Abstract
A novel sustainable, simple, sensitive, and green spectrofluorimetric method was developed for the concurrent estimation of venlafaxine and agomelatine in pharmaceuticals and biological fluids. The method relies on synchronous fluorescence spectroscopy, where venlafaxine and agomelatine were measured at 276 and 328 nm, respectively, using Δλ of 20 nm. The potential factors affecting the fluorescence intensity were optimized by the one-factor-at-a-time (OFAT) strategy, where synchronous fluorescence intensity was significantly enhanced using a 1% w/v sodium dodecyl sulfate micellar system. The method was fully validated and exhibited excellent linearity (r2 > 0.999 for both drugs) with very low limits of detection (LODs) in the range of 0.14–0.84 ng/mL. Consequently, the proposed approach was efficiently adopted to analyze the co-administered drugs in their pharmaceuticals and in spiked human plasma with excellent % recovery between 97.4 and 102.2%. Finally, the method's greenness was evaluated using different metric tools, including Green Analytical Procedure Index (GAPI) and Analytical GREEnness (AGREE), which proved its excellent greenness.
Similar content being viewed by others
Introduction
Depression (a common mental disorder) is one of the most prevalent main psychiatric disorders. Major depressive disorders are more common in those who have suffered a stroke (10–27%), myocardial infarction (40–65%), and cancer (20–25%). Antidepressant drugs have been used to treat all types of severe depressive disorders1. Over the past few years, antidepressant prescriptions have significantly increased in Egypt. The selective serotonin-norepinephrine reuptake inhibitors (SNRIs) and the serotonin-2C (5-HT2C) antagonists are two significant classes of antidepressants that are frequently prescribed in psychiatry2. They have clinical efficacy comparable to that of traditional tricyclic antidepressants but lack some of the negative cardiovascular and anticholinergic side effects frequently associated with these medications3.
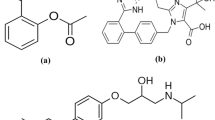
Venlafaxine (VFX), (1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol), is a second-generation SNRIs antidepressant drug (Fig. 1a)4. In comparison to the antidepressant fluoxetine, VFX has shown a rapid onset of action and improved response5. However, the most prevalent side effects of VFX use include depression, serotonin poisoning, seizures, or problems with heart conduction6,7. Agomelatine (AGM), also known as N-(2-(7-methoxy-1-naphthyl) ethyl) acetamide, is a new antidepressant medication8 that acts as both a 5HT-2C antagonist and an agonist on MT1/MT2 melatonergic receptors9 (Fig. 1b). Comparable efficacy to SSRIs like paroxetine and SNRIs like VFX has been demonstrated in clinical studies10. The drug revealed numerous merits over conventional antidepressants, including fewer sexual adverse effects than VFX, beneficial effects on sleep disruptions in depression, and independence from side effects like weight gain and serotonin syndrome11.
Thus, there seems to be a clinical justification for combining these antidepressants to manage the various symptoms of depressive syndrome. AGM is a favored medication in combination therapy with SSRIs or SNRIs due to its high tolerance and safety profile in comparison to other antidepressants12. Furthermore, although VFX and AGM have different mechanisms of action, they seem to work best together when combined13. Additionally, a proportion of patients who respond insufficiently to VFX or AGM alone may benefit from their use in combination due to their relative safety and lower risk of drug interactions14,15. To sum up, the combination of AGM and VFX for major depression was found to have a good therapeutic rationale, better tolerability, and fewer side effects14.
A trial-and-error dose titration strategy is frequently used in clinical practice to help determine an individual’s optimal dose for antidepressant medications. However, therapeutic drug monitoring16,17,18 is a broadly utilized method for determining an individual patient’s ideal dosage, which always improves efficacy and safety when utilized with antidepressant medications.
To date, few reports were applied to analyze AGM in its pharmaceuticals and in biological fluids. These reports included spectrofluorimetry19,20, HPLC–UV21, and LC–MS22. Moreover, various analytical methods were developed for the estimation of VFX in different matrices23,24,25. However, up to our knowledge, no reports were found for the concurrent determination of the co-administered drugs, AGM and VFX, either in their pharmaceuticals or biological fluids. This calls for the development of an appropriate approach for their simultaneous determination within their therapeutic drug levels.
The spectrofluorimetric approach is well-known for its outstanding sensitivity and selectivity26,27, although selectivity issues can emerge when determining multi-component samples due to the broadband spectrum overlaps. Synchronous fluorescence spectroscopy (SFS) plays a crucial role when analyzing mixtures with overlapped spectra28,29. SFS has been shown to be extremely efficient at characterizing and quantifying a huge variety of compounds30. The improved spectral resolution, better selectivity, reduced light scattering, and simplicity of SFS spectra make it preferable to the traditional fluorescence spectroscopy31. SFS has been applied for the successful quantitative estimation of various compounds in just one run, thanks to its narrow and sharp spectra32,33,34.
Consequently, our aim was to introduce a smart, sensitive, and environmentally friendly method for the simultaneous quantitation of VFX and AGM in their pharmaceuticals and biological fluids. The need to apply a sustainable method that was scientifically justified and eliminated the consumption of hazardous substances and solvents while still having a lower environmental impact was also taken into consideration. With the least degree of environmental harm, we were able to determine VFX and AGM using the well-established SFS method. A considerable micellar enhancement of the fluorescence intensities of the studied drugs using sodium dodecyl sulfate (SDS) as a micellar system was also achieved. The method could be applied for the concurrent estimation of the above-mentioned drugs in both pharmaceuticals and biological fluids at parts per billion levels. Furthermore, the strategy is simple, hazardous solvent-free, and affordable since it employs a technique that is available in the majority of research laboratories.
Experimental
Chemicals and reagents
VFX (98.0%) was purchased from Wako Pure Chemicals Ltd. (Osaka, Japan), while AGM (98.0%) was purchased from Tokyo Chemical Industries Co. (Tokyo, Japan). Britton-Robinson buffer, Brij-35, cetrimide, β-cyclodextrin, sodium dodecyl sulfate, tween-80, ethanol, methanol, and acetonitrile were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Efexor XR® capsules (75 mg VFX/capsule, Batch #FJ4605, Pfizer Ireland Pharmaceuticals, Newbridge Co. Kildare, Republic of Ireland), and Agovald® tablets (25 mg AGM/tablet, Batch #M1120121-8–24, Mash Premiere Co., New York, USA), were purchased from an Egyptian local Pharmacy. For human plasma samples, they were provided by Mansoura University Hospitals (Mansoura, Egypt) and kept at − 20 °C until use before being gently thawed. Britton-Robinson buffer (0.02 M) was prepared in double distilled water to cover pH ranges from 2 to 12. Additionally, organized media solutions at a 1.0% v/v or w/v concentration were prepared.
Instrumentation
The measurements of synchronous fluorescence spectra were carried out using an Agilent Cary Eclipse Fluorescence Spectrometer (Agilent Technologies, Santa Clara, CA 95,051, United States). The apparatus was run at a high voltage setting of 800 V with a slit width of 5 nm. At Δλ = 20 nm, spectra were recorded using a scan range of 200–400 nm. Other equipments utilized in the study included an ultrasonic bath (SS 101H 230, USA), a centrifuge (2-16P, Germany), a vortex mixer (IVM-300p, Gemmy Industrial Corp., Taiwan), and a pH-meter (NV P-901, Belgium). Membrane filters (0.45 mm) were obtained from Phenomenex, USA.
Preparation of standard solutions
Stock solutions of AGM and VFX were prepared separately, at a concentration of 100.0 μg/mL, by dissolving 10.0 mg of each medication in 100.0 mL of double-distilled water as a final volume. After that, working solutions were obtained by further diluting the stock solutions with water. The resulting solutions could be stable for at least a week when kept at 4 °C.
Construction of calibration graphs
Aliquots of stock solutions were transferred into a set of 5-mL volumetric flasks, with concentration ranges of 20.0–1000.0 and 5.0–200.0 ng/mL for VFX and AGM, respectively. One milliliter of the 1% SDS solution was then added to the solutions, which were then properly diluted with double-distilled water and carefully mixed. Both medications were scanned at Δλ = 20 nm with 5 nm windows over the 200–400 nm range. The synchronous fluorescence intensities (SFIs) were measured at 276 nm and 328 nm for VFX and AGM, respectively. Similarly, blank samples were created. The corrected SFI against the drug's final concentration (ng/mL) was plotted to get the calibration graphs and develop the regression equations.
Analysis of AGM/VFX synthetic mixture
Aliquots of each drug's stock solution were transferred to 5 mL flasks to create five synthetic mixtures with different ratios and within the linear range of VFX and AGM. After adding one milliliter of 1% SDS, the volume was adjusted using double-distilled water. The measurements were performed as described in section “Construction of calibration graphs”. The concentrations were determined using the respective regression models.
Analysis of AGM/VFX in commercial dosage forms
The equivalent of ten Agovald® tablets or Efexor XR® capsules’ content were individually weighed and finely ground. Separately, an exact amount of the powder equal to 10.0 mg of AGM or VFX was put into small flasks, and then 50 mL of double distilled water was added. The solutions were sonicated for 20 min, then filtered into clean dry 100-mL volumetric flasks and filled to the desired volume with the same solvent. The above-mentioned procedure was carried out after transferring progressively increasing amounts of the filtrate into 5-mL volumetric flasks. The corresponding regression equations were used to determine the nominal contents of tablets or capsules.
Construction of calibration curves for the studied drugs in spiked human plasma
Separately, 1.0 mL of human plasma was put into a set of 15 mL of centrifuge tubes. Plasma samples were spiked with aliquots from the VFX and AGM solutions, separately, to achieve final concentrations in the range of 50.0–200.0 and 5.0–15.0 ng/mL, respectively. The solutions were then vortexed for 2 min, followed by protein precipitation by completing up to 5 mL with methanol. After that, the tubes were centrifuged at 6000 rpm for 15 min to allow the medicines to phase separate from the plasma’s contents. One milliliter aliquots of the filtered supernatant were then transferred to 5 mL volumetric flasks. Subsequently, 1 mL of 1% SDS was added, followed by distilled water to complete it to the mark.
Quality control samples should be obtained from different stock solutions by spiking human plasma with a known quantity within the range of drug linearity. As previously mentioned, the corrected SFIs were measured in parallel with a blank sample. The corrected SFIs were then plotted against the concentrations of each drug to generate regression equations.
All experiments were performed in accordance with the Institutional Ethics Approval of the relevant University Committee (Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt).
Analysis of AGM/VFX in spiked human plasma
The previously described procedure was repeated in five replicates on a part of the produced supernatant. The corresponding regression equation was used to determine the nominal concentration of the tested drug mixtures in the spiked plasma sample. Simultaneously, a blank experiment was performed on the plasma sample without the addition of the drug solution.
Ethics declarations
All experiments were performed in accordance with relevant guidelines and regulations and this work was approved by the Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt (protocol code KFS-Ph-001-22).
Informed consent
A waiver for the informed consent for the current study was obtained from the Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt.
Results and discussion
Spectral characteristics
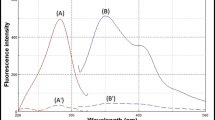
VFX and AGM aqueous solutions exhibit native fluorescence at λem of 301 and 357 nm following excitation at 272 and 226 nm, respectively. Consequently, the overlap between their emission fluorescence spectra precludes their simultaneous estimation (Fig. 2). The synchronous fluorescence spectroscopy (SFS) was so far the suitable option for analyzing the two drugs simultaneously in just one scan. SFS offers additional merits including enhanced selectivity, spectral overlap reduction, simplification, and its sharp and narrow spectrum with a considerable tolerance for exogenous contaminants, especially when analyzing biological matrices and dose forms. Accordingly, the SFS method was optimized to reduce the spectral overlap and increase the sensitivity.
Optimization of the experimental conditions
By using the one-factor-at-a-time (OFAT) strategy (changing one single factor at a time while keeping the others constant), several experimental factors that influence the SFI of the concerned drugs (including Δλ change, diluting solvents, pH, and organized media) were evaluated and optimized.
Effect of Δλ
Changing Δλ has an obvious impact on peak resolution and sensitivity. The signal strength, spectral structure, and width of the synchronous band are all directly affected. On a large Δλ scale (10–140 nm), the SFS of VFX and AGM were explored. The SF spectra of the two drugs were acquired in a single run with sufficient sensitivity using Δλ of 20 nm, which was shown to be appropriate and give reliable results for both drugs. The well-resolved spectra generated at 276 nm for VFX and 328 nm for AGM allowed for the separation of the two drugs without interference when they were simultaneously analyzed. Therefore, the optimal wavelength for both drugs was found to be Δλ = 20 nm (Fig. 3). Lower Δλ than this value revealed reduced SFI for both VFX and AGM, while values above 20 nm resulted in overlapping spectra. Consequently, Δλ of 20 nm was further used in the investigation.
Effect of diluting solvents
Various diluting solvents such as double distilled water, acetonitrile, methanol, and ethanol were investigated. The greatest SFI for both VFX and AGM with high resolution was obtained by water (Fig. 4a), which gives the proposed method an additional benefit in terms of sustainability. This impact rises with increasing solvent polarity. In contrast to nonpolar molecules, only polar fluorophores often show a stronger affinity to solvent polarity27. The dielectric constant (Ɛ) is a measure of a solvent's polarity, the greater the dielectric constant, the more polar the solvent35. Moreover, AGM and VFX are polar enough to exhibit high SFI in double distilled water. On the other hand, acetonitrile, methanol, and ethanol produced high blank readings and reduced the SFIs of the two drugs (Fig. 4a). Therefore, water was used as the diluting solvent of choice.
Effect of pH
The SFI of both AGM and VFX aqueous solutions over a wide pH range (2–12) was investigated using 0.02 M Britton-Robinson buffer. No significant effect was observed upon changing the pH, either acidic or alkaline. Moreover, the SFI of VFX gradually decreased upon increasing the pH above 7 (Fig. 4b). Therefore, no buffer was used in the consequences.
Effect of organized media
The ability of various organized media to improve the SFI of AGM and VFX was evaluated at a concentration higher than their critical micelle concentrations (CMC)34. They included SDS, cetrimide, tween-80, Brij-35, and β-cyclodextrin. SDS was found to significantly enhance the SFIs of VFX and AGM by about 53% and 16% of their native fluorescence, respectively (Fig. S1, Supplementary Material). The enhancement in this micelle is obviously caused by the inhibition of a nonradiative process, which can be attributed to one or more of the following: sequestering from water, resulting in a considerable decrease in the polarity of the fluorophore’s microenvironment as well as an increase in the micro-viscosity. This explains why the addition of 1% w/v SDS significantly improved the SFI of AGM and VFX. On the other hand, cetrimide was found to significantly decrease the FI of both drugs (Fig. 4c). As for Brij-35 and β-cyclodextrin, no enhancement was observed, and interfering peaks were detected at 285 and 278 nm, respectively. For tween-80, even an enhancement of the SFI of AGM was observed; a high blank reading was measured with interfering peaks at 321 and 282 nm. As a result, SDS was used in the consequences for the selective enhancement of the SFI of both drugs. Consequently, different volumes of SDS were investigated over the range of 0.5–3.0 mL. One milliliter of 1% SDS was found the optimal for the highest SFI of both drugs (Fig. 4d).
Method validation
The proposed method was validated according to the ICH guidelines36. Validation studies included linearity and range, precision, accuracy, limits of detection (LOD) and quantitation (LOQ), and selectivity.
Firstly, the method exhibited excellent linearity (r2 > 0.999, for both drugs) over the ranges of 20.0–1000.0 and 5.0–200.0 ng/mL for VFX and AGM, respectively (Fig. S2, supplementary material). Moreover, both LOD and LOQ were calculated from 3.3 × (Sa/b), and 10 × (Sa/b), respectively, where Sa is the standard deviation of the intercept and b is the slope of the calibration curves. The obtained low LODs and LOQs (Table 1) confirm the high sensitivity of the proposed method (at parts per billion levels). Therefore, the method was efficiently applied for the simultaneous analysis of the two co-administered drugs in human plasma.
The method’s precision and accuracy were also evaluated as intra- and inter-day precisions through the analysis of three different concentrations within the same day or on three consecutive days, respectively. Based on the obtained results, the % RSD was calculated to express the precision, while the mean %recovery of each nominal concentration (three replicates) demonstrated the method's accuracy. The low %RSD revealed the excellent precision of the proposed method (Table 2), and the great recovery demonstrated its high accuracy (Table 3). Additionally, the selectivity of our method was also evaluated through the simultaneous estimation of the studied drugs at the same time without any interference from each other (Fig. 5). The ability of the proposed method to analyze both drugs in spiked human plasma with low %RSD and excellent recovery and without any interference from the plasma components demonstrated the high selectivity of the proposed method.
Application to VFX and AGM synthetic mixtures
The proposed method was utilized to evaluate synthetic mixtures with varying VFX and AGM ratios (Fig. S3, supplementary material). The concentrations of both drugs in various ratios were estimated using the respective regression equations. The findings produced demonstrated the method’s accuracy, as shown in Table S1, supplementary material.
Application to pharmaceutical dosage forms
The optimized method was applied for the estimation of VFX and AGM in their single dosage forms. VFX was analyzed in its 75 mg capsules, while AGM was determined in its 25 mg tablets. The method exhibited high % recoveries (> 98%) with low %RSD (< 1.62) (Table 4), confirming its excellent applicability for the determination of VFX and AGM in their pharmaceuticals without interference from the co-formulated excipients.
Application to spiked human plasma
The optimized method was used to simultaneously analyze VFX and AGM in human plasma. AGM has a high bioavailability of more than 78% and is easily absorbed after oral administration10. Its maximum plasma concentration (Cmax) is reported to be 15.1 ng/mL37. Moreover, the therapeutic plasma level of VFX was reported in the range of 30–200 ng/mL38. Given that these concentrations fall within the established method's linear range, it is concluded that this method can concurrently determine both drugs in human plasma. By graphing the SFI versus the drug concentration in ng/mL, a linear relationship was generated in plasma samples spiked with VFX and AGM using the optimized method. The following equations were generated through a linear regression analysis of the data:
A linear range was obtained by spiking plasma samples with VFX and AGM at different concentrations within the ranges of 50.0 − 200.0 and 5.0 − 15.0 ng/mL, respectively. The obtained high % recoveries (> 97%) demonstrated the high efficiency of our method in such complex matrices (Table 5).
Assessment of method greenness by various metrics tools
The development of a variety of tools and related metrics to assess the environmental effects of various analytical procedures has preceded the rise in efforts to develop greener analytical techniques39. Although each published tool has its own computation and criteria, these tools and metrics might point chemists in the direction of more environmentally friendly methods40. Therefore, comparing both on an equal footing might help scientists decide whether to employ one over the other, depending on their goals and the level of precision they are looking for in these instruments39. In this context, we aim to evaluate the greenness of our proposed method using different metrics, including the Green Analytical Procedure Index (GAPI)41 and Analytical GREEnness (AGREE)42.
The GAPI was recently built for the evaluation of the whole analytical method. It is made up of five pentagrams that represent sample collection and preparation, chemicals and solvents, instruments, and technique type. The use of red color indicates that this step may be environmentally hazardous, yellow color indicates that this step has a moderate environmental effect, and green color indicates that this step is environmentally benign. From the step sample collection until the final determination, GAPI was utilized to demonstrate the proposed method’s green potential. The GAPI pictogram for the proposed procedure is presented in Fig. 6a. The only drawback is the pretreatment step in the case of plasma which uses a small amount of methanol for protein precipitation. Additionally, AGREE, a metric tool that emphasizes sample preparation, was used to assess the new method's greenness. It is based on 12 impact categories, which were then converted to subs-cores on a 0–1 scale and used to produce the assessment's final result. The factors for evaluation included sample size, throughput, waste output, energy consumption, and the choice and use of solvents, materials, and reagents. The ability to differentiate between the relevance of criteria by giving weights to them was also used in the evaluation. The findings of the AGREE analysis are presented in Fig. 6b. On a scale of 0 to 1, the AGREE analysis of the studied compounds received a general score of 0.78, confirming the excellent greenness and lower environmental impact of our procedure.
Conclusion
The first synchronous fluorescence spectroscopic method was developed for the simultaneous estimation of venlafaxine and agomelatine. The method relies on the use of water rather than any toxic or hazardous solvents. Additionally, the addition of an SDS micellar system greatly improved the method's sensitivity. The method was fully validated and exhibited low LODs in parts per billion levels. Consequently, the method could be employed for therapeutic drug monitoring in spiked human plasma. The greenness of the method was evaluated using various metric tools, confirming its excellent greenness and low environmental impact. The method has the pros of being simple, easy, green, precise, and highly sensitive. The proposed method's advantages have all demonstrated its effectiveness and suitability for use in pharmaceutical quality control laboratories.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Stafford, A. M. C. et al. The natural course of adolescent depression treatment in the primary care setting. J. Pediatr. Heal. Care 34, 38–46 (2020).
Hall, C. A. & Reynolds, C. F. Late-life depression in the primary care setting: Challenges, collaborative care and prevention. Maturitas 79, 147–152 (2014).
Alexopoulos, G. S. Mechanisms and treatment of late-life depression. Transl. Psychiatry 9, 188 (2019).
Thase, M. E., Entsuah, R., Cantillon, M. & Kornstein, S. G. Relative antidepressant efficacy of venlafaxine and SSRIs: Sex-age interactions. J. Women Heal. 14, 609–616 (2005).
Chen, T. R., Huang, H. C., Hsu, J. H., Ouyang, W. C. & Lin, K. C. Pharmacological and psychological interventions for generalized anxiety disorder in adults: A network meta-analysis. J. Psychiatr. Res. 118, 73–83 (2019).
Chabenat, A., Bidel, F., Knigge, T. & Bellanger, C. Alteration of predatory behaviour and growth in juvenile cuttlefish by fluoxetine and venlafaxine. Chemosphere 277, 130169 (2021).
Edvardsson, B. Venlafaxine as single therapy associated with hypertensive encephalopathy. Springerplus 4, 1–4 (2015).
Sweetman, Sean C. Martindale: The Complete Drug Reference. Statewide Agricultural Land Use Baseline (The Pharmaceutical Press, 2009). https://doi.org/10.1017/CBO9781107415324.004.
Howland, R. H. Agomelatine: A novel atypical antidepressant. J. Psychosoc. Nurs. Ment. Health Serv. 45, 13–17 (2007).
Girish, M. B., Bhuvana, K., Nagesh Raju, G. & Sarala, N. A novel atypical antidepressant drug: Agomelatine—A review. Int. J. Pharm. Biomed. Res. 1, 113–116 (2010).
Lapmanee, S., Charoenphandhu, J., Teerapornpuntakit, J., Krishnamra, N. & Charoenphandhu, N. Agomelatine, venlafaxine and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PLoS One 12, e0187671 (2017).
Potměšil, P. What combinations of agomelatine with other antidepressants could be successful during the treatment of major depressive disorder or anxiety disorders in clinical practice?. Ther. Adv. Psychopharmacol. 9, 2045125319855206 (2019).
Thomas, J., Khanam, R. & Vohora, D. Augmentation of antidepressant effects of venlafaxine by agomelatine in mice are independent of kynurenine pathway. Neurochem. Int. 99, 103–109 (2016).
Dahale, A. B., Narayanaswamy, J. C., Venkatasubramanian, G. & Bagewadi, V. I. Successful use of agomelatine and venlafaxine combination in major depression. Gen. Hosp. Psychiatry 36, e3 (2014).
Signorelli, M. S. et al. Venlafaxine augmentation with agomelatine in a patient with obsessive-compulsive disorder and suicidal behaviors. SAGE Open Med. Case Rep. 2, 2050313X14561778 (2014).
Ibrahim, F. et al. Simultaneous determination of four vasoactive phytochemicals in different pharmaceutical preparations by a simple HPLC-DAD method. Anal. Methods 8, 1858–1866 (2016).
El-Deen, A. K., Elmansi, H. & Shimizu, K. Natural hydrophobic deep eutectic solvent for vortex-assisted dispersive liquid-liquid microextraction of anti-prostate cancer triple therapy from water and human plasma. Microchem. J. 184, 108124 (2022).
El-Deen, A. K., Elmansi, H. & Shimizu, K. Utilization of hydrophilic and hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of bicalutamide from water and spiked human plasma. Sustain. Chem. Pharm. 29, 100825 (2022).
Ibrahim, F., El-Deen, A. K. & Shimizu, K. Application of quinone-based fluorophore and native fluorescence for the spectrofluorimetric determination of agomelatine in dosage form: Identification of acidic and alkaline- induced degradation products by LC–MS/TOF. Luminescence 33, 225–231 (2018).
Elkhoudary, M. M., Naguib, I. A., Abdel Salam, R. A. & Hadad, G. M. Comparison between two linear supervised learning machines’ methods with principle component based methods for the spectrofluorimetric determination of agomelatine and its degradants. J. Fluoresc. 27, 1149–1160 (2017).
Liu, Y., Chen, L. & Ji, Y. Quantification and structural elucidation of potential impurities in agomelatine active pharmaceutical ingredient. J. Pharm. Biomed. Anal. 81–82, 193–201 (2013).
Trawiński, J. & Skibiński, R. Photostability study of agomelatine using UHPLC-DAD-MS/MS method. Kinetics, identification of the transformation products and in silico evaluation of toxicity. J. Liq. Chromatogr. Relat. Technol. 42, 63–73 (2019).
Qin, X. Y. et al. Determination of venlafaxine in human plasma by high-performance liquid chromatography using cloud-point extraction and spectrofluorimetric detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 872, 38–42 (2008).
Shahnawaz, S., Siddiqui, Z. & Hoda, Q. Sensitive spectrofluorimetric method of analysis for venlafaxine in spiked rat plasma and formulations. J. Fluoresc. 20, 821–825 (2010).
Gu, G. et al. Validation of an LC-MS/MS method for simultaneous quantification of venlafaxine and its five metabolites in rat plasma and its application in a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1087, 29–35 (2018).
Sharaf El-Din, M. K., Ibrahim, F., El-Deen, A. K. & Shimizu, K. Stability-indicating spectrofluorimetric method with enhanced sensitivity for determination of vancomycin hydrochloride in pharmaceuticals and spiked human plasma: Application to degradation kinetics. J. Food Drug Anal. 26, 834–841 (2018).
Lakowicz, J. R. 06. Solvent and environmental effects. In Principles of Fluorescence Spectroscopy (ed. Lakowicz, J. R.) (Springer, 2006).
Andrade-Eiroa, Á., de-Armas, G., Estela, J. M. & Cerdà, V. Critical approach to synchronous spectrofluorimetry. II. TrAC—Trends Anal. Chem. 29, 902–927 (2010).
Elmansi, H., Belal, F. & Magdy, G. Determination of pholcodine alone or in combination with ephedrine in human plasma using fluorescence spectroscopy. Sci. Rep. 12, 1–11 (2022).
Jouyban, A. & Rahimpour, E. Using constant-wavelength synchronous fluorescence spectroscopy in nanoparticle-based sensors: A minireview. Anal. Methods 13, 968–973 (2021).
Patra, D. & Mishra, A. K. Recent developments in multi-component synchronous fluorescence scan analysis. TrAC—Trends Anal. Chem. 21, 787–798 (2002).
Li, Y.-Q. et al. Synchronous Fluorescence Spectroscopy and Its Applications in Clinical Analysis and Food Safety Evaluation 95–117 (Reviews in fluorescence. Springer, 2010).
Magdy, G., Belal, F., Abdel-Megied, A. M. & Abdel Hakiem, A. F. Two different synchronous spectrofluorimetric approaches for simultaneous determination of febuxostat and ibuprofen. R. Soc. Open Sci. 8, 210354 (2021).
Magdy, G., Belal, F. F., Abdel-Megied, A. M. & Abdel Hakiem, A. F. Micelle-enhanced conventional and synchronous spectrofluorimetric methods for the simultaneous determination of lesinurad and febuxostat: Application to human plasma. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 248, 119239 (2021).
Maryott, A. A. & Smith, E. R. Table of Dielectric Constants of Pure Liquids. Materials and Structures. (US Government Printing Office, 1951).
ICH Harmonised Tripartite Guideline. Validation of analytical procedures: Text and methodology, Q2(R1); Geneva. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (Accessed 23 September 2015) (2005).
Wang, X. et al. LC-MS/MS method for the determination of agomelatine in human plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 28, 218–222 (2014).
Nichols, A. I., Focht, K., Jiang, Q., Preskorn, S. H. & Kane, C. P. Pharmacokinetics of venlafaxine extended release 75mg and desvenlafaxine 50mg in healthy CYP2D6 extensive and poor metabolizers: A randomized, open-label, two-period, parallel-group, crossover study. Clin. Drug Investig. 31, 155–167 (2011).
Sajid, M. & Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 238, 123046 (2022).
Gałuszka, A., Migaszewski, Z. & Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC—Trends Anal. Chem. 50, 78–84 (2013).
Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 181, 204–209 (2018).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 92, 10076–10082 (2020).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
G.M.: Conceptualization, Methodology, Formal analysis, Validation, Writing-review & editing. F.B.: Conceptualization, Resources, Writing-review & editing, Investigation, Supervision. A.K.E: Conceptualization, Data curation, Visualization, Writing-original draft. All authors approved the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magdy, G., Belal, F. & El-Deen, A.K. Green synchronous spectrofluorimetric method for the simultaneous determination of agomelatine and venlafaxine in human plasma at part per billion levels. Sci Rep 12, 22559 (2022). https://doi.org/10.1038/s41598-022-26827-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26827-2
- Springer Nature Limited
This article is cited by
-
Ecofriendly micellar mediated spectrofluorimetric method for ultrasensitive quantification of the antiparkinsonian drug safinamide in pharmaceutical formulation and spiked human plasma
Scientific Reports (2024)
-
Development of Eco-Friendly Scattering and Fluorimetric Methods for the Determination of Clemastine Through Its Interaction with Eosin Y: Assessment of Whiteness, Blueness, and Greenness Tools
Journal of Fluorescence (2024)
-
Multiple green spectroscopic methods for erdosteine determination in bulk and dosage form with extensive greenness evaluation
Scientific Reports (2023)