Abstract
To elevate the accuracy of diagnostic results, CNV-seq is usually performed simultaneously with karyotyping or QF-PCR. Although several studies have investigated the performance of the combined use of CNV-seq with karyotyping or QF-PCR, there have been no reports focusing on the comparison of these 2 diagnostic strategies. In our study, 2507 pregnant women were included to investigate these 2 strategies. The detection rates of foetal genetic abnormalities and turnaround time were compared between these 2 groups. Moreover, the detection rates of foetal genetic abnormalities in different indications were analyzed. Our results unveiled that the detection rates of numerical chromosomal abnormalities were nearly the same in these 2 groups. In addition to numerical chromosomal abnormalities, 39 balanced karyotypic changes and chromosome polymorphisms were detected via the combined use of karyotyping and CNV-seq. Further investigation revealed that the vast majority of these karyotypic changes were inherited from parents. Compared with the karyotyping group, the combination of QF-PCR and CNV-seq reduced the reporting time from 31.593 ± 4.944 days to 11.460 ± 4.894 days. Meanwhile, NIPT, maternal serum screening and ultrasound scan significantly improved the detection of foetal genetic abnormalities. In conclusion, our results revealed that parental karyotyping is a useful supplementary method for CNV-seq and systematic prenatal examinations improved the detection of foetal genetic defects.
Similar content being viewed by others
Introduction
According to the World Health Organization, birth defects affect 4–8% of births worldwide, and their incidence varies between different countries1. In China, the incidence of birth defects is approximately 5.6%2. Birth defects usually lead to foetal death, perinatal death, infant death or child disabilities. Chromosome aberrations, including aneuploid, triploid and deletion or duplication of large chromosome segments, are a major cause of birth defects3,4. Additionally, copy number variant syndromes (CNV syndromes), which are caused by pathogenic copy number variations (pCNVs), lead to intellectual disability, multiple congenital anomalies, autistic spectrum disorders and other diseases5. Due to a lack of cures for most birth defects caused by genetic abnormalities, termination of pregnancies with a prenatal diagnosis of foetuses with genetic aberrations is the primary response6.
Copy number variation sequencing (CNV-seq), based on high-throughput sequencing, was developed in 20097. Through whole genome low-coverage sequencing, CNV-seq is able to detect genetic aberrations, including chromosome aneuploidies and CNVs larger than 100 kb8. Combined with the advantages of using small amounts of genomic DNA and detecting low-level mosaicism, CNV-seq has been used increasingly widely for prenatal diagnosis9,10,11,12. However, due to its low sequencing depth, CNV-seq cannot detect balanced karyotypic changes and polyploidies such as 69, XXX8. Additionally, maternal cell contamination affects the accuracy of CNV-seq and may lead to misinterpretation of testing results. Therefore, other detection methods need to be used in combination with CNV-seq for prenatal diagnosis.
Currently, CNV-seq is usually performed in combination with karyotyping or QF-PCR for prenatal diagnosis9,11,13. Karyotyping, the gold standard for detecting chromosome abnormalities, has been used for prenatal diagnosis since the 1960s14. With a resolution of approximately 5–10 Mb, karyotyping is able to detect karyotypic abnormalities, euploidies and abnormalities involving large chromosomal segments15. CNV-seq performed simultaneously with karyotyping is able to detect balanced karyotypic abnormalities, chromosomal polymorphisms and numerical chromosomal abnormalities13. However, the combined use of CNV-seq and karyotyping in prenatal diagnosis is labor-intensive and has a long turnaround time. Quantitative fluorescent polymerase chain reaction (QF-PCR) has been used as a rapid prenatal detection method for common aneuploidies and euploidies since 1999 by analyzing highly polymorphic short tandem repeats16. Moreover, maternal cell contamination, a potential hazard to the accuracy of CNV-seq, can also be identified by QF-PCR17. In 2019, experts recommended the combined use of CNV-seq and QF-PCR for prenatal diagnosis in China18. However, the combined use of CNV-seq and QF-PCR could not detect balanced karyotypic changes. Taken together, the combined use of CNV-seq and karyotyping and the combined use of CNV-seq and QF-PCR have their advantages and disadvantages in prenatal diagnosis. Until recently, no studies have focused on the comparison of these 2 diagnostic strategies. In our study, the detection rates of different abnormalities and turnaround time of pregnant women accepting prenatal diagnosis by CNV-seq and karyotyping or QF-PCR were analyzed. Moreover, we also analyzed the abnormalities detected in pregnant women with different indications. Our results revealed that parental karyotyping is a useful supplementary method of CNV-seq for the first time. Meanwhile, systematic prenatal examinations, including NIPT, maternal serum screening and ultrasound scans, helped to detect chromosome abnormalities and pCNVs.
Results
Clinical characteristics
A total of 2507 participants were included in our study. Of these, 2019 and 488 were included in the karyotyping group and QF-PCR group, respectively. Although the maternal age and gestational age of these 2 groups were not significantly different, the difference in indications between the karyotyping and QF-PCR groups was statistically significant (Table 1). Advanced maternal age and increased maternal serum screening risk were more common in the QF-PCR group, while ultrasound abnormalities and increased NIPT risk were more common in the karyotyping group.
Detection of chromosomal abnormalities in the karyotyping and QF-PCR groups
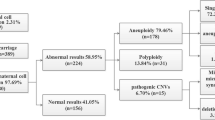
In the karyotyping group, 39 chromosome aneuploidies, 2 triploids, 8 mosaic aneuploidies and 3 derivative chromosomes, were detected (Table 2). Trisomy 21 and sex chromosome aneuploidies were the most common aneuploidies, accounting for nearly 90% of chromosome aneuploidies. Meanwhile, 10 numerical chromosomal abnormalities, including 9 chromosome aneuploidies and 1 mosaic aneuploidy, were detected in the QF-PCR group (Table 2). Consistent with the results in the karyotyping group, sex chromosome aneuploidies and trisomy 21 accounted for nearly 90% of chromosome aneuploidies. Detailed information on the chromosome abnormalities detected in these 2 groups is listed in Table 2. Our results revealed that the detection rates of numerical chromosomal abnormalities were not significantly different between these 2 groups.
The detection rates of numerical chromosomal abnormalities, including aneuploidies, polyploidies and mosaic aneuploidies, in pregnancies with different indications were different (Table 3). For pregnancies with an increased NIPT risk, the detection rate of aneuploidies and derivative chromosomes was significantly higher than others (22.64%, 24/106). Meanwhile, the detection rates of aneuploidies and triploidies in pregnancies with abnormal ultrasound results (2.00%, 14/701), advanced maternal ages (1.52%, 10/657) and mixed indications (2.78%, 3/108) were significantly higher than those in pregnancies with an increased maternal serum screening risk (0.77%, 5/652). This implied that ultrasound examination and prenatal diagnosis of pregnancies at advanced maternal age improved the detection of chromosome aneuploidies.
In addition to unbalanced karyotypic abnormalities, karyotyping was able to detect balanced karyotypic changes and chromosome polymorphisms. In the karyotyping group, translocation (33.33%, 13/39), inversion of chromosome 9 (25.64%, 10/39), extended heterochromatin area (15.38%, 6/39) and polymorphisms in satellites (25.64%, 10/39) were detected in 38 foetuses (Table 4). Because of a fetus carrying 9qh+ and 22pss simultaneously, there were 39 balanced karyotypic abnormalities and chromosomal polymorphisms in 38 foetuses. To investigate the inheritance pattern of these chromosomal changes, parents of 30 foetuses accepted karyotyping. Our results revealed that the vast majority of these karyotypic changes (93.3%, 28/30) were inherited, implying that the combined use of CNV-seq and karyotyping was more suitable for couples carrying balanced karyotypic changes.
Comparison of reporting time between the karyotyping and QF-PCR groups
The turnaround time was compared between these 2 strategies. Theoretically, the reporting time of the combined CNV-seq and karyotyping or QF-PCR was approximately 3 weeks and 1–2 weeks, respectively. Based on these timings, we expected that the reporting time for the karyotyping group would be much longer than that of the QF-PCR group. Consistent with this assumption, our results revealed that QF-PCR reduced the reporting time from 31.593 ± 4.944 days to 11.460 ± 4.894 days (Fig. 1). The gestational ages of participants with different indications were also analyzed. Our results revealed that pregnancies with ultrasound abnormalities were at more advanced gestational ages than others (Fig. 2). For these pregnancies, the combined use of QF-PCR and CNV-seq was a better choice than the combined use of karyotyping and CNV-seq.
The gestational weeks of pregnancies with different indications. UA, ultrasound abnormalities; AMA, advanced maternal age; IMSSR, increased maternal serum screening risk; INIPTR, increased NIPT risk. Other indications included previous fetus/child with genetic or phenotypic abnormalities, carriers of monogenic genetic diseases, medication during pregnancy, embryos stopping developing, and intellectual disability of pregnant women. ***Represented a P < 0.001.
Detection of CNVs in pregnancies with different indications
With the use of CNV-seq, CNVs were effectively detected in both groups. As a result, a total of 253 CNVs, including 133 microdeletions and 120 microduplications, were detected in 233 foetuses (Fig. 3A, Supplement Table 1). Most foetuses (91.42%, 213/233) carried only one CNV and the average length of CNV detected in our study was 1.88 Mb. Among these microdeletions, 24, 3 and 106 were classified as pathogenic, likely pathogenic and VUS, respectively. Meanwhile, 16, 1 and 103 microduplications were classified as pathogenic, likely pathogenic and VUS, respectively. The distribution tendencies of microdeletions and microduplications were different. Microdeletions were more likely to occur on chromosomes 1, 7 and 15, while microduplications were more likely to occur on chromosomes 15, 22 and X (Fig. 3B and C).
The detection rates of CNVs in pregnancies with different indications were also analyzed. Although the detection rates of VUS in pregnancies with different indications were not significantly different, pCNVs and likely pathogenic CNVs (lpCNVs) were more likely to be identified in pregnancies with increased NIPT risk, increased maternal serum screening risk and ultrasound abnormalities (Table 5). As shown in Fig. 3D, pCNVs and lpCNVs were more likely to occur on chromosomes 22, 15 and 16. Subsequent analysis revealed that the incidence of pCNVs and lpCNVs varied in pregnancies with different indications and microduplication of 22q11.21 and microdeletion of 15q11.2 were the most common pCNVs (Table 6).
To investigate the inheritance pattern of CNVs detected in our study, CNV-seq was performed for parents of 123 foetuses. Because of 9 foetuses carrying 2 CNVs simultaneously, a total of 132 CNVs were detected in 123 foetuses. Subsequent results revealed that 46, 71 and 15 of these CNVs were inherited from their father, their mother and de novo, respectively (Supplement Table 2). The inheritance pattern of pCNVs, lpCNVs and VUS was further analyzed. Among 21 pCNVs and lpCNVs, 17 and 4 were inherited from their mother and de novo, respectively. Meanwhile, among 111 VUS, 46, 54 and 11 were inherited from their father, their mother and de novo, respectively. Although VUS were more likely to be inherited, the difference in the ratio of inherited CNVs was not statistically significant between VUS and pCNVs, lpCNVs (P = 0.226). In conclusion, our results revealed that the majority of CNVs detected in foetuses were inherited.
Discussion
To systematically compare the combined use of CNV-seq and karyotyping and the combined use of CNV-seq and QF-PCR, a total of 2507 pregnant women were included in our study and divided into a karyotyping group and QF-PCR group. The most common indications in our study were ultrasound abnormalities, advanced maternal age and increased maternal serum screening risk, accounting for approximately 80% of all pregnancies. In our study, the most common unbalanced karyotypic change was trisomy 21 (37.10%, 23/62), followed by sex chromosome aneuploidies (32.26%, 20/62), mosaic aneuploidies (14.52%, 9/62), trisomy 18 (8.06%, 5/62), derivative chromosome (4.84%, 3/62) and triploidies (3.23%, 2/62). The detection rates of these karyotypic abnormalities were not significantly different between these 2 groups, revealing that both strategies were able to detect unbalanced karyotypic changes effectively. Compared with previous reports19,20, the detection rate of trisomy 21 and sex chromosome abnormalities decreased and increased significantly in our study, respectively. As revealed by Zhao et al.21, NIPT was an effective method for prenatal screening of sex chromosome aneuploidies. Including more pregnancies with increased NIPT risk in our study may lead to this disparity. In addition to numerical chromosomal abnormalities, the combined use of CNV-seq and karyotyping was also able to detect balanced karyotypic changes and chromosome polymorphisms. In our study, the most common balanced karyotypic abnormality was translocation (33.33%, 13/39), followed by inversion of chromosome 9 (25.64%, 10/39), polymorphisms in satellites (25.64%, 10/39) and extended heterochromatin area (15.38%, 6/39). Consistent with previous reports22,23, we found that most of these balanced karyotypic changes and chromosome polymorphisms were inherited. Therefore, parental karyotyping is vital for choosing a supplementary method of CNV-seq in prenatal diagnosis. For couples carrying balanced karyotypic changes, using karyotyping and CNV-seq was a better choice.
Turnaround time was an important factor to take into account when choosing prenatal diagnostic strategies. For pregnant women, especially those at advanced gestational ages, shorter turnaround time helped to relieve maternal anxiety and gave them more time for post-test counseling and interventions. Therefore, the turnaround time was also compared between these 2 strategies. Our results revealed that QF-PCR significantly reduced the turnaround time. Interestingly, consistent with previous reports24, our results revealed that pregnancies with abnormal ultrasound results were usually at more advanced gestational ages. For these women, a shorter turnaround time helped to relieve maternal anxiety and provided more time for further interventions. Therefore, for pregnancies at advanced gestational age, such as those with ultrasound abnormalities, QF-PCR was a better supplement to CNV-seq.
Although the detailed mechanism remained elusive, microdeletions and microduplications occurred in different hot spots. Subsequent analysis revealed that pCNVs and lpCNVs were more likely to occur on chromosomes 22, 15 and 16. Although previous reports showed that the Xp22.31 microdeletion and 22q11.2 microdeletion were the most common pCNVs13,19, the microdeletion of 15q11.2 and microduplication of 22q11.21 were the most prevalent pCNVs in our study. Interestingly, most microduplications of 22q11.21 were detected in pregnancies with increased NIPT risk. Therefore, including more pregnancies with increased NIPT risk in our study may elevate the detection rate of microduplication of 22q11.21. Additionally, conducting research in different districts may also lead to this inconsistency. The inheritance pattern of CNVs detected in our study was also investigated. Although the ratio of inherited CNVs was not statistically different between pCNVs, lpCNVs and VUS, our results hinted that pCNVs and lpCNVs were more likely to be de novo. Future research with a larger sample size may elucidate the inheritance pattern of CNVs with different pathogenicity.
NIPT, maternal serum screening and prenatal ultrasound examination are routinely used for prenatal detection of foetal abnormalities. The detection of chromosome abnormalities and pCNVs in pregnancies with different indications was analysed in our study. Growing evidence has revealed that NIPT dramatically improves the detection of common aneuploidies and pCNVs25,26. Consistent with these reports, the detection rates of aneuploidies and pCNVs in pregnancies with an increased NIPT risk were elevated significantly in our study. Moreover, compared with previous reports, including more pregnancies with an increased NIPT risk elevated the detection rates of sex chromosome aneuploidies and microduplication of 22q11.219,20. Maternal serum screening has been used for prenatal screening of foetal aneuploidies since the 1950s27. Recent evidence has shown that abnormal serum screening results are associated with foetal pCNVs28. In our study, although the detection rates of aneuploidies in pregnancies with an increased maternal serum screening risk were not significantly increased, the detection rates of pCNVs and lpCNVs in these pregnancies elevated significantly. Growing evidence revealed that the detection rates of foetal aneuploidies and pCNVs in pregnancies with ultrasound abnormalities increased significantly12,24,29,30, and CNV-seq was an effective way to detect foetal pCNVs in pregnancies with ultrasound abnormalities24. Consistent with these reports, our results revealed that the detection rates of foetal karyotypic anomalies and pCNVs in pregnancies with ultrasound abnormalities was increased. Taken together, although the detection efficiency of chromosomal abnormities and pCNVs varied between different screening methods, our results revealed that systematic prenatal examinations improved the detection of genetic abnormalities in foetuses and reduced the incidence of birth defects.
In conclusion, the combined use of CNV-seq and karyotyping was able to detect balanced karyotypic changes and chromosome polymorphisms, while the combined use of CNV-seq and QF-PCR was able to detect maternal cell contamination and significantly reduced the turnaround time. Therefore, parental karyotyping is important in selecting a supplementary method of CNV-seq. For couples carrying balanced karyotypic changes, karyotyping was a better supplement for CNV-seq. For couples with normal karyotypes and pregnant women at advanced gestational ages, the combined use of CNV-seq and QF-PCR was the best option studied. Moreover, our results revealed that NIPT, maternal serum screening and prenatal ultrasound scans improved the detection rates of chromosome anomalies and pCNVs.
Materials and methods
Study design and participants
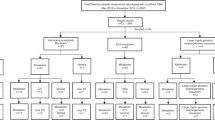
A total of 2507 pregnant women within the 13+4th to 34+1th gestational weeks were included in our study from September 2019 to December 2021 at the Deyang People’s Hospital. All pregnancies were singleton. Indications for prenatal diagnosis were ultrasound abnormalities, advanced maternal age (> 35 years), increased maternal serum screening risk, increased non-invasive prenatal testing (NIPT) risk, mixed indications and other indications. Ultrasound abnormalities included increased fetal nuchal translucency (NT) thickness, nasal bone hypoplasia, short femur length, thicken nuchal fold, ventriculomegaly, echogenic bowel, echogenic intracardiac focus, choroid plexus cysts, aberrant right subclavian artery and single umbilical artery. Mixed indications were pregnant women with two or more indications. Other indications included previous fetus/child with genetic or phenotypic abnormalities, carriers of monogenic genetic diseases, medication during pregnancy, embryos stopping developing, and intellectual disability of pregnant women. Detailed clinical information of the participants is listed in Table 1. According to prenatal diagnosis strategies, participants were divided into a QF-PCR group and a karyotyping group. In the karyotyping group, 2019 participants chose to accept CNV-seq and karyotyping. CNV-seq and QF-PCR were performed for 488 participants in the QF-PCR group. All methods were performed following the relevant guidelines and regulations in our study. This study was approved by the Ethical Committee of Deyang People’s Hospital, and informed consent was obtained from all participants.
QF-PCR assay
Rapid detection of common foetal chromosome aneuploidy was performed using the STR Genotyping Kit for Chromosomes 13/18/21/X/Y (DaRui Biotech Co., Guangzhou, China) according to the manufacturer’s instructions. Briefly, the experimental process included genomic DNA extraction, PCR amplification and capillary electrophoresis. Genomic DNA was extracted from 8 ml of amniotic fluid using the TIANamp Genomic DNA kit (Tiangen Biotech Co., Beijing, China) according to the manufacturer’s instructions. The concentrations of genomic DNA were measured using Qubit 1 × dsDNA High Sensitivity (HS) and Broad Range (BR) Assay Kits (Thermo Fisher Scientific Inc., Rockford, USA). Subsequently, the concentration of genomic DNA was diluted to 5–10 ng/μl. PCR amplification of 20 highly polymorphic short tandem repeats (STRs) (Table 7) was performed using a Bio–Rad PTC 200 PCR system (Bio–Rad Laboratories, Hercules, USA). The PCR profile was pre-denaturation at 95 °C for 5 min followed by 95 °C for 30 s, 58 °C for 40 s, and 72 °C for 50 s, for 25 cycles with a final extension at 72 °C for 10 min. After PCR amplification, 1 μl PCR products were mixed with 13.5 μl HiDi formamide (Thermo Fisher) and 1 μl LIZ600 (Thermo Fisher), and then capillary electrophoreses were performed using the 3500 ABI Genetic Analyser (Applied Biosystems, Waltham, USA). Finally, the electrophoresis results were interpreted according to the manufacturer’s instructions.
CNV-seq
CNV-seq was performed to detect chromosome aneuploidy and pCNVs. The workflow of CNV-seq included extracting genomic DNA, constructing a library, quality control, pooling, sequencing, bioinformatics analysis and interpreting the results. First, genomic DNA was extracted from 2 to 4 ml of amniotic fluid using the TIANamp Genomic DNA kit (Tiangen) according to the manufacturer’s instructions. Then, 20 ng genomic DNA was fragmented by NEBNext dsDNA Fragmentase (New England Biolabs, Ipswich, USA) at 37 °C for 50 min. Subsequently, end filling, adaptor ligation and PCR amplification were conducted to construct a DNA library using Foetal Chromosome Aneuploidy (T21, T18, and T13) Testing Kits (Annoroad, Beijing, China). The concentrations of the libraries were measured by Qubit 1X dsDNA High Sensitivity (HS) and Broad Range (BR) Assay Kits (Thermo Fisher). Agilent 2100 Bioanalyzed (Agilent Technologies, Palo Alto, USA) was also used for quality control of libraries. Subsequently, qualified libraries were pooled together and subjected to massively parallel sequencing using NextSeq 550AR (Illumina, San Diego, USA). For each sample, at least 4.5 million raw reads with a length of 40 bp were generated for further analysis. The quality control criteria of the sequencing results were as follows: reads > 4.5 Mb, GC content: 38.5–45.5%, Q30 ratio > 85%, alignment ratio > 62.5%, unique read ratio > 60%, and duplication ratio < 10%. Qualified sequencing results were mapped to the grch37 version of the human genome using the Burrows–Wheelers algorithm31, and copy number variations (CNVs) were detected by bioinformatics analysis.
The pathogenicity of CNVs was classified as pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign and benign according to American College of Medical Genetics guidelines32. Public databases, including ClinGen, Database of Genomic Variation and Phenotype in Humans Using Ensembl Resources (DECIPHER), Online Mendelian Inheritance in Man (OMIM), 1000 Genomes, and the Database of Genomic Variants, were used to interpret the pathogenicity of these CNVs. CNVs were described according to ISCN 2020 guidelines.
Karyotyping
A total of 20 ml amniotic fluid was collected from each pregnant woman and then equally divided into 2 parts. After centrifugation at 1000 rpm for 10 min, the supernatant was discarded. Then, precipitated amniocytes were resuspended in 3 ml BIO-AMF-3 complete medium (Biological Industries, Cromwell, USA) and incubated at 37 °C in a Thermo 3111 CO2 incubator (Thermo Fisher). For each sample, 2 independent cultures were established. Subsequently, amniocytes were harvested for G banding after culturing for 9–14 days. For each sample, 20 metaphase images were captured and counted using a Zeiss automatic karyotyping scanning system (Carl Zeiss, Jena, Germany). Among these metaphases, 5 were analysed using IKAROS software (Carl Zeiss). Karyotypes were described according to ISCN 2020 guidelines.
Statistics
Statistical analysis was performed using SPSS 19.0 (IBM, New York, USA). Qualitative data were analysed by the chi-square test, while normally distributed quantitative data were analysed by t-test and one-way ANOVA. A P < 0.05 indicated that the difference was statistically significant. The schematic diagrams of distributions of CNVs on chromosomes and boxplots were drawn using R packages RIdeogram33 and ggplot2, respectively.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
References
World Health Organization. The Global Burden of Disease: 2004 Update (World Health Organization, 2008).
Xu, W. et al. National perinatal prevalence of selected major birth defects—China, 2010–2018. China CDC Week. 2, 711–717 (2020).
Harris, B. S. et al. Risk factors for birth defects. Obstet. Gynecol. Surv. 72, 123–135. https://doi.org/10.1097/OGX.0000000000000405 (2017).
Evans, M. I., Wapner, R. J. & Berkowitz, R. L. Noninvasive prenatal screening or advanced diagnostic testing: Caveat emptor. Am. J. Obstet. Gynecol. 215, 298–305. https://doi.org/10.1016/j.ajog.2016.04.029 (2016).
Nevado, J. et al. New microdeletion and microduplication syndromes: A comprehensive review. Genet. Mol. Biol. 37, 210–219. https://doi.org/10.1590/s1415-47572014000200007 (2014).
Krstic, N. & Obican, S. G. Current landscape of prenatal genetic screening and testing. Birth Defects Res. 112, 321–331. https://doi.org/10.1002/bdr2.1598 (2020).
Xie, C. & Tammi, M. T. CNV-seq, a new method to detect copy number variation using high-throughput sequencing. BMC Bioinform. 10, 80. https://doi.org/10.1186/1471-2105-10-80 (2009).
Liang, D. et al. Copy number variation sequencing for comprehensive diagnosis of chromosome disease syndromes. J. Mol. Diagn. 16, 519–526. https://doi.org/10.1016/j.jmoldx.2014.05.002 (2014).
Qiao, J. et al. Combined diagnosis of QF-PCR and CNV-Seq in fetal chromosomal abnormalities: A new perspective on prenatal diagnosis. J. Clin. Lab. Anal. 2022, e24311. https://doi.org/10.1002/jcla.24311 (2022).
Lan, L., She, L., Zhang, B., He, Y. & Zheng, Z. Prenatal diagnosis of 913 fetuses samples using copy number variation sequencing. J. Gene Med. 23, e3324. https://doi.org/10.1002/jgm.3324 (2021).
Zhao, X. & Fu, L. Efficacy of copy-number variation sequencing technology in prenatal diagnosis. J. Perinat. Med. 47, 651–655. https://doi.org/10.1515/jpm-2019-0005 (2019).
Lan, L. et al. Analysis of copy number variation by sequencing in fetuses with nuchal translucency thickening. J. Clin. Lab. Anal. 34, e23347. https://doi.org/10.1002/jcla.23347 (2020).
Zhang, J. et al. Investigation on combined copy number variation sequencing and cytogenetic karyotyping for prenatal diagnosis. BMC Pregnancy Childbirth 21, 496. https://doi.org/10.1186/s12884-021-03918-y (2021).
Steele, M. W. & Breg, W. R. Chromosome analysis of human amniotic-fluid cells. Lancet 1, 383–385. https://doi.org/10.1016/s0140-6736(66)91387-0 (1966).
Saldarriaga, W., Garcia-Perdomo, H. A., Arango-Pineda, J. & Fonseca, J. Karyotype versus genomic hybridization for the prenatal diagnosis of chromosomal abnormalities: A metaanalysis. Am. J. Obstet. Gynecol. 212, 330. https://doi.org/10.1016/j.ajog.2014.10.011 (2015).
Pertl, B. et al. Rapid prenatal diagnosis of aneuploidy by quantitative fluorescent PCR on fetal samples from mothers at high risk for chromosome disorders. Mol. Hum. Reprod. 5, 1176–1179. https://doi.org/10.1093/molehr/5.12.1176 (1999).
Stojilkovic-Mikic, T., Mann, K., Docherty, Z. & Mackie-Ogilvie, C. Maternal cell contamination of prenatal samples assessed by QF-PCR genotyping. Prenat. Diagn. 25, 79–83. https://doi.org/10.1002/pd.1089 (2005).
Clinical Genetics Group Of Medical Genetics Branch Chinese Medical, A., Professional Committee For Prenatal Diagnosis Of Genetic Diseases Medical Genetics Branch Of Chinese Medical A., Group Of Genetic Disease P., Control Birth Defect P. & Control Committee Of Chinese Society Of Preventive M. Expert consensus on the application of low-depth whole genome sequencing in prenatal diagnosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 36, 293–296. https://doi.org/10.3760/cma.j.issn.1003-9406.2019.04.001 (2019).
Xu, L. et al. The comprehensive comparison of bacterial artificial chromosomes (BACs)-on-beads assay and copy number variation sequencing in prenatal diagnosis of southern Chinese Women. J. Mol. Diagn. 22, 1324–1332. https://doi.org/10.1016/j.jmoldx.2020.07.005 (2020).
Wang, J. et al. Prospective chromosome analysis of 3429 amniocentesis samples in China using copy number variation sequencing. Am. J. Obstet. Gynecol. 219, 287e1-287e18. https://doi.org/10.1016/j.ajog.2018.05.030 (2018).
Zhao, G. et al. Clinical application of noninvasive prenatal testing for sex chromosome aneuploidies in Central China. Front. Med. Lausanne 8, 672211. https://doi.org/10.3389/fmed.2021.672211 (2021).
Wang, L. F., Wang, K. Y., Tu, H. J., Lin, K. & Lin, H. Clinical investigation of chromosome karyotype analysis with amniotic fluids cell and parental peripheral blood. Clin. Lab. 2022, 68. https://doi.org/10.7754/Clin.Lab.2021.210643 (2022).
Zhang, H. G. et al. Balanced reciprocal translocation at amniocentesis: Cytogenetic detection and implications for genetic counseling. Genet. Mol. Res. 2016, 15. https://doi.org/10.4238/gmr.15038556 (2016).
Wang, J. et al. Identification of copy number variations among fetuses with ultrasound soft markers using next-generation sequencing. Sci. Rep. 8, 8134. https://doi.org/10.1038/s41598-018-26555-6 (2018).
Benn, P., Cuckle, H. & Pergament, E. Non-invasive prenatal testing for aneuploidy: Current status and future prospects. Ultrasound Obstet. Gynecol. 42, 15–33. https://doi.org/10.1002/uog.12513 (2013).
Yang, J. et al. Performances of NIPT for copy number variations at different sequencing depths using the semiconductor sequencing platform. Hum. Genomics 15, 41. https://doi.org/10.1186/s40246-021-00332-5 (2021).
Rink, B. D. & Norton, M. E. Screening for fetal aneuploidy. Semin. Perinatol. 40, 35–43. https://doi.org/10.1053/j.semperi.2015.11.006 (2016).
Bornstein, E. et al. Microarray analysis: First-trimester maternal serum free beta-hCG and the risk of significant copy number variants. Prenat. Diagn. 38, 971–978. https://doi.org/10.1002/pd.5350 (2018).
Benacerraf, B. R., Barss, V. A. & Laboda, L. A. A sonographic sign for the detection in the second trimester of the fetus with Down’s syndrome. Am. J. Obstet. Gynecol. 151, 1078–1079. https://doi.org/10.1016/0002-9378(85)90385-0 (1985).
Nyberg, D. A. & Souter, V. L. Sonographic markers of fetal trisomies: Second trimester. J. Ultrasound Med. 20, 655–674. https://doi.org/10.7863/jum.2001.20.6.655 (2001).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. https://doi.org/10.1093/bioinformatics/btp324 (2009).
Riggs, E. R. et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet. Med. 22, 245–257. https://doi.org/10.1038/s41436-019-0686-8 (2020).
Hao, Z. et al. RIdeogram: Drawing SVG graphics to visualize and map genome-wide data on the idiograms. PeerJ Comput. Sci. 6, e251. https://doi.org/10.7717/peerj-cs.251 (2020).
Funding
This study was supported by grants from the Science and Technology Bureau of Deyang (grant no. 2021SZ15).
Author information
Authors and Affiliations
Contributions
H.Z. designed the study, performed experiments, analyzed data and wrote the manuscript. Z.X. supervised the study and revised manuscript. Q.C. and X.D. designed the study and analyzed data. H.C., L.L. and Y.X. performed experiments and collected data. All authors read and approved the final version of the manuscript. All authors have read and approved the content and agree to submit it for consideration of publication in the journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, H., Xu, Z., Chen, Q. et al. Comparison of the combined use of CNV-seq and karyotyping or QF-PCR in prenatal diagnosis: a retrospective study. Sci Rep 13, 1862 (2023). https://doi.org/10.1038/s41598-023-29053-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29053-6
- Springer Nature Limited