extracellularly attached H. pylori. The potential mechanisms of BCG-induced phagocytosis of H. pylori require further understanding. These in vitro results prompt further study on in vivo model of Caviae porcellus with experimental H. pylori infection, which was well characterized in terms of inflammatory and immune responses46. Using this model it will be possible to determine whether inoculation of animals with BCG before H. pylori infection or during ongoing infection with these bacteria will prevent or diminish colonization, respectively. Our preliminary results on Caviae porcellus model showed that inoculation of animals per os with onco-BCG resulted in diminished colonization of gastric mucosa with H. pylori, which was related to diminished amount of mucin containing receptors for H. pylori adhesins36. The current in vitro study showed that BCG may enhance phagocytic capacity of monocytes/macrophages which was affected by H. pylori and the expression of an important activity markers of macrophages.
Material and methods
Cell cultures
Human THP-1 monocytes (purchased in American Type Culture Collection, ATCC TIB-202, Manassas, VA, USA) (TIB 202). Cells were grown in RPMI (Roswell Park Memorial Institute) -1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 U/mL streptomycin, 2 mM/ml L-glutamine at 37 °C, (all in Biowest, Nuaillé, France), in an atmosphere of cell culture incubator, containing 5% CO2. Cells were passaged every 3 days to maintain cell density < 2 × 106 cells/mL.
Stimulation of THP-1 derived macrophages
In several experiments human monocytes THP-1 were differentiated into macrophages with 50 nM phorbol-12-myristate-13-acetate (PMA; Sigma-Aldrich, Saint Louis, USA) for 72 h at 37 °C in the condition of cell culture incubator. After 72 h, the adherent macrophages were exposed to the Bacillus Calmette–Guérin (BCG)-onco vaccine (onco-BCG, Biomed, Lublin, Poland) containing Mycobacterium bovis bacilli (M. bovis BCG) or to Helicobacter pylori CCUG 17874 (purchased from Culture Collection Univeristy of Gotehnburg, Sweden). Different time schedules of macrophage priming or restimulation were used to determine the immunomodulatory activity of microbial formulations. In this study we used the procedure described by Bekkering et al.57, in own modification: exposure for 24 h to BCG or H. pylori; exposure for 24 h to BCG or H. pylori and then restimulation for 5 days with homological or heterological microbial agent (BCG/H. pylori); exposure for 24 h to BCG or H. pylori, restimulation for 5 days with homological or heterological bacterial agent (H. pylori/BCG); and additional restimulation for 24 h (BCG/H. pylori). The viability of control macrophages compatible with cells, which were stimulated according to the following schedule: 24 h priming, 24 h priming and 5 days restimulation as well as 24 h priming, 5 days restimulation and 24 h of additional restimulation, was evaluated in the reference 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay according to ISO norm as previously described58.
M. bovis BCG bacilli were suspended in RPMI-1640 culture medium to the density of 5 × 107 CFU (colony-forming unit)/mL; H. pylori were grown for 72 h on commercial blood agar plates dedicated to these bacteria (Becton Dickinson East Rutherford, New Jersey, USA), in microaerophilic conditions, suspended in RPMI-1640 to the density of 5 × 107 CFU/mL. Both M. bovis and H. pylori were used for stimulation of THP-1 cells as multiplicity of infection (MOI) of 10:1. Lipopolysaccharide (LPS) Escherichia coli (O55:B5 serotype) (Merck Millipore, Burlington, USA), at the concentration 1 µg/mL was used as positive control. After cell priming or priming and restimulation the cells were used in phagocytosis assay, and to assess selected cell surface integrins/receptors and DNA global methylation while cell culture supernatants were used to determine the concentration of selected soluble mediators.
Phagocytosis
The suspension of THP-1 monocytes in RPMI-1640 culture medium (5 × 106 cells/mL) was applied to the wells of 96-well plate (100 µL/well), and stimulated with live BCG and/or H. pylori at MOI of 10:1 in the following variants: fluorescently labeled H. pylori 15 or 30 min, fluorescently labeled BCG 15 or 30 min, first unlabeled BCG 15 min and next fluorescently labeled H. pylori 15 or 30 min, or simultaneously with fluorescently labeled H. pylori and unlabeled BCG mix 15 or 30 min. H. pylori rods and onco-BCG mycobacteria were stained with the commercial LIVE/DEAD BacLight (ThermoFisher, Waltham, USA) tracer for 30 min at room temperature33. After labeling, the bacteria were centrifuged 2 times to wash away excess dye. The pre-labeled fluorescent bacteria were plated into the wells containing the phagocytes and stimulated as described above. At selected time points, cell culture supernatants were collected and the fluorescence was measured using a multifunctional reader SpectraMax i3 (Molecular Devicesat, San Jose, CA, USA). Cytospin preparations were made from the remaining cells (cells were collected with medium and 300 µl of cell suspension was centrifuged at 300 × g for 10 min). Cells were fixed with 4% formaldehyde, for 20 min, at room temperature, washed 3 times with phosphate buffered saline (PBS) and stained with DAPI fluorochrome (Sigma-Aldrich, Saint Louis, USA) (1μ DAPI/1000 μL PBS), for 15 min under the conditions as above. Cells were imaged under confocal microscopy (Leica TCS SPE, Wetzlar, Germany) at the wavelength for each fluorochrome: fluorescein isothiocyanate (FITC)/ BacLight (excitation 495 nm, emission 519 nm), DAPI (excitation 345 nm, emission 455 nm) at a magnification of 63 × .
Phagocytic activity of THP-1 cells was also assessed using fluorescently labeled Escherichia coli bioparticles from Vybrant Phagocytosis Assay Kit (ThermoFisher Scientific, Waltham, USA), as recommended by the manufacturer. For this purpose the various variants of cells were used: stimulated with H. pylori 15 or 30 min, stimulated with BCG 15 or 30 min, exposed first to BCG 15 min and next to H. pylori 15 or 30 min, or exposed simultaneously to H. pylori and BCG mix 15 or 30 min. To see how longer and repeated exposure of cells to BCG or H. pylori will influence the phagocytic activity of THP-1 derived macrophages we used different variants of cells, which were primed with BCG or H. pylori for 24 h, and then restimulated for 5 days or additional 24 h with homologous or heterologous bacterial agent (BCG/H. pylori). Intensity of phagocytosis was measured by using a multifunctional reader SpectraMax i3 (Molecular Devices, San Jose, CA, USA) reader at 495 nm (excitation) and 515 nm (emission).Three independent experiments were performed in triplicate for each variant.
Determination of cell surface markers: immunofluorescence
The macrophages were prepared for staining with primary and secondary antibodies fluorescently labeled, as previously described7. To assess macrophage surface markers of activation, we used the following monoclonal antibodies: rabbit anti-CD11b, mouse anti-CD11d, rat anti-CD14, rabbit anti-CD18 (all from ThermoFisher Waltham, USA), diluted 1:200 in 1% bovine serum albumin (BSA) in PBS. The macrophages were then treated with the appropriate secondary antibody: goat anti-rabbit, goat anti-mouse or goat anti-rat labeled with Alexa Fluor488 or Alexa Fluor 568 (Invitrogen, CA USA) diluted 1:200 I 1% BSA/PBS. The intensity of fluorescence was measured by using a multifunctional reader SpectraMax i3 (Molecular Devices, San Jose, CA, USA) at the appropriate wavelengths: for Alexa Fluor488 (excitation 495 nm, emission 519 nm), for Alexa Fluor 568 (excitation 591 nm, emission 608 nm). Three independent experiments were performed in triplicate.
Determination by the ELISA of soluble mediators delivered by macrophages
Cell culture supernatants were tested for soluble CD14 (sCD14), and macrophage chemotactic protein (MCP)-1 by the commercial Enzyme Linked Immunosorbent Assay—ELISA (ThermoFisher, Waltham, USA), with a sensitivity of 6 pg/mL (sCD14), and 3.3 pg/mL (MCP-1). The ELISA assays dedicated to each mediator were developed as recommended by the manufacturer. Three independent experiments were performed in triplicate.
Isolation of macrophage DNA and estimation of its global methylation
The suspensions of macrophages, which underwent priming or priming and restimulation as described above were transferred to individual tubes and centrifuged (400 × g for 10 min, at room temperature). Cellular pellets were suspended in 200 μL of TRIS buffer and were used for DNA isolation according to the Genomic Mini DNA purification protocol (A&A Biotechnology, Gdansk, Poland). Briefly, samples were transferred to 2 mL DNAse free tubes, treated with lysis buffer and proteinase K for 20 min, and for 5 min with 5 μL of RNAase A (10 mg/mL). Following incubation (5 min at 70 °C) with TRIS elution buffer, 20 s vortexing and centrifugation (250 × g) the supernatants were transferred to microcolumns. Purified DNA samples were stored at − 20 °C. The efficiency and purity of DNA were verified spectrophotometrically by Nanophotometer Pearl (Implen, Westlake, USA), 100–125 ng/μL, and A 260/280 ratio = 1.6, respectively. DNA Methylation enzyme‐linked immunosorbent assay (ELISA; Epigenetek, Farmingdale, USA), with high DNA affinity strip wells, and capture as well as detection antibodies specific for 5 hmC were used. The quantity of hydroxymethylated DNA fragments was detected colorimetrically at OD = 450 nm. The percentage of 5hmC in DNA samples was calculated in reference to standard curve ranging from 0.02 to 1%. To calculate the percentage of 5 hmC in total DNA the following formula was used: 5‐hmC% = (sample OD − negative control OD/slope × 100 ng) × 100%
Statistical analysis
Data were expressed as the median ± range. The differences between groups were tested using the non-parametric Mann–Whitney U test. For statistical analysis the Statistica 13 PL software (https://statistica.software.informer.com/13.3software (Krakow, Poland), was used. Results were considered statistically significant when p < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article or supplementary file.
References
Warren, J. R. & Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1275 (1983).
Rudnicka, K., Backert, S. & Chmiela, M. Genetic polymorphisms in inflammatory and other regulators in gastric cancer: Risks and clinical consequences. Curr. Top. Microbiol. Immunol. 421, 53–76. https://doi.org/10.1007/978-3-030-15138-6_3 (2019).
Chmiela, M. & Kupcinskas, J. Pathogenesis of Helicobacter pylori infection. Helicobacter 24, e12638. https://doi.org/10.1111/hel.12638 (2019).
Papagiannakis, P. et al. The role of Helicobacter pylori infection in hematological disorders. Eur. J. Intern. Med. 24, 685–690. https://doi.org/10.1016/j.ejim.2013.02.011 (2013).
Park, J. S. et al. Gastric autoantigenic proteins in Helicobacter pylori infection. Yonsei Med. J. 54, 1342–1352. https://doi.org/10.3349/ymj.2013.54.6.1342 (2013).
Gonciarz, W. et al. Autoantibodies to a specific peptide epitope of human Hsp60 (ATVLA) with homology to Helicobacter pylori HspB in H. pylori-infected patients. APMIS 127, 139–149. https://doi.org/10.1111/apm.1292 (2019).
Gonciarz, W. et al. The effect of Helicobacter pylori infection and different H. pylori components on the proliferation and apoptosis of gastric epithelial cells and fibroblasts. PLoS ONE 14, e0220636. https://doi.org/10.1371/journal.pone.0220636 (2019).
Mnich, E. et al. Impact of Helicobacter pylori on the healing process of the gastric barrier. World J. Gastroenterol. 22, 7536–7558. https://doi.org/10.3748/wjg.v22.i33.7536 (2016).
Li, B. et al. Proton-pump inhibitor and amoxicillin-based triple therapy containing clarithromycin versus metronidazole for Helicobacter pylori: A meta-analysis. Microb. Pathog. 142, 104075. https://doi.org/10.1016/j.micpath.2020.104075 (2020).
Savoldi, A., Carrara, E., Graham, D. Y. & Tacconelli, C. E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 155, 1372–1382. https://doi.org/10.1053/j.gastro.2018.07.007 (2018).
Cui, R. et al. Correlation analysis among genotype resistance, phenotype resistance and eradication effect of Helicobacter pylori. Infect. Drug Resist. 14, 1747–1756. https://doi.org/10.2147/IDR.S305996 (2021).
Bujanda, L. et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics (Basel) 10, 1058. https://doi.org/10.3390/antibiotics10091058 (2021).
Chmiela, M., Czkwianianc, E., Wadstrom, T. & Rudnicka, W. Role of Helicobacter pylori surface structures in bacterial interaction with macrophages. Gut 40, 20–24 (1997).
Ramarao, N. & Meyer, T. F. Helicobacter pylori resists phagocytosis by macrophages: Quantitative assessment by confocal microscopy and fluorescence-activated cell sorting. Infect. Immun. 69, 2604–2611. https://doi.org/10.1128/IAI.69.4.2604-2611.2001 (2001).
Allen, L. A. Phagocytosis and persistence of Helicobacter pylori. Cell Microbiol. 9, 817–828. https://doi.org/10.1111/j.1462-5822.2007.00906.X (2007).
Grębowska, A. et al. Anti-phagocitic activity of Helicobacter pylori lipopolysaccharide (LPS)—Possible modulation of the innate immune response to these bacteria. Pol. J. Microbiol. 57, 185–192 (2008).
Rudnicka, K. et al. Helicobacter pylori-driven modulation of NK cell expansion, intracellular cytokine expression and cytotoxic activity. Innate Immun. 21, 127–139. https://doi.org/10.1177/1753425913518225 (2015).
Paziak-Domańsk, B., Chmiela, M., Jarosińska, A. & Rudnicka, W. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell Immunol. 202, 136–139 (2002).
Matusiak, A. et al. Putative consequences of exposure to Helicobacter pylori infection in patients with coronary heart disease in terms of humoral immune response and inflammation. Arch. Med. Sci. 12, 45–54. https://doi.org/10.5114/aoms.2015.50772 (2016).
Miszczyk, E. et al. Antigen-specific lymphocyte proliferation as a marker of immune response in guinea pigs with sustained Helicobacter pylori infection. Acta Bioch. Pol. 62, 295–303 (2014).
Saroj, P., Verma, M. & Jha, K. K. An overview on immunomodulation. J. Adv. Sci. Res. 3, 1–12 (2012).
Netea, M. G. et al. Trained immunity: A program of innate immune memory in health and disease. Science 352, aaf1098. https://doi.org/10.1126/science.aaf1098 (2016).
Sohrabi, Y. et al. Trained immunity as a novel approach against COVID-19 with a focus on Bacillus Calmette–Guérin vaccine: Mechanisms, challenges and perspectives. Clin. Transl. Immunol. 9, e1228. https://doi.org/10.1002/cti2.1228 (2020).
Teunissen, A. J. P. et al. Targeting trained innate immunity with nanobiologics to treat cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 41, 1839–1850. https://doi.org/10.1161/ATVBAHA.120.315448 (2021).
Rusek, P., Wala, M., Druszczyńska, M. & Fol, M. Infectious agents as stimuli of trained innate immunity. Int. J. Mol. Sci. 19, 456. https://doi.org/10.3390/ijms19020456 (2018).
van der Heijden, C. D. C. C. et al. Epigenetics and trained immunity. Antioxid. Redox Signal. 10(29), 1023–1040. https://doi.org/10.1089/ars.2017.7310 (2018).
Masihi, K. N. Fighting infection using immunomodulatory agents. Expert Opin. Biol. Ther. 1, 641–653. https://doi.org/10.1517/14712598.1.4.641 (2001).
Yamazaki-Nakashimada, M. A. et al. BCG: A vaccine with multiple faces. Hum. Vaccin. Immunother. 16, 1841–1850. https://doi.org/10.1080/21645515.2019.1706930 (2020).
Freyne, B., Marchant, A. & Curtis, N. BCG-associated heterologous immunity, a historical perspective: Intervention studies in animal models of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 109, 52–61. https://doi.org/10.1093/trstmh/tru197 (2015).
Aaby, P. et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period?. J. Infect. Dis. 15(204), 245–252. https://doi.org/10.1093/infdis/jir240 (2011).
Wardhana, D. et al. The efficacy of Bacillus Calmette–Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 43, 185–190 (2011).
Hana, J., Gua, X., Lia, Y. & Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 129, 110393. https://doi.org/10.1016/j.biopha.2020.110393 (2020).
Gonciarz, W., Krupa, A. & Chmiela, M. Proregenerative cctivity of IL-33 in gastric tissue cells undergoing Helicobacter pylori-induced apoptosis. Int. J. Mol. Sci. 21, 1801. https://doi.org/10.3390/ijms21051801 (2020).
Ernst, J. D. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66, 1277–1281 (1998).
Vinod, V., Vijayrajratnam, S., Vasudevan, A. K. & Biswas, R. The cell surface adhesins of Mycobacterium tuberculosis. Microbiol. Res. 232, 126392. https://doi.org/10.1016/j.micres.2019.126392 (2020).
Gonciarz, W., Chyb, M. & Chmiela, M. Modulation of the Helicobacter pylori interaction with host cells through Mycobacterium bovis onko-BCG vaccine on in vitro and in vivo models. Microb. Health Dis. 4, e735. https://doi.org/10.26355/mhd_20229_735 (2022).
Squeglia, F., Ruggiero, A., De Simone, A. & Berisio, R. A structural overview of mycobacterial adhesins: Key biomarkers for diagnostics and therapeutics. Protein Sci. 27, 369–380. https://doi.org/10.1002/pro.3346 (2017).
Diaz-Silvestre, H. et al. The 19 kD antigen of Mycobacterium tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytic cells and promotes phagocytosis of mycobacteria. Microb. Pathog. 39, 97–107. https://doi.org/10.1016/j.micpath.2005.06.002 (2005).
Sun, H., Zhi, K., Hu, L. & Fan, Z. The activation and regulation of Beta2 integrins in phagocytes and phagocytosis. Front. Immunol. 12, 633639. https://doi.org/10.3389/fimmu.2021.633639 (2021).
Vandendriessche, S., Cambier, S., Proost, P. & Marques, P. E. Complement receptors and their role in leukocyte recruitment and phagocytosis. Front. Immunol. 10, 2318. https://doi.org/10.3389/fimmu.2021.62402 (2021).
Yao, X. et al. Leukadherin-1-mediated activation of CD11b inhibits LPS-induced pro-inflammatory response in macrophages and protects mice against endotoxic shock by blocking LPS-TLR4 interaction. Front. Immunol. 10, 215. https://doi.org/10.3389/fimmu.2019.00215 (2019).
Abdelbaqi, M. et al. Regulation of dextran sodium sulfate induced colitis by leukocyte beta 2 integrins. Lab. Investig. 86, 380–390. https://doi.org/10.1038/labinvest.3700398 (2006).
Ehirchiou, D. et al. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J. Exp. Med. 204, 1519–1524. https://doi.org/10.1084/jem.20062292 (2007).
Ganguly, N. et al. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex inhibit lipopolysaccharide induced NF-kappa B transactivation by downregulation of reactive oxygen species (ROS) production. Immunol. Cell Biol. 86, 98–106. https://doi.org/10.1038/sj.icb.7100117 (2008).
Feng, X. et al. Lipopolysaccharide inhibits macrophage phagocytosis of apoptotic neutrophils by regulating the production of tumour necrosis factor α and growth arrest-specific gene 6. Immunology 132, 287–295. https://doi.org/10.1111/j.1365-2567.2010.03364.x (2011).
Walencka, M. et al. The microbiological, histological, immunological and molecular determinants of Helicobacter pylori infection in guinea pigs as a convenient animal model to study pathogenicity of these bacteria and the infection dependent immune response of the host. Acta Bioch. Pol. 62, 697–706. https://doi.org/10.18388/abp.2015_1110 (2015).
Ciesielska, A., Matyjek, M. & Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 78, 1233–1261. https://doi.org/10.1007/s00018-020-03656-y (2021).
Deng, M. et al. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J. Immunol. 15(190), 5152–5160. https://doi.org/10.4049/jimmunol.1300496 (2013).
Zhang, M. et al. Toll-like receptor 4 is essential to preserving cardiac function and survival in low-grade polymicrobial sepsis. Anesthesiology 121, 1270–1280. https://doi.org/10.1097/ALN.0000000000000337 (2014).
Poltorak, A. et al. Deffective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Moran, A. P. et al. Phenotypic variation in molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. Acid-induced phase variation in Lewis(x) and Lewis(y) expression by H. pylori lipopolysaccharides. J. Biol. Chem. 22(277), 5785–5795. https://doi.org/10.1074/jbc.M108574200 (2002).
Grębowska, A. et al. H. pylori lipopolysaccharide activity in human peripheral blood mononuclear leukocytes cultures. J. Physiol. Pharmacol. 61, 437–442 (2010).
Keating, S. T. & El-Osta, A. Epigenetics and metabolism. Circ. Res. 116, 715–736. https://doi.org/10.1161/CIRCRESAHA.116.303936 (2015).
Yang, Y. et al. PSTPIP2 connects DNA methylation to macrophage polarization in CCL4-induced mouse model of hepatic fibrosis. Oncogene 37, 6119–6135. https://doi.org/10.1038/s41388-018-0383-0 (2018).
Ji, J. et al. Methionine attenuates lipopolysaccharide-induced inflammatory responses via DNA methylation in macrophages. ACS Omega 4, 2331–2336. https://doi.org/10.1021/acsomega.8b0357 (2019).
Natoli, G. & Ostuni, R. Adaptation and memory in immune responses. Nat. Immunol. 20, 783–792. https://doi.org/10.1038/s41590-019-0399-9 (2019).
Bekkering, S. et al. In vitro experimental model of trained innate immunity in human primary monocytes. Clin. Vaccine Immunol. 23, 926–933. https://doi.org/10.1128/CVI.00349-16 (2016).
Brzeziński, M. et al. Nanocarriers based on block copolymers of l-proline and lactide: The effect of core crosslinking versus its pH-sensitivity on their cellular uptake. Eur. Polym. J. 156, 110572. https://doi.org/10.1016/j.eurpolymj.2021.110572 (2021).
Funding
This research was financially supported by University of Lodz, Grant Number 15/GNZPA/2022 (B2111001000027.07). Development of chitozan biopolimer with Mycobacterium bovis BCG-onko vaccine mycobacteria, with immunomodulatory properties, to improve immune response towards Helicobacter pylori and Student Research Grants financed by University of Lodz.
Author information
Authors and Affiliations
Contributions
M.C. and W.G.: conceptualization, writing manuscript, W.G.: methodology, performing all experiments, validation, formal analysis, data analysis, M.C. performing of phagocytosis experiments on THP-1 monocytes, M.C.: scientific supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonciarz, W., Chyb, M. & Chmiela, M. Mycobacterium bovis BCG increase the selected determinants of monocyte/macrophage activity, which were diminished in response to gastric pathogen Helicobacter pylori. Sci Rep 13, 3107 (2023). https://doi.org/10.1038/s41598-023-30250-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30250-6
- Springer Nature Limited
This article is cited by
-
Drug repurposing for cancer therapy
Signal Transduction and Targeted Therapy (2024)
-
Spray-dried pH-sensitive chitosan microparticles loaded with Mycobacterium bovis BCG intended for supporting treatment of Helicobacter pylori infection
Scientific Reports (2024)
-
Bacillus Calmette-Guérin (BCG)-Induced Protection in Brain Disorders
Inflammation (2024)