Abstract
Assessment of burn extent and depth are critical and require very specialized diagnosis. Automated image-based algorithms could assist in performing wound detection and classification. We aimed to develop two deep-learning algorithms that respectively identify burns, and classify whether they require surgery. An additional aim assessed the performances in different Fitzpatrick skin types. Annotated burn (n = 1105) and background (n = 536) images were collected. Using a commercially available platform for deep learning algorithms, two models were trained and validated on 70% of the images and tested on the remaining 30%. Accuracy was measured for each image using the percentage of wound area correctly identified and F1 scores for the wound identifier; and area under the receiver operating characteristic (AUC) curve, sensitivity, and specificity for the wound classifier. The wound identifier algorithm detected an average of 87.2% of the wound areas accurately in the test set. For the wound classifier algorithm, the AUC was 0.885. The wound identifier algorithm was more accurate in patients with darker skin types; the wound classifier was more accurate in patients with lighter skin types. To conclude, image-based algorithms can support the assessment of acute burns with relatively good accuracy although larger and different datasets are needed.
Similar content being viewed by others
Introduction
Burns are defined as the destruction of tissues (skin or other organs) due to energy transfer caused by heat, friction, cold, radiation, electricity or chemicals1. They are a common injury of daily life globally with an estimated number of 153,000 deaths annually globally2, and a much larger number of non-fatal cases. Not only are they common, they also often affect the most vulnerable parts of populations such as children, elderly, those with poor living conditions, low income or immigrant status residence3. Correctly assessing the extent and depth of an acute burn is difficult and misdiagnosis has large consequences for both the patients and the burn centres4,5. Indeed, there is evidence that visual assessment of burn depth at bedside by front line clinicians6,7 and surgeons7,8 can be inaccurate6 with errors occurring as often as in 25–39% of cases. Distinguishing early those burns that will heal spontaneously and can be managed conservatively (often at a lower level of care) from those that require surgical intervention (e.g. excision and skin grafts) is critical not only to reduce the risk of over- and undertreatment of the patient, but also to not overwhelm scarce specialized resources9.
The most equipped burns centres have access to technologies like laser doppler or optical coherence tomography to enhance their performances in order to diagnose depth and estimate the need for surgery10. Such equipment is however very expensive and uncommon in both high-income11 and low-income settings12. Besides being ill equipped, burns centres in low-income settings struggle also with high caseloads and under-resourced emergency care services13,14. Indeed, in those settings it is not only the equipment that is lacking, it is also the number of specialists who can provide accurate diagnosis for all those who need it15.
For front line clinicians at the point of care it is critical that they receive timely diagnostic assistance from burns experts in order to improve patient outcome and perform triage. Remote consultation through mobile health (mHealth) applications which are now deployed in many clinical environments is a potential solution16. Studies reveal that they both provide front-line clinicians with accurate diagnosis for burn depth17 and extent18 and that they receive acceptance at both ends, i.e. from front-line clinicians and burns specialists19.
A step further from remote image-based diagnostic assistance given by burns experts is through automated processes, as was suggested in recent studies20. Several automated procedures for burn depth estimation have been suggested with different levels of technological sophistication21,22,23,24,25,26,27,28,29,30,31,32,33. At an early stage, hand-crafted image features from a small number of images were used with the aim to segment the burn with the help of the user, and then classify the burn depth into three or four categories24,25. More recent approaches take advantage of deep-learning methods such as convolutional neural networks (CNNs) with transfer learning to differentiate burn areas from either normal skin21,22,23 or other types of wounds26,27. Regarding burn depth classification, the reported deep-learning algorithms resulted in accuracies between 81 and 95% when classifying depth, including healthy skin28,30. These studies however often use images collected through online searches and lack appropriate burn diagnosis30,31,32,33. Indeed, through a systematic review, we have identified that despite the increased accuracy in burn automated diagnosis, especially for burn segmentation, there are currently large risks of bias in studies at hand, and improved results are needed20. Another limitation is the fact that most studies were based on images from fair types of skin. This has implications severe for training of algorithms which will likely not be representative of all populations. It is also a big limitation from a clinical perspective as most cases occur in the most vulnerable populations, including in South-East Asia and Sub-Saharan Africa, who could benefit the most from this types of technologies34. In fact, only one study has included burns from Caucasian and African patients and demonstrated the complexity of training an algorithm in a mixed skin types environment21.
Given the current evidence for the development of automated burn diagnosis, but the lack of appropriate training material, especially including patients with different skin types, this study was embarked with an aim that was three-fold: to develop and assess image-based deep-learning algorithms that identify burn wounds, classify them based on their depth whether they need surgery (such as skin grafting) or not, and assess accuracy of the algorithms among several skin types.
Methods
Hereafter we will present the methods used for database generation, annotation, model development and statistical analyses.
Image database and annotations
A database of images has been assembled and is composed of two separate datasets in order to represent patients with different skin colour (also known as Fitzpatrick skin types35). There are six Fitzpatrick skin prototypes, which are a constitutional characteristic of the patient from birth, and which characterize the colour of the skin as well as its reaction to ultraviolet radiation exposure35. Our two datasets consist of one collected in Sweden and including mostly patients of North-Germanic origin with lighter skin types (Fitzpatrick skin types 1–2). The second was collected in South Africa and includes mostly patients of South African origins with mixed and darker skin types (Fitzpatrick skin types 3–6).
The Swedish cases were all collected at the burn centre at Uppsala University Hospital between 2006 and 2019. Pictures were taken as part of routine care from arrival to discharge of the patient in order to follow up on wound progression. Patients were then contacted in 2018 to obtain informed consent of these images for the current study.
The South African cases were collected from 2016 to 2018 at one of three burns centres in the country: Tygerberg Hospital and Red Cross Children’s War Memorial Hospital in Cape Town, Western Cape Province, and Edendale Hospital in Pietermaritzburg in Kwa-Zulu Natal. Referrals to these burns centres through the burn section of an image-based local mHealth App: Vula Mobile (www.vulamobile.com) submitted between 2017 and 2018 were also included. Consent was obtained at point of care as part of routine care.
All images and associated information were pseudonymized and stored on a secure server (ownCloud, ownCloud Inc, Lexington, MA) managed by Karolinska Institutet and located in Stockholm, Sweden. Patient information included age group (children and adults) and sex, injury information included body part involved, burn mechanism, and burn depth. Burn depth was assessed by a burn expert as part of the routine clinical evaluation, either at bedside on arrival of the patient to the respective burns centre, or by a burn expert remotely when giving an image-based remote diagnosis based on images through the Vula Mobile app17
In order to be included, images had to present acute burns (photographed within 48 h post injury, which is defined as the period of shock36). In order to mimic most appropriately the clinical settings where only clean, scrubbed wounds are accepted for remote consultation, wounds included had to be undressed, cleaned and scrubbed (all blisters removed) prior to the picture being taken. All pictures not fulfilling those criteria were excluded.
The final database comprised of 391 (35%) images collected in Sweden and 714 (65%) of images collected in South Africa. A total of 387 patients were included, of which 198 (51%) were children. This resulted in 1105 images representing various body parts of which 339 (31%) required surgery.
To improve the discrimination between the burn wound, normal skin and background such as clothes, bed sheets and various objects in the surrounding, a number of background images were obtained from two publicly available online datasets37,38. Images were specifically selected to contain some human body parts with skin present, and with a diversity of skin types.
To mimic “real-life” setting, no standardization protocols were provided for image capture. Indeed, it is believed that if there were to be such protocols in some settings, point-of-care clinicians would not provide images for remote consultation. Images were therefore collected with varying background, devices used, distance from the wound, orientation, flash use, or size. Using previously defined anthropomorphic measurements39,40, the pixel size of all images was approximated and set individually on the algorithm training platform (Aiforia Hub, Aiforia Technologies, Helsinki, Finland) in order to adjust the scale of the photographed body parts and the variable range of age groups.
All images were individually and manually annotated to segment the burn wound from normal skin (or background) on a pixel-by-pixel level using binary masks created with an image annotation software (ImageJ, NIH, Bethesda, MD), or directly on the algorithm training platform (Aiforia Create, Aiforia Technologies, Helsinki, Finland). Annotations done using ImageJ were programmatically imported to the training platform. Annotations were performed by trained nurses and medical students familiar with burn injuries under the supervision of CB (South Africa) and JF (Sweden). Several images were annotated by at least two annotators and verified through close collaboration with burn experts. Regarding the surgical classification annotations which requires extreme levels of competences in the field, these were made on an image-level based on the burn’s expert depth diagnosis which is considered gold standard. Previous work using some thirty images also included in this study has shown acceptable agreement in over two thirds of the images17.
Training of the deep learning algorithms
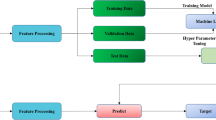
Considering the latest development of research in the field, as well as the complexity of the wound patterns to be classified, we assumed that a probabilistic model would be the best approach, we therefore decided to train CNNs using a commercially available software (Aiforia Create, Aiforia Technologies, Helsinki, Finland). Two independent and separate models were developed. The first algorithm identified and segmented the burn wounds from the background (everything in the image that is not a burn wound). The second algorithm classified each burn area based on their depth into one of two categories: surgical burns which require surgical intervention, for example for skin grafting, because they are of deep-partial or full thickness; or non-surgical burns which are superficial and superficial partial thickness burns and are manageable with conservative treatment possibly at lower levels of care. Keeping in proportions of images from each skin type and for each severity status, the images were split into two sets: one training and validation dataset consisting 70% (n = 773) of all the images, and a set-aside test set with the remaining 30% (n = 332 images) (see Fig. S1).
For the wound identification algorithm, the training area included the whole image and the burn was the area to be segmented. The 536 background images were only added for training (none of the images were included in the test set) and had no annotations. These images were not used with the aim of specifically recognizing other objects, but rather with the only aim to improve the algorithm for burn segmentation. The hypothesis behind this aim is that this type of algorithm would only be used in the context of an acute burn, rather than for other purposes. For the second algorithm of wound classification, only the wound areas were used in the training, separated into two categories based on the burn depth, either surgery needed or no surgery needed. For both algorithms, the training was performed three times using a random selection of 70% of the images directly by the software, and the remaining 30% was used as a validation set for evaluation of the results obtained as well as for hyperparameter selection. After the verification that all three trainings obtained similar results, a last training was performed using the same settings and 100% of the training set prior to be predicted on the set-aside test set.
In addition to the training and testing performed on the complete dataset, separate trainings with the same settings (see Table 1) and subsequent testing were performed on the datasets separated according to skin type (Fitzpatrick skin types 1–2 vs 3–6).
For all algorithms, hyperparameters are presented in Table 1. Feature size was predefined at 125 units for the wound detection algorithms, and 190 units for the surgery classification algorithms. A unit corresponds to an approximated pixel size following manual adjustment according to anthropomorphic measurements as described above.
Statistical analyses
Statistical analyses were all performed using Stata for Mac version 15. Average measures with standard deviations were calculated for the results of all three folds of training and validation sets. Wound identification was measured on a pixel-by-pixel level for each image and aggregated measures for all images were used. This assessment was made using sensitivity (percentage of pixels identified as burn areas out of the whole burn area), precision (percentage of pixels of burnt area out of all those identified as a burn by the algorithm), and F1 score (the harmonic mean of the sensitivity and the precision). For background images, the percentage of images in which a burn area was identified was recorded as well as the number of images in which the identified burn area represented more than 5% of the image itself. Analyses for the two stratified trainings and testing by skin type of the patients were performed and statistical difference in sensitivity was measured using a non-parametric Mann–Whitney U-test.
For burn surgical classification, while the outcome is binary (surgical burns of deep-partial or full thickness and non-surgical burns of superficial and superficial-partial thickness) the algorithm defines for each image an area in pixels that would require surgery or not. Receiver Operating Characteristic (ROC) curve as well as area under the ROC curve (AUC) were measured. Images were then classified as a surgical burn when ≥ 1% of the wound’s pixels were identified as such. The success rate was the number of images correctly classified over the total number of images in a given set. Sensitivity represents the percentage of images which had ≥ 1% of pixels identified as surgical burn out of all images that required surgery. Specificity was measured as the number of images which had < 1% of identified surgical burn pixels out of all images that did not require surgery. This was measured first for all training and validation sets, and then in the set-aside test set overall; and for the set stratified by skin type of the patients. Sensitivity and specificity are presented with 95% confidence intervals (C.I.) measured using the Clopper–Pearson (exact) estimation.
Ethical approval
Ethical approval was granted by the Regional Ethics Board in Uppsala (Dnr 2016/279) for the Swedish part of the study, and by both the Stellenbosch Health Research Ethics Committee (N13/02/024) and the University of Kwa-Zulu Natal Biomedical Research Ethics Committee (BCA106/14) for the South African part of the study. All methods were carried out in accordance with relevant guidelines and regulations.
Results
The following section will present the results obtained for both algorithms: first that of burn area identification, and secondly of burn severity classification.
Burn area identification and segmentation
In the three-fold trainings (where three training and validation runs were performed using a random 70% vs 30% of the images), a burn area was identified in 13.1% of the images which did not contain any burns in the training set, whereas 0.5% of images with a burn were not identified as such. In the non-burn images, the area identified was larger than 5% of the images’ total pixels in only 6 out of 1147 images. In the validation set, a burn area was identified in 20.0% of the non-burn images, however this area was larger than 5% of the images’ pixels in only 6 out of the 464 background images. Table 2 presents the results in terms of sensitivity and specificity for the number of images in which a burn area was identified for both burn and non-burn images.
Figure S3 presents the results for each of the training and validation sets in the three-fold training. Table 3 presents the accuracy measures for the pooled three-fold training and validation sets as well as for the final training and test datasets. The burn identification algorithm could identify 92.5% and 85.1% of the burn area across all three-fold training and testing sets respectively. In the final training, the sensitivity was of 93.2% while in the test set it was of 86.9%. Figure S2 illustrates graphically the results of the burn identification algorithm.
When the results were split by skin types of the patients, the average sensitivity was higher in cases with a darker skin type than in those with a lighter skin type (Mann–Whitney U test; P < 0.001; Table 4).
Classification of burn depth according to the need of surgery
Across the three training folds, almost all the burns requiring skin grafting were identified as such, with a sensitivity of 98%. The specificity was also high with 88%, overall. In the validation sets, the sensitivity was 96% and the specificity was 71%. In the last training, sensitivity was of 99.6% and specificity was of 93.4%. The ROC curves for the set-aside test set as a whole as well as for the two separate skin types are presented in Fig. 1. The areas under the curves were of 0.885, 0.863 and 0.875 in the complete set, and in the images of lighter and darker skin types respectively.
Table 5 presents the summary accuracy statistics for the surgery classification algorithms. The success rate overall was 64.7%, and 78.0% and 66.8% respectively in patients of lighter and darker skin types. The overall sensitivity was 92.5% and the specificity was 53.8%. Indeed, out of the 93 burns which required surgery, only 7 were misdiagnosed. On the other hand, 128 out of 238 burns which did not require surgery were diagnosed as such by the algorithm.
Discussion
This study, designed as a proof-of-concept to assist in the diagnosis of extremely complex real-world medical images, evaluates a deep-learning approach for burn wound identification and classification in patients with a full range of skin types. Our results indicate that burns can be identified and segmented with a reasonable accuracy in highly variable pictures captured in real-life settings, whereas classification according to the need of surgery is more challenging. While the burn identification algorithm showed better performance for patients with darker Fitzpatrick skin types, the algorithm for the classification of surgery was less accurate for those patients compared to patients of lighter skin types. We believe the differences in results obtained between skin types and algorithms could be explained by the fact that the difference in colour between burn and normal skin is probably larger for patients of darker-skin types and therefore the wound identifier algorithm performing better in that dataset. On the contrary, the wound classifier performed better in the lighter skin-types which could partly be explained by a higher quality of the images and less diverse image capture settings.
The aggregated results of the accuracy of the wound identification are in line with previous deep-learning studies, as reflected in an F1 score of 83.0%22,23,41. Requesting the front-line user to indicate where the burn is prior to segmentation, as it has been done in the past24,25 might improve the results; yet, it is doubtful that this procedure would be viable in real life settings.
Including images without burns in the data revealed a propensity for the algorithm to identify small burn areas where there were none in as much as a fifth of the images in the validation set. A slightly larger proportion of false positives was also reported in a previous deep-learning study23. Although images without any burn wounds are unlikely to be submitted by a front-line healthcare professional for diagnostic assistance, false positives could later on have consequences if the algorithm is used in sequence with a wound classifier algorithm in a fully automated approach. This could be the focus of future studies whereby training and testing with non-burn images is an integral part of the research question, and with the hypothesis that non-burn images could be provided to such a model by a clinician. In fact, previous studies have already studied the distinction between burn and other types of skin wounds26,27. Focus of further research could also include replicating the obtained results using an open-source software.
With regards to burn severity classification, most of the algorithms published did not apply CNNs but rather, handcrafted features were used to train mathematical or Support Vector Machine (SVM) models24,25,29. Using those methods, two studies also discriminated between the need for surgery or not, and classified accurately about 80% of the images24,29. These results are comparable to ours for patients of North-Germanic origin which had similar (lighter) skin types as in those previous studies, but higher than what we obtained with all cases aggregated. Nonetheless, there has been more recent CNN models that have classified burn depth in several categories including normal skin, with promising results between 80 and 95%28,30,31,42. Given the differences in category definition, these results are difficult to compare to the ones we have obtained.
The algorithm was trained using a commercially available software which did not permit to describe the actual architecture of the CNN models. Nonetheless, we believe that with the parameter information provided in this publication it should be possible to replicate the experiment. If a researcher requires access to the exact same algorithm, the corresponding author can be contacted for inquiries. Further, this study did not investigate other types of approaches to analyse the data such as SVM or decision trees. Differences in performances between approaches was not the aim of the study, and our choice of CNNs was based on previous literature where deep-learning algorithms outperformed other cited methods for image segmentation and classification43.
The depth of the burn correlates with the time to healing and scarring (and need for surgical intervention) and has therefore consequences on the clinical protocol. However, as indicated earlier, clinical evaluation of the depth is a challenging task. Our results suggest we could reduce this error to one in four cases among the lighter skin types but likely not among the darker ones. Image-based assessment of whether surgery is indicated or not has been tested on physicians of a range of competences, including referring physicians with a higher specificity (76.2%) but a much lower sensitivity than our algorithm (39.9% versus 92.5% respectively)44. It is also of note that in order to give an appropriate diagnosis, burn wounds should be cleaned and undressed. This is the case not only for clinical bed-side evaluations, but also for remote consultation. Therefore, we have only included such type of wound images in our dataset. It is possible that in the future less stringent criteria can be applied, and for an algorithm to be trained on uncleaned wounds, nonetheless, this will require some more complex data collection, annotation and model training.
Contrary to what was suggested previously by Abubakar and colleagues who also investigated two skin types, performing separate model trainings did not improve the results compared to the hybrid algorithm with regards to the AUC26. They however suggested that providing skin-type information as input to the algorithms would improve the results, which might also be the case in our algorithm since we find differences in results between skin types. It is also possible that all datasets collected to date are too small for this type of complexity level. It is nonetheless important to include patients of various skin types, and especially those of darker skin types as they are those where burn injuries are most prevalent2 and where care is the least available15.
The main strength of this study is that it is based on a large dataset with patient images diversified as regards type of skin, setting (with several centres from South Africa and Sweden), injured population (adults versus children), injured body parts, and burn mechanisms. An additional quality of the dataset is that it consists of images from real-life settings, acquired using various cameras and smartphones, in the same way they would be sent to a specialist for assessment.
A drawback of the dataset is that the classification as to whether surgery was needed or not was image-based rather than on specific areas of the images. The training was therefore weakly supervised, a procedure that will introduce noise in the labels and thereafter a loss in accuracy as the algorithm returns a value for each pixel. It is however also a strength in that it is a better representation of how a real-life remote consultation would proceed with a burn specialist. Both algorithms were analysed and presented separately in this study, it is however possible to envision that in the future they will form part of a single assessment, with the regions identified would then be fed for severity classification. Yet, this would require detailed pixel-level annotation even for the severity classification algorithm.
An additional issue is the differences between the material collected from both settings. The images from South Africa surpassed in number the ones from Sweden and contained most of the cases requiring surgery. Furthermore, their origin and quality were more heterogeneous as several burns centres and their referrals were included. These differences may have resulted in a slight overtraining towards the dark-skinned cases. However, the results of the wound classifier algorithm with much higher accuracy for images captured in Sweden shows this is probably not the case.
Among the annotations, whether surgery was required or not was established by a burn expert, a method that can lead to discrepancies between physicians8, but which was measured previously on a similar dataset with acceptable results17. Given the high number of images and the retrospective use of clinical diagnosis, difference between annotators could unfortunately not be measured in this study. A potential alternative would have been to wait for 21 days for wound closure, which would be particularly relevant given the dynamic nature of the burn wound in the first hours following a burn. This is however not feasible in settings where hospital beds are extremely limited and risk of infection is high9. Furthermore, additional variables such as the mechanism of the injury, or physical parameters such as blanching or capillary refill might have improved the surgical classification algorithm’s performances. On a similar note, in the first few days following the injury, the borders of the superficial burn areas can be difficult to define. Training of the annotators as well as close collaboration with the burn experts were intended to reduce the error in area definitions, nonetheless annotation errors cannot be excluded. However, given the high number of pixels included in a burn image and the fact that the majority of the pixels within the drawn polygon are likely to represent the true burn wound, this should not have affected the results in a significant manner.
Results in other disciplines such as dermatology, or ophthalmology have highlighted the potential added value of deep learning algorithms for improved diagnosis45,46. This study is a first step towards the development of such a system in burns care where, globally, the number of cases far exceeds the number of specialists available for assistance. To augment the accuracy of the algorithms, there might be a need for training in more homogeneous and refined subsets of data. However, these subsets might come short of being a good reflection in real-life settings. Additional challenges ahead would relate to the acceptance and trust of procedures of the like among all users , their seamless integration into health care services47, and the full respect of crucial ethical principles like patient autonomy and safety48.
From the front-line clinicians’ perspective, the accuracy results obtained are comparable to bedside levels of assessment, thus the algorithm could assist with their management of burns patients, relieving most of the stress usually observed. From the burns specialist’s perspective, a high sensitivity would minimize the risk of missing a burn that would require surgery, enhancing the probability of a good outcome for the patient as it would provide an accurate diagnosis while still reducing the number of referrals to a burns specialist who cannot attend all cases.
Conclusion
This article provides evidence that two steps critical to burn assessment can be supported by image-based deep-learning algorithms among various skin types with high levels of accuracy for wound identification but also for wound classification with respect to the need for surgery, especially in lighter skin types. For an automated diagnosis to become a viable option to be used by frontline clinicians at point-of-care, the performance of deep learning algorithms must be re-trained and assessed on extended datasets representing even more variable clinical settings. Training using pixel-level annotations, identification and segmentation of burn depths could also be beneficial from a clinical standpoint in order to have a more precise diagnosis. Furthermore, for such systems to be implemented, acceptability and usability issues will have to be looked into.
Data availability
The data that support the findings of this study were used under a license for the current study, and some restrictions apply to their availability. The data are available from the authors upon reasonable request.
Change history
27 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-31508-9
References
Jeschke, M. G. et al. Burn injury. Nat. Rev. Dis. Prim. https://doi.org/10.1038/s41572-020-0145-5 (2020).
World Health Organization. Global Health Estimates 2016: Estimated deaths by cause and region, 2000 and 2016. (2017).
Peck, M. D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 37(7), 1087–1100. https://doi.org/10.1016/j.burns.2011.06.005 (2011).
den Hollander, D. & Mars, M. Smart phones make smart referrals. Burns https://doi.org/10.1016/j.burns.2016.07.015 (2016).
den Hollander, D., Albertyn, R. & Amber, J. Palliation, end-of-life care and burns; concepts, decision-making and communication—A narrative review. Afr. J. Emerg. Med. 10(2), 95–98. https://doi.org/10.1016/j.afjem.2020.01.003 (2020).
Hlava, P., Moserova, J. & Konigova, R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir. Plast. 25(4), 202–208 (1983).
Heimbach, D. M., Afromowitz, M. A., Engrav, L. H., Marvin, J. A. & Perry, B. Burn depth estimation—Man or machine. J. Trauma 24(5), 373–378 (1984).
Niazi, Z. B. M. et al. New laser doppler scanner, a valuable adjunct in burn depth assessment. Burns 19(6), 485–489. https://doi.org/10.1016/0305-4179(93)90004-R (1993).
Singh, V., Devgan, L., Bhat, S. & Milner, S. M. The pathogenesis of burn wound conversion. Ann. Plast. Surg. 59(1), 109–115. https://doi.org/10.1097/01.sap.0000252065.90759.e6 (2007).
Paul, D. W. et al. Noninvasive imaging technologies for cutaneous wound assessment: A review. Wound Repair Regen. 23(2), 149–162 (2015).
Resch, T. R., Drake, R. M., Helmer, S. D., Jost, G. D. & Osland, J. S. Estimation of burn depth at burn centers in the United States: A survey. J. Burn Care Res. 35(6), 491–497. https://doi.org/10.1097/BCR.0000000000000031 (2014).
Calland, J. F. et al. Burn management in sub-Saharan Africa: Opportunities for implementation of dedicated training and development of specialty centers. Burns 40(1), 157–163. https://doi.org/10.1016/j.burns.2013.05.015 (2014).
Tyson, A. F. et al. Survival after burn in a sub-Saharan burn unit: Challenges and opportunities. Burns 39(8), 1619–1625. https://doi.org/10.1016/j.burns.2013.04.013 (2013).
Allorto, N. L., Wall, S. & Clarke, D. L. Quantifying capacity for burn care in South Africa. Burns Open 2(4), 188–192. https://doi.org/10.1016/j.burnso.2018.07.002 (2018).
Allorto, N. L., Zoepke, S., Clarke, D. L. & Rode, H. Burn surgeons in South Africa: A rare species. S. Afr. Med. J. 106(2), 186. https://doi.org/10.7196/SAMJ.2016.v106i2.9954 (2016).
Blom, L. mHealth for image-based diagnostics of acute burns in resource-poor settings: Studies on the role of experts and the accuracy of their assessments. Glob. Health Action 13(1), 1802951. https://doi.org/10.1080/16549716.2020.1802951 (2020).
Blom, L. et al. Accuracy of acute burns diagnosis made using smartphones and tablets: A questionnaire-based study among medical experts. BMC Emerg. Med. 17(1), 39. https://doi.org/10.1186/s12873-017-0151-4 (2017).
Tocco-Tussardi, I., Presman, B. & Huss, F. Want correct percentage of TBSA burned? Let a Layman do the assessment. J. Burn Care Res. https://doi.org/10.1097/BCR.0000000000000613 (2017).
Crumley, I., Blom, L., Laflamme, L. & Alvesson, H. M. What do emergency medicine and burns specialists from resource constrained settings expect from mHealth-based diagnostic support? A qualitative study examining the case of acute burn care. BMC Med. Inform. Decis. Mak. 18(1), 71. https://doi.org/10.1186/s12911-018-0647-1 (2018).
Boissin, C. & Laflamme, L. Accuracy of image-based automated diagnosis in the identification and classification of acute burn injuries. A systematic review. Eur. Burn J. 2(4), 281–292 (2021).
Abubakar, A., Ugail, H. & Bukar, A. M. Noninvasive assessment and classification of human skin burns using images of Caucasian and African patients. J. Electron. Imaging. 29(4), 041002 (2020).
Jiao, C., Su, K., Xie, W. & Ye, Z. Burn image segmentation based on Mask Regions with Convolutional Neural Network deep learning framework: More accurate and more convenient. Burns Trauma 7, 1–14. https://doi.org/10.1186/s41038-018-0137-9 (2019).
Badea, M.-S., Vertan, C., Florea, C., Florea, L. & Badoiu, S. Automatic burn area identification in color images. In 2016 International Conference on Communications (COMM) (IEEE, 2016).
Acha, B., Serrano, C., Fondon, I. & Gomez-Cia, T. Burn depth analysis using multidimensional scaling applied to psychophysical experiment data. IEEE Trans. Med. Imaging 32(6), 1111–1120. https://doi.org/10.1109/TMI.2013.2254719 (2013).
Serrano, C., Acha, B., Gomez-Cia, T., Acha, J. I. & Roa, L. M. A computer assisted diagnosis tool for the classification of burns by depth of injury. Burns 31(3), 275–281. https://doi.org/10.1016/j.burns.2004.11.019 (2005).
Abubakar, A., Ajuji, M. & Usman, Y. I. Comparison of deep transfer learning techniques in human skin burns discrimination. Appl. Syst. Innov. 3(2), 20. https://doi.org/10.3390/asi3020020 (2020).
Khalil, A., Elmogy, M., Ghazal, M., Burns, C. & El-Baz, A. Chronic wound healing assessment system based on different features modalities and non-negative matrix factorization (NMF) feature reduction. IEEE Access 7, 80110–80121. https://doi.org/10.1109/ACCESS.2019.2923962 (2019).
Cirillo, M. D., Mirdell, R., Sjöberg, F. & Pham, T. D. Time-independent prediction of burn depth using deep convolutional neural networks. J. Burn Care Res. 40(6), 857–863. https://doi.org/10.1093/jbcr/irz103 (2019).
Yadav, D. P., Sharma, A., Singh, M. & Goyal, A. Feature extraction based machine learning for human burn diagnosis from burn images. IEEE J. Transl. Eng. Health Med. 7(July), 1–7. https://doi.org/10.1109/JTEHM.2019.2923628 (2019).
Abubakar, A., Ugail, H., Smith, K. M., Bukar, A. M. & Elhmahmudi, A. Burns depth assessment using deep learning features. J. Med. Biol. Eng. 40, 923–933 (2020).
Chauhan, J. & Goyal, P. BPBSAM: Body part-specific burn severity assessment model. Burns 46(6), 1407–1423. https://doi.org/10.1016/j.burns.2020.03.007 (2020).
Chauhan, J. & Goyal, P. Convolution neural network for effective burn region segmentation of color images. Burns 12, 12. https://doi.org/10.1016/j.burns.2020.08.016 (2020).
Pabitha, C. & Vanathi, B. Densemask RCNN: A hybrid model for skin burn image classification and severity grading. Neural Process. Lett. 53(1), 319–337. https://doi.org/10.1007/s11063-020-10387-5 (2021).
Owoyemi, A., Owoyemi, J., Osiyemi, A. & Boyd, A. Artificial intelligence for healthcare in Africa. Front. Digit. Health https://doi.org/10.3389/fdgth.2020.00006 (2020).
Fitzpatrick, T. B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 124, 869–871 (1988).
Bilwani, P. Unfavourable results in acute burn management. Indian J. Plast. Surg. 46(2), 428–433 (2013).
Deng, J., Dong, W., Socher, R., Li, L., Kai, L., Li, F.-F., editors. ImageNet: A large-scale hierarchical image database. In 2009 IEEE Conference on Computer Vision and Pattern Recognition (2009).
Huang, G. B., Ramesh, M., Berg, T. & Learned-Miller, E. Labeled Faces in the Wild: A Database for Studying Face Recognition in Unconstrained Environments. Technical Report 07-49. (University of Massachusetts, 2007).
National Aeronautics and Space Administration. Anthropometry and Biomecanics. 1995 [cited 29.01.2019]. In: Document NASA-STD-3000: Man-Systems Integration Standards [Internet]. [cited 29.01.2019]. Available from: https://msis.jsc.nasa.gov/sections/section03.htm
Fryar, C. D., Gu, Q., Ogden, C. L. & Flegal, K. M. Anthropometric reference data for children and adults: United States, 2011–2014. Vital Health Stat. 39, 1–46 (2016).
Dai, F., Zhang, D., Su, K. & Xin, N. Burn images segmentation based on Burn-GAN. J. Burn Care Res. 18, 18. https://doi.org/10.1093/jbcr/iraa208 (2020).
Khan, F. A. et al. Burnt human skin segmentation and depth classification using deep convolutional neural network (DCNN). J. Med. Imaging Health Inform. 10(10), 2421–2429. https://doi.org/10.1166/jmihi.2020.3258 (2020).
Ehteshami Bejnordi, B. et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318(22), 2199–2210. https://doi.org/10.1001/jama.2017.14585 (2017).
Hop, M. J. et al. Photographic assessment of burn size and depth: Reliability and validity. J. Wound Care 23(3), 144–152. https://doi.org/10.12968/jowc.2014.23.3.144 (2014).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542(7639), 115–118. https://doi.org/10.1038/nature21056 (2017).
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA J. Am. Med. Assoc. 316(22), 2402–2410. https://doi.org/10.1001/jama.2016.17216 (2016).
Wallis, L. et al. A roadmap for the implementation of mHealth innovations for image-based diagnostic support in clinical and public-health settings: A focus on front-line health workers and health-system organizations. Glob. Health Action 10(sup3), 1340254. https://doi.org/10.1080/16549716.2017.1340254 (2017).
Laflamme, L. et al. Targeting ethical considerations tied to image-based mobile health diagnostic support specific to clinicians in low-resource settings: The Brocher proposition. Glob. Health Action 12(1), 1666695. https://doi.org/10.1080/16549716.2019.1666695 (2019).
Acknowledgements
The authors would like to thank Miss Shira Kimberley and Mr Melvin Amandusson for their assistance in data collection, and Mr Hakan Kücükel for his technical assistance during the whole process.
Funding
Open access funding provided by Karolinska Institute. Funding was provided by Vetenskapsrådet (Grant No. 2016-01819).
Author information
Authors and Affiliations
Contributions
Study design and research questions were determined by C.B., L.L., J.F., F.H., L.W., N.A. and J.L. Data collection and image annotations were performed by C.B. and J.F. with the assistance of L.L., L.W., F.H. and N.A. Algorithm training and data analyses were performed by C.B. with the help of J.F., M.L., and J.L. C.B. was in charge of drafting the manuscript together with L.L. All authors contributed to the interpretation of the results and approved to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Mikael Lundin and Johan Lundin are founders and co-owners of Aiforia Technologies Oy, Helsinki, Finland. Other authors have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors. The names of the authors Jian Fransén, Fredrik Huss, Lee Wallis, Nikki Allorto and Johan Lundin were incorrectly given as Fransén Jian, Huss Fredrik, Wallis Lee, Allorto Nikki and Lundin Johan.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boissin, C., Laflamme, L., Fransén, J. et al. Development and evaluation of deep learning algorithms for assessment of acute burns and the need for surgery. Sci Rep 13, 1794 (2023). https://doi.org/10.1038/s41598-023-28164-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28164-4
- Springer Nature Limited
This article is cited by
-
Dense Mesh RCNN: assessment of human skin burn and burn depth severity
The Journal of Supercomputing (2024)
-
Spatial attention-based residual network for human burn identification and classification
Scientific Reports (2023)