Abstract
The objective of this study was to investigate the impact of lactation stage and parity number on fatty acid and non-volatile polar metabolite profiles in sow colostrum and milk using a metabolomics approach. A total number of 63 colostrum, transient and mature milk were collected from primiparous and multiparous Landrace × Yorkshire crossbred sows. Macrochemical, fatty acid and non-volatile polar metabolite compositions of samples were analyzed using infrared spectrometry, gas chromatography coupled with mass-spectrometry and proton nuclear magnetic resonance spectroscopy, respectively. Univariate and multivariate statistical analysis demonstrated significant impacts of lactation stage and parity number on colostrum and milk compositions. Chemometric analysis revealed significant influences of sow parity on the distinction in fatty acid profiles of mature milk while the distinction in non-volatile polar metabolite profiles was more evident in colostrum. Alterations in the concentration of linoleic (C18:2n6), lignoceric (C24:0), behenic (C22:0), caprylic (C8:0) and myristoleic (C14:1) acid together with those of creatine, creatinine phosphate, glutamate and glycolate were statistically suggested to be mainly affected by sow parity number. Variations in the concentration of these compounds reflected the physiological function of sow mammary gland influenced. This information could be applied for feed and feeding strategies in lactating sows and improving lactating performances.
Similar content being viewed by others
Introduction
Ability to produce colostrum and milk are some of the most important performance traits for lactating sows to ensure the survival of their piglets1,2. The main composition of sow colostrum and milk includes fat, proteins, lactose, vitamins, minerals as well as a series of immunoglobulins, which provide a good source of nutrients and passive immunity that support the growth and survival of piglets3,4. The chemical and nutritional composition of sow colostrum and milk are predominantly influenced by many factors, including animal individuality, genetic variants, physiological and health status, lactation stage, feeding regimen, farming practices as well as other environmental factors linked to specific swine production systems5,6. The lactation stage, parity, feed supplementations and animal housing conditions affected the colostrum and milk quality and quantity related the sow and piglet performances3,4. Beside the gross composition, a more comprehensive insight on dynamic changes in the porcine milk biomolecular profile is another point of interest that remains to be investigated.
Recently, metabolomics, a comprehensive characterization of low molecular weight metabolite (< 1.5 kDa) present in biological system7, is well acknowledged in milk and dairy research8. Mass spectrometry (MS)-based and nuclear magnetic resonance (NMR)-based metabolomics has been extensively applied for characterization of metabolites present in colostrum and milk from human, bovine as well as other small ruminant animals8,9,10. To date, more than 400 metabolites have been identified in colostrum and milk from various animal sources (https://lmdb.ca/) including sows11,12,13,14,15. In addition to polar metabolite compounds, variations in the lipid components of milk have been documented to be associated with feeding regimen, physiological and health status of sows16,17,18. Nevertheless, a global characterization of compounds present in both lipid and non-lipid fractions of sow milk has never been reported. The current study hypothesized that combined information on milk fat composition and milk serum metabolome could provide a better reflection on reproductive traits and lactation performance of the sows. Therefore, the objective of this study was to investigate the impact of lactation stage and parity number of sows on fatty acid and non-volatile polar metabolite profiles in colostrum and milk using a complementary GC–MS and non-targeted 1H-NMR metabolomics approach.
Methods
Animals
A total of 21 Landrace × Yorkshire crossbred sows was included in the present study. The sows were randomly allocated according to parity numbers including 1 (n = 7), 2–6 (n = 7) and 7 (n = 7). The gestating sows were moved from gestation unit at 7 days before expected farrowing dates. All lactating sows were kept in an evaporative cooling system of a commercial swine herd in Thailand. The lactating sows were kept in individual farrowing pens (2.5 × 3.0 m) on a slatted floor. In each pen, sows were housed in a crate (1.95 × 0.75 m) at the center of the pen whose floor was partly concrete. The piglets were kept at both sides of farrowing crate with access to a warm creep area (1.0 × 0.6 m). Sows had access to water ad libitum and were fed a commercial lactation diet twice a day before farrowing and four times daily after farrowing. The parturition process was supervised and sow interventions during parturition were limited as much as possible.
Sample collection
On total, 21 sows were collected the milk sample at parturition and days 3 and 17 of lactation from all functional mammary glands. A total number of 63 colostrum and milk samples were included in this study, with a minimum of 30 mL of the sample. Colostrum samples were collected within 1 h after the onset of parturition as the first piglet was born by hand milking. Transient and mature milk samples were collected on days 3 and 17 of lactation. During samples collection, sows were slowly administered with oxytocin (VetOne®; 5 USP Units/head IV; MWI Veterinary Supply Co., Ltd., Idaho, USA) in the median auricular vein. Samples from all teats were filtered through gauze, pooled and stored at –20 °C until the analysis.
Determination of colostrum and milk compositions
Macrochemical composition analysis
Frozen colostrum and milk samples were thawed in a water bath at 40 °C for 20 min before the analysis. The macrochemical composition including fat, protein, lactose and dry matter content (%) of samples were determined by means of infrared spectroscopic technique using a MilkoScan FT2 instrument (Foss MilkoScan, Hillerød, Denmark).
Fatty acid profile analysis by GC–MS
Fatty acid (FA) profiles in sow colostrum and milk were determined using a gas chromatography coupled with mass spectrometry for fatty acid methyl ester (GC–MS-FAME) analysis according to method of O'Fallon et al.19 with modifications. All chemical used at this step were obtained from Sigma-Aldrich (Steinheim, Germany) and Merck (Darmstadt, Germany). In detail, one gram of sample was placed into a screw cap Pyrex culture tube, added with 1 mL of internal standard solution consisting of 0.5 mg of C13:0/mL of MeOH, and 0.7 mL of 10 N KOH and 5.3 mL of MeOH and incubated in a water bath at 55 °C for 1.5 h with a periodically vigorous shaking every 20 min to promote the hydrolysis process. The samples were cooled down to room temperature (30 ± 2 °C), added with 0.58 mL of 24 N H2SO4 and incubated again in a water bath at 55 °C with a periodically vigorous shaking every 20 min for another 1.5 h. After fatty acid methyl ester (FAME) formation, samples were cooled to room temperature, added with 3 mL of hexane and subjected to a vortex mixer for 5 min. The hexane layer was separated by centrifugation at 3000 × g for 5 min, then collected and placed into a glass GC vial. The vials were capped and placed at −20 °C until the analysis. Fatty acid composition of the FAME fraction was determined by capillary GC on a SP-2560, 100 m × 0.25 mm × 0.20 µm capillary column (Supelco, Bellefonte, PA, USA). The initial oven temperature was maintained at 140 °C for 5 min, subsequently increased at the rate of 4 °C/min to 260 °C and maintained for 20 min. The carrier gas was helium fed with a constant flow rate at 0.5 mL/min. The column head pressure was adjusted at 280 kPa. Both injector and detector were set at 260 °C. The split ratio was at 100:1. Fatty acids were identified by comparing their specific retention time and m/z model with a fatty acid methyl ester standard (Supelco 37 Component FAME mix, Sigma-Aldrich, Steinheim, Germany). Automated peak integration was performed. The concentrations of fatty acids were calculated using calibration curves fitted by linear regression analysis and finally expressed as mg/100 g.
Non-volatile polar metabolite profile analysis by 1H-NMR
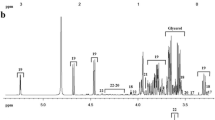
Non-volatile polar metabolite profiles in sow colostrum and milk were determined using non-targeted 1H-NMR technique adapted from Settachaimogkon et al.20. In brief, the pH of samples was adjusted to 6.0 and lipid phase was removed by dichloromethane extraction followed by centrifugation (4100 × g for 20 min at 4 °C). Milk protein fractions were removed by ultra-centrifugation (74,200 × g for 60 min at 4 °C) and ultra-filtration through a Pall Nanosep® centrifugal device with 3 kDa molecular weight cutoffs (Pall Life Sciences, Ann Arbor, MI). The clear milk serum was mixed 1:1 (v/v) with phosphate buffer pH 6.0 consisting of 1 mM 3-(Trimethylsilyl) propionic-2, 2, 3, 3-d4 acid sodium salt (TSP) (Merck, Darmstadt, Germany) as internal standard. Finally, the mixture (400 μL) was subjected to a 500 MHz NOESY-GPPR-1D-1H-NMR spectrometer (Bruker, Rheinstetten, Germany) equipped with full automation and operated with similar parameters as described in our previous study21. 1H-NMR spectra were phase/based line corrected, pre-treated, and chemical shift (δ = 0.00–10.00 ppm) was segmented using binning technique with a 0.02 ppm interval21. Metabolite identification was performed by consulting Chenomx NMR suite 8.2 library (Chenomx Inc., Canada), Livestock Metabolome Database (www.lmdb.ca), Milk Composition Database (www.mcdb.ca), and literatures13,14,17,18,22. The sum of signal intensity corresponding to respective metabolites was expressed in arbitrary units22. The 1H-NMR signal intensities of respective compounds were expressed as log10 transformed [arbitrary unit] and introduced as variables in statistical analysis.
Statistical analysis
Univariate statistical analysis was performed using SAS 9.0 (SAS, 2002). A general linear mixed model procedure was used to analyze the effects of sow parity number on milk composition. The model included the fixed effects of sow parity numbers1,2,3,4,5,6,7, time3,20. The following model was applied to analyze the data:
where Yijk is the response variable, m is the overall mean, Pi is the fixed effect of parity number (i.e., 1, 2–6 and 7), Tj is the fixed effect of time (i.e., 0, 3 and 17), Sk is a random component related to the sow and Oijk is the residual error component. The sow was included as a random effect. The data is presented as least–squared means. A probability value of P < 0.05 was regarded as being statistically significant.
GC-derived fatty acid and 1H-NMR-derived non-volatile polar metabolite data were normalized by median-centering and log-scaling before subjecting to multivariate analysis21 in MetaboAnalyst 5.0 software (www.metaboanalyst.ca). Partial least square discriminant analysis (PLS-DA) was applied to visualize the overall distinctive patterns among fatty acid and non-volatile polar metabolite profiles of colostrum and milk obtained from sows with different parity number. The quality of PLS model was expressed by R2 (accuracy) and Q2 values (predictability) captured by the model. Finally, compounds with variable importance in projection (VIP) score higher than 1.0 and significant P values were considered to be accountable for the discrimination23.
Ethical declaration
The observational study followed guidelines documented in the ethical principles and guidelines for the use of animals for scientific purposes published by the National Research Council of Thailand and the study is reported in accordance with ARRIVE guidelines. All procedures were approved by the Chulalongkorn University Animal Care and Use Committee (animal use protocol number 2031009).
Results
Composition of sow colostrum and milk
Results in this study confirmed the significant influence of lactation day on the macrochemical composition of porcine milk (Table 1). Significant increases in fat and lactose and decreases in total proteins could be notably observed in samples through the lactation period (P < 0.001). Regarding the effect of parity number, only the fat content in milk samples from sow parity number 1 (9.0 ± 0.5 g/ 100 g) was significantly higher than sow parity number 2–6 (7.4 ± 0.5 g/ 100 g, P = 0.022) and had a tendency to be higher than sow parity number 7 (7.6 ± 0.6 g/100 g, P = 0.054). No evidence of an interaction between day after parturition and parity number was observed for fat, protein and lactose content of milk samples in this study.
Fatty acid profiles of sow colostrum and milk
A total of 31 fatty acids were identified and quantified (mg/100 g) in colostrum and milk samples in this study (Table1). Results showed that the day after parturition provided significant influence on the concentration of most fatty acids except for those of eicosatrienoic acid (C20:3n3), cis-13,16-docosadienoic acid (C22:2), docosapentaenoic acid (C22:4), eicosapentaenoic acid (C20:5n3), and docosahexaenoic acid (C22:6n3). Various increasing and decreasing trends of fatty acid concentrations could be observed in samples with the increased day postpartum. The individual effect of sow parity number was significantly related to the decreases in myristoleic acid (C14:1) (P = 0.005), palmitic acid (C16:0) (P = 0.042), cis-10-heptadecarnoic acid (C17:1) (P = 0.003), linoleic acid (C18:2n6c) (P = 0.019), linolenic acid (C18:3n3) (P = 0.039) and lignoceric acid (C24:0) (P = 0.026) content in milk. Moreover, interaction between the two main effects provided significant impacts on the variations of caprylic acid (C8:0) (P = 0.048), myristoleic acid (C14:1) (P = 0.016) and behenic acid (C22:0) (P = 0.030) (Table 1) content of milk samples in this study.
To visualize the overall distinction pattern among fatty acid profiles of samples, a PLS-DA score plot was constructed with a prediction accuracy of 76.20%, R2 = 0.672 and Q2 = 0.454 (Fig. 1A). It was evident that day after farrowing provided substantial impact on the fatty acid profiles of sow milk. Colostrum samples could be well distinguished from milk collected on day 3 while samples collected on day 17 were overlapped between the two groups. However, it should be mentioned that the influence of sow parity number was not clearly observed in this overall PLS-DA result. To overcome this hindrance, three separated PLS-DAs were performed for comparison of samples collected within the same day (Fig. 2). Results revealed a progressive separation among samples from different parity number with the increased time postpartum. Although the influence of sow parity number on fatty acid profiles could not be observed in colostrum (Fig. 2A), a better separation among different parities was remarkably noticed in transient (Fig. 2B) and mature milk samples (Fig. 2C). The most distinguish PLS-DA pattern was found in samples collected on day 17 with a prediction accuracy of 64.63%, R2 = 0.488 and Q2 = 0.380 (Fig. 2C). A good distinction was observed between milk samples of primiparous and multiparous sows along component 1. Based on the VIP scores, a list of fatty acids potentially responsible for the discrimination could be suggested (Fig. 2D). Taken together, with statistically significant levels demonstrated in Table 1, the variations in the concentration of linoleic acid (C18:2n6c), lignoceric acid (C24:0), behenic acid (C22:0), caprylic acid (C8:0) and myristoleic acid (C14:1) were significantly affected by either individual effect of sow parity number or its interaction with day after parturition (P < 0.05). Their concentrations were compared and visualized through a box-whicker plot summary (Fig. 3).
PLS-DA score plots for overall comparison of fatty acid (panel A) and non-volatile polar metabolite (panel B) profiles of colostrum (day 0; red), transient milk (day 3; blue) and mature milk (day 17; green) samples collected from sows in different parity numbers. The blocks in ( ), (
), ( ) and (
) and ( ) shape correspond to the samples collected from sows in parity 1, parity 2–6 and parity 7, respectively.
) shape correspond to the samples collected from sows in parity 1, parity 2–6 and parity 7, respectively.
PLS-DA score plots for the comparison of fatty acid profiles of colostrum (day 0; panel A), transient milk (day 3; panel B) and mature milk (day 17; panel C) samples collected from sows in parity 1 ( ), parity 2–6 (
), parity 2–6 ( ) and parity 7 (
) and parity 7 ( ). VIP scores derived from the comparison among mature milk samples (day 17) with indicative fatty acids accountable for the discrimination and their relative abundances are visualized in panel D.
). VIP scores derived from the comparison among mature milk samples (day 17) with indicative fatty acids accountable for the discrimination and their relative abundances are visualized in panel D.
Box-whisker plots of comparative quantification of indicative fatty acids (mg/100 g) responsible for discrimination among mature milk samples (day 17) collected from sows in in parity 1 (red), parity 2–6 (green) and parity 7 (blue). The lower and upper edge of the box denote 25th and 75th percentile of observation, respectively; the bold line within box denotes median value; the rhombus spot within box ( ) denotes average value; whiskers denote 5th and 95th percentiles. For interpretation of the significant difference among mean values of samples, the reader is referred to the MIXED model statistical tests in Table 1.
) denotes average value; whiskers denote 5th and 95th percentiles. For interpretation of the significant difference among mean values of samples, the reader is referred to the MIXED model statistical tests in Table 1.
Non-volatile polar metabolite profiles of sow colostrum and milk
A total of 35 non-volatile polar metabolites including amino acids, carbohydrates, alcohols, organic acids and lipid derivatives were identified in colostrum and milk samples in this study (Table 2). Results demonstrated a highly significant influence of day after parturition on the non-volatile polar metabolite profiles (P < 0.001) of samples. It could be noticed that the concentrations of all metabolites significantly decreased with the following days after farrowing. Beside this, neither individual effect of parity number nor its interaction with day after parturition provided significant effect on the variations of non-volatile polar metabolite profiles of milk samples in this study (Table 2).
To compare the overall non-volatile polar metabolite profiles among samples, a PLS-DA score plot was constructed with a prediction accuracy of 84.17%, R2 = 0.537 and Q2 = 0.488 (Fig. 1B). Unlike fatty acid composition, a clear distinction was observed among the 1H-NMR metabolite profiles of colostrum and mature milk samples. This result demonstrated a significant impact of day after farrowing on the metabolome of sow milk while the influence of parity number seemed to be unremarkable. To investigate the impact of sow parity, three separated PLS-DA analyses were performed for comparison of samples collected on the same day (Fig. 4). The clearest separation between primiparous and multiparous sows was found in colostrum samples revealed by a PLS-DA score plot with a prediction accuracy of 54.62%, R2 = 0.476 and Q2 = 0.343 (Fig. 4A). Based on the VIP scores, a list of non-volatile polar metabolites potentially responsible for the discrimination could be suggested (Fig. 4B). Taken together with statistically significant levels demonstrated in Table 2, the variations in the concentration of creatine, creatine phosphate, glutamate and glycolate seemed to be mostly affected by the interaction between day after farrowing and sow parity number with P values ranged from 0.151 to 0.194. Their concentrations were compared and visualized through a box-whicker plot summary (Fig. 5). On the other hand, it should be mentioned that the metabolite profiles of mature milk samples became less distinguished with the increased time postpartum (Fig. 4C,D).
PLS-DA score plots for the comparison of non-volatile polar metabolite profiles of colostrum (day 0; panel A), transient milk (day 3; panel C) and mature milk (day 17; panel D) samples collected from sows in parity 1 ( ), parity 2–6 (
), parity 2–6 ( ) and parity 7 (
) and parity 7 ( ). VIP scores derived from the comparison among colostrum samples (day 0) with indicative metabolites accountable for the discrimination and their relative abundances are visualized in panel B.
). VIP scores derived from the comparison among colostrum samples (day 0) with indicative metabolites accountable for the discrimination and their relative abundances are visualized in panel B.
Box-whisker plots of comparative quantification of indicative non-volatile polar metabolites (log10 [peak area in arbitrary unit]) responsible for discrimination among colostrum samples (day 0) collected from sows in in parity 1 (red), parity 2–6 (green) and parity 7 (blue). The lower and upper edge of the box denote 25th and 75th percentile of observation, respectively; the bold line within box denotes median value; the rhombus spot within box ( ) denotes average value; whiskers denote 5th and 95th percentiles. For interpretation of the significant difference among mean values of samples, the reader is referred to the MIXED model statistical tests in Table 2.
) denotes average value; whiskers denote 5th and 95th percentiles. For interpretation of the significant difference among mean values of samples, the reader is referred to the MIXED model statistical tests in Table 2.
Discussion
Variations in macrochemical composition
The mammary secretion as colostrum and milk was classified into 3 parts by the component of milk included colostrum, transition milk and mature milk. The colostrum is generally high in immunoglobulins whereas transition and mature milk are high in concentrations of fat and lactose3,24, which was also found in the current study. In accordance with previous studies, the parity number did not relate both colostrum and milk composition3,25. However, the present study found that fat concentration declined from primiparous sows to multiparous sows in milk secretion, regardless collection day. This result is similar with previous studies, showing that colostral fat in sow parity 6 was lower than in 1st parity sows26. Peter and Mahan25 reported that sows with parity 3–4 have lower milk fat concentration than sow parity number 1. Indeed, the effect of animal parity number on the minor components of sow colostrum and milk is still controversial. Therefore, changes in biomolecular profiles, i.e., fatty acid and non-volatile polar metabolite compositions, were investigated in our study to provide more insights on potential biochemical variations of sow colostrum and milk. To the authors’ best knowledge, this is the first report that a complementary GC–MS and non-targeted 1H-NMR metabolomics approach was applied to investigate the overall biomolecular profiles in both lipid and non-lipid fractions of sow colostrum and milk.
Variations in fatty acid profiles
Milk fat is the major energy source that contribute about 50% of the total energy required by suckling piglets27. It is well recognized that supplementing diets of lactating sows with suitable fat sources could enhance their reproductive performance and alters colostrum and milk FA composition25,26,30. Dynamic changes in FA profiles of sow milk during lactation have been reported by Hu et al.16. Similar to their results, increases of caprylic (C8:0), capric (C10:0), lauric (C12:0), palmitic (C16:0) and myristoleic acid (C14:1), and decrease of linoleic acid (C18:2n6c) in sow milk along lactation period were also found in this study. Indeed, our results demonstrated significant influence of lactation stage on the variations of 20 fatty acids in total. Continued increases in short- and medium chain FAs and decreases in long chain FAs with the increased day postpartum were notably observed. This could be linked to the secretory activation explained by the closure of cellular tight junctions and activation of de novo FA synthesis in the mammary gland15. Precisely, the acquisition of secretory activation results in higher levels of FAs with ≤ 16 carbons, de novo synthesized in the mammary gland, and reduction of FAs with ≥ 18 carbons, usually mobilized from diets or maternal adipose stores15. Consequently, the overall fatty acid component changes from larger carbon chains to smaller carbon chains as lactation progressed. Chemometric analysis revealed that overall, FA profiles of milk at day 3 were more distinctive from colostrum samples compared with those of mature milk at day 17. This pattern recognition corresponded well with the significantly highest fat content determined by MilkoScan in transient milk samples collected on day 3.
The influence of parity on variations in colostrum and milk fat linked to sow reproductive and litter performances has been reported3,17,31. Most studies found that the content of milk fat was negatively related with sow parity number. In the present study, we also found that the total fat content and concentrations of most FA in mature milk were likely decreased as parity number increased. In total, the levels of nine FAs were significantly influenced by sow parity or its interaction with lactation stage. An adverse relation between milk fat and lactose contents when the parity number increased was also observed and agreed with literature17. Chemometric analysis revealed that the influence of sow parity on overall milk fatty acid profiles was gradually distinguishable with progress of lactation. A well distinction between primiparous and 7th parity sows was notably observed in mature milk collected on day 17, which could be linked to significant variations in linoleic, lignoceric, behenic, caprylic and myristoleic acid contents.
Linoleic acid (C18:2n6) is an essential fatty acid (EFA) in sow milk which supports piglet’s brain, neural, vision, digestive tract and immune system development and function29. A high level of linoleic acid in milk has been reported to be positively correlated with reproductive performance of sows and survivability of piglets32. However, it should be noted that this statement was derived from studies dedicated to supplementing lipids from various sources to sow diets. Regarding the influence of animal parity, our results demonstrated that primiparous sows had higher milk linoleic acid concentration than multiparous sows. This observation was aligned with literature stating that, without lipid supplementation, sows advance in parity could encounter a negative EFA scenario during their lactation29,33. Because linoleic acid (C18:2n6c) is a precursor for synthesis of hormones involved in diverse stages of reproduction, dietary EFA supplementation is thus recommended to mature sows29,33. Lignoceric (C24:0) and behenic acid (C22:0) are very long-chain saturated fatty acids (VLSFA) present in milk of various mammal species34. Variations in these VLSFA in sow milk as affected by lactation stage and dietary supplementation have been reported16,35. It is well acknowledged that mobilization of long-chain FA from body reserves may trigger a mechanism to supply energy during negative balance condition36. This could be a possible explanation for the higher levels of lignoceric (C24:0) and behenic acid (C22:0) in milk of primiparous sows found in this study. Unfortunately, information on the role of VLSFA in swine health and nutrition has received little attention and therefore remains to be investigated. The presence of caprylic acid (C8:0) in sow milk has been reported to be beneficial for digestive tract modulation and health development of piglets37. Our results showed a significant interaction between parity number and lactation stage on the reduction of caprylic acid (C8:0) in mature milk from older sows. A significant decrease of milk caprylic acid (C8:0) across sow parity orders were also observed by Vieira et al.38, along with butyrate supplementation in sow diets. The presence of myristoleic acid (C14:1n5) has been reported in sow colostrum and milk16,35. Interestingly, it should be highlighted that myristoleic acid was the only FA that the concentration was significantly altered by the two main effects and their interaction in this study. A significant decrease of milk myristoleic acid (C14:1) was found in 7th parity sows. An elevated level of milk myristoleic acid (C14:1) has been reported to be likely correlated with negative energy balance in dairy cows39. It should be mentioned that alterations in milk fatty acid composition reflected the sources of lipids and metabolic activity of the mammary gland15. Based on lactation biology, it is well recognized that short- and medium-chain FAs are synthesized de novo in the mammary gland while long-chain FAs are typically released from maternal body fat reserves during negative energy balance15,40. Therefore, an increase in the milk long-chain FA level should be a more suitable indicator of negative energy balance status in lactating animals40. This could explain the higher levels of linoleic (C18:3n3), lignoceric (C24:0), behenic (C22:0) and myristoleic acid (C14:1) found in mature milk of primiparous sow in this study.
Variations in non-volatile polar metabolite profiles
This complementary approach should provide a more comprehensive insight regarding the variation in overall biomolecular composition, i.e., accounting compounds present in both lipid and non-lipid fractions, in sow colostrum and milk. Unlike the FA profiles, results revealed a progressive discrimination pattern of non-volatile polar metabolite composition of samples corresponding with the increased day postpartum. Regarding the influence of sow parity number, a good distinction of non-volatile polar metabolite profiles was observed in colostrum samples but became less noticeable in transient and mature milk. Interestingly, it should be underlined that these results were in contradiction with the discrimination patterns of FA mentioned above, in which the influence of sow parity number became more evident with progress of lactation. Regarding metabolite quantification, our results corresponded with other studies which reported no significant or slight influence of sow parity on the concentrations of colostrum and milk metabolites14,18. However, we found that variations in creatine, creatine phosphate, glutamate and glycolate contents of colostrum could be tentatively altered by the interaction between stage of lactation and sow parity. Comparative box-whisker plots showed that their concentrations were slightly raised in the colostrum from sows with high parity number.
Changes in the concentrations of colostrum and milk metabolites have been correlated with metabolic status and lactation performance of the sows12,14. Creatine and creatine phosphate are important energy sources for muscle and brain that could be essentially involved in the survival, health and growth performance of suckling piglets41. Conversion of the two metabolites into creatinine can be associated with a mobilization of stored proteins and body mass fat in high metabolic demand sows42. Therefore, high abundances of creatine and creatine phosphate in milk may indicate a good metabolic status of the mature sows in this study. Moreover, the content of creatine could be positively correlated with % Brix estimate of IgG in sow colostrum14. Although we have found a significantly lower level of IgG in colostrum from primiparous sows than in multiparous sows6, only a slight change in creatine was noticed in this study. Glutamate is an abundant amino acid in milk which provides energy source for epithelial cells especially in piglet small intestine43. An adequate amount of glutamate can maintain gut-health and prevents intestinal dysfunction resulting in better growth performance and survival of piglets43. Significantly higher levels of creatine and glutamate in milk have been reported to be associated with the high lactation performance of sows12. Considering the result in this study, a tendency to increase of creatine and glutamate in colostrum may suggest a development in lactation performance of sows with increased parity number. Since creatine is transferred from blood to milk by the mammary gland, a greater creatine level has been reported in early post-farrowing plasma of second parity compared to primiparous sows corresponding with their higher reproductive performance44. Indeed, we have found that multiparous sows can yield more colostrum than primiparous sows in our previous work3. Additionally, glycolate concentration in sow milk also influenced the litter performances13.
Conclusions
The present study demonstrated the impacts of lactation stage and parity number on biomolecular profiles in sow colostrum and milk from a metabolomic perspective. Results revealed significant impact of sow parity number on the distinction of fatty acid profiles especially in mature milk. On the other hand, in case of non-volatile polar metabolite profiles, the distinction among different parity numbers could be better noticed in colostrum samples. Variations in the contents of linoleic, lignoceric, behenic, caprylic and myristoleic acid in mature milk as well as creatine, creatinine phosphate, glutamate and glycolate in colostrum differed statistically across sow parities. Variations in the concentration of these compounds reflected the physiological function of sow mammary gland influenced by lactation period and animal parity number. This information could be applied for feed and feeding strategies in lactating sows and improving lactating performances.
Data availability
Data supported to this study can be available from the corresponding author on a reasonable request.
Abbreviations
- FA:
-
Fatty acid
- FAME:
-
Fatty acid methyl ester
- GC–MS:
-
Gas chromatography coupled with mass spectrometry
- PLS-DA:
-
Partial least square discriminant analysis
- 1H-NMR:
-
Proton Nuclear Magnetic Resonance
- TSP:
-
3-(Trimethylsilyl) propionic-2, 2, 3, 3-d4 acid sodium salt
- VIP:
-
Variable importance in projection
References
Skrzypczak, E. et al. Impact of fat and selected profiles of fatty acids contained in the colostrum and milk of sows of native breeds on piglet rearing. Anim. Sci. J. 86(83–91), 2015. https://doi.org/10.1111/asj.12241 (2015).
Theil, P. K., Krogh, U., Bruun, T. S. & Feyera, T. Feeding the modern sow to sustain high productivity. Mol. Reprod. Dev. https://doi.org/10.1002/mrd.23571 (2022).
Nuntapaitoon, M., Juthamanee, P., Theil, P. K. & Tummaruk, P. Impact of sow parity on yield and composition of colostrum and milk in Danish Landrace × Yorkshire crossbred sows. Prev. Vet. Med. 181, 105085. https://doi.org/10.1016/j.prevetmed.2020.105085 (2020).
Nuntapaitoon, M., Muns, R., Theil, P. K. & Tummaruk, P. Factors influencing colostrum consumption by piglets and relationship with survival and growth in tropical climates. Livest. Sci. 224, 31–39. https://doi.org/10.1016/j.livsci.2019.04.008 (2019).
Quesnel, H. Colostrum production by sows: Variability of colostrum yield and immunoglobulin G concentrations. Animals 5, 1546–1553. https://doi.org/10.1017/S175173111100070X (2011).
Nuntapaitoon, M. et al. Impact of parity and housing conditions on concentration of immunoglobulin G in sow colostrum. Trop. Anim. Health Prod. 51, 1239–1246. https://doi.org/10.1007/s11250-019-01816-2 (2019).
Wishart, D. S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Tech. 19, 482–493. https://doi.org/10.1016/j.tifs.2008.03.003 (2008).
Rocchetti, G. & O’Callaghan, T. F. Application of metabolomics to assess milk quality and traceability. Curr. Opin. Food Sci. 40, 168–178. https://doi.org/10.1016/j.cofs.2021.04.005 (2021).
Slupsky, C.M. Metabolomics in human milk research. Nestle Nutr Inst Workshop Ser. 90, 179–190. https://doi.org/10.1159/000490305 (2019).
Caboni, P. et al. A gas chromatography-mass spectrometry untargeted metabolomics approach to discriminate Fiore Sardo cheese produced from raw or thermized ovine milk. J. Dairy Sci. 102, 5005–5018. https://doi.org/10.3168/jds.2018-15885 (2019).
Curtasu, M. V., Theil, P. K. & Hedemann, M. S. Metabolomic profiles of colostrum and milk from lactating sows. J. Anim. Sci. 94, 272–275. https://doi.org/10.2527/jas.2015-9769 (2016).
Tan, C. et al. Metabolomic profiles reveal potential factors that correlate with lactation performance in sow milk. Sci. Rep. 8, 10712. https://doi.org/10.1038/s41598-018-28793-0 (2018).
Picone, G. et al. Metabolomics characterization of colostrum in three sow breeds and its influences on piglets’ survival and litter growth rates. J. Anim. Sci. Biotech. 9, 23. https://doi.org/10.1186/s40104-018-0237-1 (2018).
Luise, D. et al. Investigation of the defatted colostrum1H-NMR metabolomics profile of gilts and multiparous sows and its relationship with litter performance. Animals 10, 154. https://doi.org/10.3390/ani10010154 (2020).
Suarez-Trujillo, A. et al. Changes in sow milk lipidome across lactation occur in fatty acyl residues of triacylglycerol and phosphatidylglycerol lipids, but not in plasma membrane phospholipids. Animal 15, 100280. https://doi.org/10.1016/j.animal.2021.100280 (2021).
Hu, P. et al. Dynamic changes of fatty acids and minerals in sow milk during lactation. J. Anim. Physiol. Anim. Nutr. (Berl) 103, 603–611. https://doi.org/10.1111/jpn.13040 (2019).
Amatucci, L., Luise, D., Correa, F., Bosi, P. & Trevisi, P. Importance of breed, parity and sow colostrum components on litter performance and health. Animals 12, 1230. https://doi.org/10.3390/ani12101230 (2022).
Craig, J. R., Dunshea, F. R., Cottrell, J. J., Wijesiriwardana, U. A. & Pluske, J. R. Primiparous and multiparous sows have largely similar colostrum and milk composition profiles throughout lactation. Animals https://doi.org/10.3390/ani9020035 (2019).
O’Fallon, J. V., Busboom, J. R., Nelson, M. L. & Gaskins, C. T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 85, 1511–1521. https://doi.org/10.2527/jas.2006-491 (2007).
Settachaimogkon, S., Wannakajeepiboon, N., Arunpunporn, P., Mekboonsonglarp, W. & Makarapong, D. Changes in bovine colostrum metabolites during early postpartum period revealed by 1H-NMR metabolomics approach. Trop. Anim. Sci. J. 44, 229–239. https://doi.org/10.5398/tasj.2021.44.2.229 (2021).
Luangwilai, M., Duangmal, K., Chantaprasarn, N. & Settachaimongkon, S. Comparative metabolite profiling of raw milk from subclinical and clinical mastitis cows using 1H-NMR combined with chemometric analysis. Int. J. Food Sci. 56(493–503), 2021. https://doi.org/10.1111/ijfs.14665 (2021).
Settachaimongkon, S., van Valenberg, H.J.F. & Smid, E.J. Metabolomics as an emerging strategy for the investigation of yogurt components. In Yogurt in Health and Disease Prevention (ed Shah, N. P.) 427–449 (Academic Press, 2017).
Cui, J. et al. The combined use of 1H and 2D NMR-based metabolomics and chemometrics for non-targeted screening of biomarkers and identification of reconstituted milk. J. Sci. Food Agric. 99, 6455–6461. https://doi.org/10.1002/jsfa.9924 (2019).
Hurley, W.L. Composition of sow colostrum and milk. In The gestating and lactating sow gestating and lactating sows (ed Farmer, C.). 193–229 (Wageningen Academic Publishers, 2015).
Peter, J. C. & Mahan, D. C. Effects of dietary organic and inorganic trace mineral levels on sow reproductive performance and daily mineral intake over six parities. J. Anim. Sci. 86, 2247–2260. https://doi.org/10.2527/jas.2007-0431 (2008).
Goransson, L. The effect on late pregnancy feed allowance on the milk composition of the sow’s colostrum and milk. Acta. Vet. Scand. 31, 109–115. https://doi.org/10.1186/BF03547583 (1990).
Ren, C., Jin, J., Wang, X., Zhang, Y. & Jin, Q. Evaluation of fatty acid profile of colostrum and milk fat of different sow breeds. Int. Dairy J. 126, 105250. https://doi.org/10.1016/j.idairyj.2021.105250 (2022).
Bai, Y. S., Wang, C. Q., Zhao, X., Shi, B. M. & Shan, A. S. Effects of fat sources in sow on the fatty acid profiles and fat globule size of milk and immunoglobulins of sows and piglets. Anim. Feed Sci. Technol. 234, 217–227. https://doi.org/10.1016/j.anifeedsci.2017.10.006 (2017).
Holen, J. P. et al. Evaluation of essential fatty acids in lactating sow diets on sow reproductive performance, colostrum and milk composition, and piglet survivability. J. Anim. Sci. https://doi.org/10.1093/jas/skac167 (2022).
Jin, C. et al. Influence of dietary fat source on sow and litter performance, colostrum and milk fatty acid profile in late gestation and lactation. Anim. Sci. J. 88, 1768–1778. https://doi.org/10.1111/asj.12836 (2017).
Segura, M., Martínez-Miró, S., López, M. J., Madrid, J. & Hernández, F. Effect of parity on reproductive performance and composition of sow colostrum during first 24 h postpartum. Animals 10, 1–12. https://doi.org/10.3390/ani10101853 (2020).
Jang, K. B., Purvis, J. M. & Kim, S. W. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiome of offspring. J. Anim. Sci. 98, skaa227. https://doi.org/10.1093/jas/skaa227 (2020).
Rosero, D. S. et al. Impact of dietary lipids on sow milk composition and balance of essential fatty acids during lactation in prolific sows. J. Anim. Sci. 93, 2935–2947. https://doi.org/10.2527/jas.2014-8529 (2015).
Yang, J., Zheng, N., Wang, J. & Yang, Y. Comparative milk fatty acid analysis of different dairy species. Int. J. Dairy Technol. 71, 544–550. https://doi.org/10.1111/1471-0307.12443 (2018).
Ma, C. et al. Branched chain amino acids alter fatty acid profile in colostrum of sows fed a high fat diet. J. Anim. Sci. Biotechnol. 11, 9. https://doi.org/10.1186/s40104-019-0423-9 (2020).
Raclot, T. Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog. Lipid Res. 42, 257–288. https://doi.org/10.1016/S0163-7827(02)00066-8 (2003).
Świątkiewicz, M., Hanczakowska, E., Okoń, K., Kowalczyk, P. & Grela, E. R. Effect of maternal diet and medium chain fatty acids supplementation for piglets on their digestive tract development, structure, and chyme acidity as well as performance and health status. Animals 10, 834. https://doi.org/10.3390/ani10050834 (2020).
Vieira, E. H. M. et al. Dietary supplementation of sodium butyrate for mixed-parity sows during lactation. Livest. Sci. 232, 103915. https://doi.org/10.1016/j.livsci.2020 (2020).
Ducháček, J., Vacek, M., Stádník, L., Beran, J. & Okrouhlá, M. Changes in milk fatty acid composition in relation to indicators of energy balance in Holstein cows. Acta Univ. Agric. Silvic. Mendelianae Brun. 60, 29–38. https://doi.org/10.11118/actaun201260010029 (2012).
Churakov, M., Karlsson, J., Rasmussen, A. E. & Holtenius, K. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animal 15, 100253. https://doi.org/10.1016/j.animal.2021.100253 (2021).
Kennaugh, L. M., Arthur, P. G. & Hartmann, P. E. The concentration of creatine and creatine phosphate in sow colostrum and milk throughout lactation and weaning. Aust. J. Agric. Res. 48, 1105–1110. https://doi.org/10.1071/A96077 (1997).
van Niekerk, B. D. H., Reid, J. T., Bensadoun, A. & Paladines, O. L. Urinary creatinine as an index of body composition. J. Nutrit. 79, 463–473. https://doi.org/10.1093/jn/79.4.463 (1963).
Hou, Y. & Wu, G. L-Glutamate nutrition and metabolism in swine. Amino Acids 50, 1497–1510. https://doi.org/10.1007/s00726-018-2634-3 (2018).
Rempel, L. A., Vallet, J. L. & Nonneman, D. J. Characterization of plasma metabolites at late gestation and lactation in early parity sows on production and post-weaning reproductive performance. J. Anim. Sci. 96, 521–531. https://doi.org/10.1093/jas/skx066 (2018).
Acknowledgements
We acknowledge a commercial swine farm for the samples of sow colostrum and milk.
Funding
The present scientific research was financially afforded by Ratchadapisek Sompot Endowment Research Fund (CU_GR_63_45_31_60) and Thailand Science Research and Innovation Fund (CU_FRB65_food(27)_191_31_10), Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
M.N. and S.S. conceived and designed the experiment, writing-review and editing manuscript. K.H. and W.M. carried out the GC–MS and 1H-NMR analysis, respectively. M.N., S.S., K.H., P.S., T.L., P.P. and P.K.T. analyzed, interpreted the data and wrote the original draft of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Settachaimongkon, S., Homyog, K., Mekboonsonglarp, W. et al. Dynamics of fatty acid and non-volatile polar metabolite profiles in colostrum and milk depending on the lactation stage and parity number of sows. Sci Rep 13, 1989 (2023). https://doi.org/10.1038/s41598-023-28966-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28966-6
- Springer Nature Limited