Abstract
Aerobic granular sludge (AGS) is a proven resource for the recovery of biopolymers like alginate-like polymers (ALP). This is the first report on the dynamics of ALP produced by AGS (ALP-AGS) in a full-scale wastewater treatment plant (WWTP), optimization of ALP recovery from AGS, and adsorption of cadmium (Cd2+) by ALP. Recovery of ALP was highest when using 120 mL of 0.2 M Na2CO3 at 70 °C for 45 min. Seasonal (1.5 years, over 3100 cycles) and intra-cycle changes in ALP-AGS in the WWTP were monitored. The ALP content in AGS increased in the transition period between winter and spring, reaching over 150 mg/g MLSS. In the batch reactor cycle, the ALP-AGS level peaked 2 h after the start of aeration (mean peak level: 120 mg/g MLSS), then decreased about two-fold by the end of the cycle. The ALP-AGS had a small surface area and a lamellar structure with crystalline outgrowths. The optimal conditions of Cd2+ adsorption with ALP were a dosage of 7.9 g d.m./L, a pH of 4–8, and an equilibrium time of 60 min. Carboxyl and hydroxyl groups were the key functional groups involved in Cd2+ adsorption. According to the Sips model, the maximum Cd2+ adsorption capacity of ALP-AGS was 29.5 mg/g d.m., which is similar to that of commercial alginate. AGS is a richer source of ALP than activated sludge, which ensures the cost-effectiveness of ALP recovery and increases the sustainability of wastewater treatment. Information on the chemical properties and yields of ALP from full-scale WWTPs is important for downstream applications with the recovered ALP.
Similar content being viewed by others
Introduction
Aerobic granular sludge (AGS) technology has been widely applied in full-scale wastewater treatment plants (WWTPs). Field reports indicate that AGS enables more stable and efficient wastewater treatment than conventional activated sludge due to higher biomass concentrations in the reactors, better resistance to unfavorable environmental conditions, and more effective sludge separation from the treated wastewater1. During granulation, operational parameters are selected to favor the production of extracellular polymeric substances (EPS), which support bacterial aggregation and form a matrix that encapsulates and protects the bacteria2. EPS have a critical functional role in the formation and stabilization of sludge granules, influencing the granules’ structure, settling performance, surface charge, flocculation, and dewatering properties3. EPS consist of a very complex mixture of biomolecules (proteins, humic-like substances, polysaccharides, uronic acid, nucleic acid, lipids, glycoproteins), which can be excreted by microorganisms, produced from cell lysis and hydrolysis, and adsorbed from wastewater4,5,6. Extracting EPS from excess AGS reduces the amount of sludge discarded by 20–35%7 and thus significantly decreases the amount of sludge that requires management. After EPS extraction, waste sludge has better biodegradability, making anaerobic digestion of the sludge more efficient8.
The main fraction of EPS is alginate-like exopolymers (ALE), which are formed not only by polysaccharides, but also by other polymers such as proteins, humic substances, and lipids9. During ALE extraction, some intracellular polysaccharides and proteins can be released by cell lysis10; this combination of ALE and intracellular polymers is referred to as alginate-like polymers (ALP)9.
Although there are reports on the effect of environmental conditions on ALP production in pure bacterial cultures, studies of ALP production in wastewater treatment systems are scarce. The extraction and recovery of ALP from AGS have attracted great interest. Currently, most extractions and purifications of ALP are performed with AGS, while fewer studies have investigated the recovery of ALP from activated sludge11. This is because the content of ALP is higher in AGS than in activated sludge12. Extractable ALP can account for up to 25% of the mass of AGS13. The production of ALP in an AGS system depends on many factors; thus, to ensure highly effective ALE recovery, the operating parameters must be optimized. In lab-scale reactors fed with wastewater containing propionate and acetate, the biomass contained significantly more ALP (up to 261 ± 33 mg volatile solids (VS)/g VS sludge) than the biomass in a reactor fed with volatile-fatty-acid–free wastewater. dos Santos et al., reported that the highest content of biopolymers (418.7 mg ALP/g VSS) was found in AGS in acetate-fed reactors. The production of ALP was also significantly higher when propionate was used as the substrate than when glycerol, glucose, or sucrose were used14. The composition of ALP was similar in activated sludge flocs and aerobic granules9. ALP production was highest when a lab-scale AGS reactor was operated with a short anoxic phase in the anaerobic/oxic/anoxic cycle and a short solid retention time of about 10 days15. Increasing the COD:N ratio increased the amount of ALP in the biomass, but when the ratio reached 30:1, ALP production decreased11. de Carvalho et al.16 indicate that the conditions for ALP production are most favorable in reactors with mature AGS that are operated with a variable organic load, a long sludge retention time (up to 20 days), a COD/N ratio of 20, and moderate salinity of the influent wastewater. Many studies on the production and utilization of ALP still focus on the use of synthetic influents, which do not truly represent the applicability of the system on a real scale12. In a study conducted in a pilot-scale WWTP with AGS treating municipal sewage containing approximately 25% slaughterhouse wastewater, the yield of extractable ALE reached 160 ± 4 mg/g VSS. The ALE were characterized by a high percentage of poly-guluronic acid blocks and formed rigid, non-deformable gels in CaCl217.
Due to the complex structure of ALP, it is practically impossible to extract all of their components with a single method. Various physical methods (e.g., centrifugation, sonication, heating) and chemical methods (extraction with formamide, NaOH, EDTA, Na2CO3) have been used to extract and recover polymers from AGS13,18. The choice of extraction method affects not only the total amount of polymers obtained but also their composition. Currently, the most suitable technique for extracting ALP is alkaline extraction using sodium carbonate (Na2CO3) at high temperatures16, which is similar to the extraction of alginate from brown algae. When dealing with tightly bound extracellular polymers in AGS, ALP extraction and recovery can be improved by acidic, microwave, or ultrasonic pretreatment18.
Polymers recovered from waste AGS can be used to produce flame retardants, absorbing gels, ink thickeners, gluing agents for fertilizer pellets, corrosion inhibitors, coatings for improving the water resistance of paper, or fire-resistant boards19,20,21. The structural fraction of EPS (sEPS) can be extracted and recovered from AGS and used to form hydrogels via a cross-linking process employing Ca2+ solutions. These hydrogels have a water-holding capacity of up to 99 gH2O/g sEPS and can be used in the chemical, paper, textile, or agronomic industrial sectors22. ALP recovered from AGS provide sorption sites for various compounds23 like methylene blue24 and for phosphorus25 and heavy metals (Pb2+, Cu2+, Ni2+, and Zn2+)26,27. Cd2+ is a priority pollutant due to its extreme toxicity and adverse effects on human health, and it should be eliminated from the environment. A previous study confirmed that Cd2+ was very effectively removed from water by EPS extracted from excess activated sludge from a municipal WWTP after short-duration aerobic digestion28.
ALP are regarded as one of the most promising bioproducts that can be recovered from WWTPs29. So far, no investigations have been done regarding the effect of seasonal and intra-cycle changes on ALP content in AGS from full-scale WWTPs. Defining the best periods for recovery of biopolymers from AGS may ensure high polymer yields and favor the economy of the process in full-scale applications. Moreover, the sorption potential of ALP recovered from AGS from a full-scale system has also not been tested in the context of heavy metal removal. Therefore, the objectives of this study were to optimize the extraction of ALP from AGS, to monitor the content of ALP in AGS during granular batch reactor cycles and seasonal temperature variations in a large-scale wastewater treatment plant, and to characterize the ALP and their ability to adsorb Cd2+ from aqueous solutions. The content of ALP in AGS was monitored for 1.5 years in a full-scale WWTP treating municipal wastewater. This is the first time that a potential valorization strategy for waste AGS has been studied in a full-scale technological system. Knowledge about the yield and chemical characteristics of the obtained ALP is important because these features are essential for downstream applications.
Materials and methods
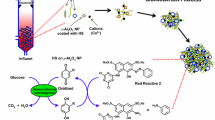
The experiments were performed in 3 steps: (1) optimization of ALP extraction from AGS, (2) evaluation of ALP-AGS dynamics in a real wastewater treatment system and (3) application of ALP-AGS for the removal of Cd2+ from aqueous solution. The flowchart of the experimental setup is shown in Fig. 1.
AGS sampling
Granular sludge was collected from WWTP in Lubawa (Poland). This plant operates at a low organic load and uses AGS technology to treat wastewater in amounts corresponding to 15,000 PE (population equivalent). The average wastewater flow is about 3200 m3/day; about 30–40% of the influent is wastewater from the dairy industry. The volumetric loading rate of the WWTP is 0.77 m3/(m3 day). The wastewater contained about 1395 ± 65 mg COD/L, 18.1 ± 1.3 mg TP/L, 90.7 ± 15.2 mg TN/L, and 55.8 ± 20.0 mg Norg/L. The technological line of the WWTP consisted of a stepped grid, grit chamber, flow equalization tank, two identically operated biological rectangular reactors with aerobic granules (i.e. GSBRs), a tank equipped with a stirrer, where phosphorus precipitation can be performed if necessary, and four secondary clarifiers, from which the effluent was discharged into a river. Waste (excess) sludge from GSBR was aerobically stabilized. The stabilized sludge and the waste sludge from the secondary clarifiers were collected in a sludge thickener and then dewatered using a mechanical belt thickener.
The cycle of a biological reactor begins with simultaneous, piston feeding of raw wastewater and withdrawal of effluent. The whole reactor cycle lasted for 4.8 h and consisted of 0.8 h of anoxic filling with simultaneous wastewater withdrawal, an aeration period of 3.4 h, and 0.6 h of settling and biomass discharge. The volumetric exchange rate was about 25%. The AGS was sampled from February 2018 to August 2019 from both GSBRs at the end of the aeration phase in the cycle (every 25 days on average). Intra-cycle measurements of ALP content in AGS were performed in two independently operated full-scale GSBRs during the aeration phase of cycle 916th. Before analysis, the sludge was stored at 4 °C for no longer than 24 h. In the investigated period, the average biomass concentrations were 6.8 g MLSS/L (GSBR#1) and 7.0 g MLSS/L (GSBR#2). The sludge volumetric index in both GSBRs was at a level of 50 mL/g MLSS.
ALP extraction from AGS
The content ALP in AGS was measured according to Lin et al.17, but the procedure was optimized to ensure the highest yield of ALP from AGS. Therefore, 2.5 g of MLSS was homogenized using Ultra Turrax T25 basic (IKA-WERKE) for 5 min at 9.500 1/min, and then incubated from 15 to 60 min at temperatures varying from 50 to 80 ºC in 0.2 M Na2CO3—a volume of solution from 80 to 400 mL. Biomass was then centrifuged at 12,000 rpm for 20 min. The reaction of the supernatant was adjusted to pH 2 using 0.1 M HCl. The supernatant was centrifuged at 12,000 rpm for 20 min and the ALP was dissolved in 0.1 M NaOH. ALP was precipitated using cold EtOH adjusting the solution to 80% (v/v), lyophilized, and weighed. The extracted ALP was used in further experiments.
Adsorbent preparation
Cd2+ adsorption experiments were performed with ALP-AGS which was in the form of beads. Sodium alginate (ALG) purchased from Sigma-Aldrich was used as a reference adsorbent. The beads were prepared by dropwise addition of a viscous 2.5% (w/w) ALG or ALP-AGS aqueous solution to 0.03 M CaCl2 solution with the help of an injection syringe. The gelation process was continued for 30 min. During the gelation process, the alginate reacts with the Ca2+ ions from the CaCl2 solution to form a cross-linked Ca-alginate. The obtained beads were left in the solution for 24 h, and then kept in distilled water at 4 °C in the refrigerator. The experiments on Cd2+ adsorption were performed with fresh beads.
Batch adsorption experiments
Commercial stock standard Cd2+ solution at a concentration of 1000 mg Cd2+/L (Sigma-Aldrich) was used. Working standards of required concentration were prepared by diluting the stock solution in distilled water.
Cd2+ removal from aqueous solution was conducted at room temperature, in a function of adsorbent dosage (0.7, 2.6, 5.3, 7.9, 10.6, 13.2, and 15.9 g d.m./L), contact time (5, 15, 30, 60, 90, 120, 150, 180 min.), pH (2, 3, 4, 5, 6, 8) and initial Cd2+ concentration (5, 10, 15, 30, 50, 75, 100, 150, 200 mg/L). The experiments were conducted in 125-mL Erlenmeyer flasks. The adsorbent dosage for pH and contact time experiments was set to 2.6 g d.m./L, while for the effect of initial Cd2+ concentration it was fixed to 7.9 g d.m./L. The pH of the Cd2+ working solution in the experiments, except for the effect of pH, was adjusted to 5.0 ± 0.2 using 0.1 M HNO3 or 0.1 M NaOH. The samples were agitated on a Gerhard shaker at room temperature water bath at 120 rpm for 2 h. The solutions thus obtained were filtered through a 0.45 µm filter and analyzed for Cd2+ concentration. Before metal analysis, the supernatants were acidified with HNO3. The residual adsorbent from the sorption experiment at selected conditions was dried to constant mass at 70 °C and characterized with advanced imaging and spectroscopic techniques.
Analytical methods
Total Cd2+ concentration was measured with a flame atomic absorption spectrometer (FAAS) (Varian, AA28OFS) at 228.8 nm. The accuracy of the Cd2+ analysis was validated with the reference material (CRM 142 R). The limit of Cd2+ detection (LOD) was 0.07 mg/L, while the limit of Cd2+ quantification (LOQ) was 0.21 mg/L. The functional groups on the surface of ALP-AGS and ALG, before and after Cd2+ sorption, were analyzed in the range of 3800–400/cm using an FTIR spectroscope (Nicolet 6700, Thermo Scientific) equipped with a Smart Multi-Bounce HATR™. The surface morphology of adsorbents was examined with an LEO 1430VP scanning electron microscope (SEM) (Carl Zeiss). Qualitative and quantitative analysis of elemental surface composition (SEM–EDX) was performed with an energy-dispersive X-ray spectrometer (EDX, Quantax 200; detector: XFlash 4010, Bruker AXS, Berlin, Germany). Elemental mapping was performed on different micro-areas on the surface of ALP-AGS and ALG and the content of each element (in mass %) was averaged.
The Brunauer-Emmet-Teller (BET) specific surface area of adsorbents was determined by fitting the BET equation to the linear portion of the BET plot; the pore size distribution was calculated on the basis of the desorption plot of the N2 adsorption–desorption isotherm using the Barret-Joyner-Halenda method (Micrometrics ASAP 2000, USA).
Calculations
The amount of Cd2+ adsorbed onto the adsorbent in the equilibrium (qe, mg/g d.m.) was calculated according to the following formula:
The efficiency (%) of Cd2+ adsorption was calculated according to the following formula:
where C0 is the initial Cd2+ concentration in the solution (mg/L), Ce is the equilibrium Cd2+ concentration (mg/L), m is the mass of adsorbent (g d.m.), and V is the volume of metal solution (L).
Equilibrium isotherm models
Three models were employed to interpret the adsorption isotherm data onto tested adsorbents:
where: Freundlich model: KF is the constant in the Freundlich equation (L/mg), 1/n is a measure of adsorption intensity (–); Langmuir model: qmax is the maximum monolayer adsorption capacity (mg/g d.m.), b is adsorption equilibrium constant (L/mg); Sips model: b is Sips isotherm constant related to the energy of adsorption (L/mg), n is a constant related to the grade of surface heterogeneity (–).
Kinetics model
The pseudo-second-order kinetic model was used to describe the kinetics of Cd2+ adsorption onto adsorbents:
where: qt is the amount of Cd2+ adsorbed at a specific adsorption time (mg/g d.m.); k is a rate constant (g d.m./(mg min)), and t is the adsorption time (min). The initial adsorption rate (r) was calculated as k‧qe2.
The fitting of the adsorption and kinetics isotherms to experimental data and the adsorption (KF, qmax, b, n) and kinetics parameters (qe, k) were calculated by the Levenberg–Marquardt optimization method included in the STATISTICA® software (version 13.3, TIBCO Software Inc.). The appropriateness of the adsorption and kinetic isotherm models was determined based on the sum of squared errors (SSE) and coefficient of determination (R2).
Results and discussion
Optimization of ALP recovery from AGS
The yield of ALP-AGS was highest with an extraction temperature of 70 °C (Fig. 2a), an extraction time of 45 min (Fig. 2b), and 120 mL of 0.2 M Na2CO3/2.5 g MLSS (131 mg/g MLSS; significant differences, ANOVA, Tukey’s HSD test, p = 0.05) (Fig. 2c). This time was shorter and the temperature of extraction was lower than in the original methodology presented by Lin et al.17, which creates some opportunities for decreasing the costs of ALP recovery from AGS in full-scale installations.
Seasonal and intra-cycle changes in ALP content in AGS
The concentration of ALP-AGS was investigated for over 3100 cycles of stable performance in two independently operated full-scale GSBRs at a municipal WWTP. In this period, no serious operational problems were reported, and wastewater composition did not vary significantly. Both GSBRs were operated at similar biomass concentrations, which caused the main operational parameters of the GSBRs, such as the organic loading rate, to be nearly identical. At the respective beginnings of spring 2018 and spring 2019, the ALP-AGS content reached nearly 100 mg/g MLSS and over 150 mg/g MLSS (Fig. 3a). These values were slightly higher than the ALP-AGS content reported in another study conducted in a full-scale WWTP30 and similar to values observed in lab-scale reactors, which are usually higher than in full-scale systems23. For example, in lab-scale reactors fed with primary effluent from municipal wastewater, AGS contained 184 ± 18 mg VS ALP/g VSS9.
In this study, the ALP-AGS concentration in sludge increased significantly in the transition periods between winter and spring. During these periods, the temperature dropped to 9 °C and was the lowest of the entire experimental period (Fig. S1, Supplementary Materials). Bacteria may produce EPS as a strategy for survival in cold environments, as the presence of EPS significantly reduces cell lysis31. In pure strains of Lactobacillus paracasei, low temperatures increased the content of the high molecular weight fraction of EPS and the total amount of EPS produced32. Our results indicate that low temperatures also affect the ALP content in AGS—the highest yields of this biopolymer from waste sludge at the WWTP can be recovered at the end of winter. The average concentrations of ALP in biomass were higher in 2019 than in 2018; this may be explained by gradual granule maturation, which favors biopolymer production33,34. In a study by Huang et al.35, ALP were only found in mature granules.
Variations in the amounts and characteristics of ALP (mostly the molecular weight, MW) during the operational cycle of industrial-scale batch reactors have been reported36. In the present study, the amount of ALP varied considerably during the cycle, from about 50 to over 120 mg ALP/g MLSS, and two peaks of ALP content in biomass were observed, 1 and 2 h from the beginning of aeration (Fig. 3b).
ALP production has been reported to follow almost the same trend as bacterial growth37. Similarly, in the present study, the peak activity of bacteria, as indicated by the 16S rRNA levels in cells (results not shown), overlapped with the peaks of ALP production. During the introduction of wastewater to the reactors, the dissolved oxygen concentrations in the bulk liquid dropped significantly to below 0.5 mg/L in the GSBRs. Such low oxygen concentrations in the environment stimulate anoxic and anaerobic metabolism in the middle and core granule layers38. Therefore, the first peak in ALP formation may have resulted from anaerobic bacteria, such as phosphate-accumulating and glycogen–accumulating microorganisms, intensively producing ALP after the start of aeration15. As alginate contains glucuronic acid, it can act as a barrier to oxygen diffusion, limiting its transfer to the enzyme complexes in bacterial cells and protecting the metabolic activity of strict anaerobes39.
In the present study, ALP production started to increase in the 75th minute of the GSBR cycle and peaked at 2 h, which can be explained by an increase in ALP biosynthesis after the depletion of easily biodegradable acetate present in the wastewater introduced to the GSBR (data not shown). This explanation is consistent with a report of reduced ALP production in bacterial cells after the addition of sodium acetate to pure cultures of Azotobacter vinelandi cultivated on glucose-based media40. A similar pattern of ALP production has been reported in lab-scale reactors operated with an anaerobic/oxic/anoxic cycle and fed with wastewater containing sodium acetate15. In that study, ALP content was highest after 90 min of aeration. In the present study, the reduction in ALP levels in AGS at the end of the GSBR cycle can be explained by ALP lyase activity, which degrades ALP in the post-polymerization step41. This reduction in ALP levels is consistent with reports of batch tests, in which ALP production and its molecular weight dropped over time42,43. Practically speaking, our results indicate that, in a full-scale operation aimed at ALP recovery, the sludge must be discharged about 2 h after the start of aeration.
Adsorbent characteristics
ALP-AGS and ALG had a low surface area (Table 1). The pore volume of ALP-AGS was about 24 times larger than that of ALG, but both adsorbents had similar pore diameters, the size of which indicated that they had microporous structures44. The surface area of ALG can vary considerably, depending on ALG conditions (e.g., the sodium alginate concentration, the type and concentration of gelation agent, etc.) or ALG modification. ALP-AGS that was produced with much more concentrated CaCl2 (12.5%) than that in the present study had a much larger surface area (76.2 m2/g) and a slightly larger pore volume (0.0623 cm3/g), but the pores had a smaller diameter (0.0177 nm)25. Aziz et al.45 have shown that the surface area of pristine ALG was low (4.1 m2/g), but that it increased 76 times (on average) after ALG modification with natural clay, natural phosphate, or activated charcoal. Because the aim of our preliminary study was to compare the usability of waste ALP with commercial ALG for metal removal, both adsorbents were used without any modification.
Structural morphology
SEM images of the surface of ALG and ALP-AGS (Fig. 4) showed that both adsorbents had a very dense appearance without much porosity, which can indicate strong cross-linking caused by Ca2+ ions during the reaction with CaCl246. Similarly, Torres-Caban et al.47 used SEM analysis to find that calcium alginate beads had a smooth surface without much porosity. At higher magnifications, the surface of the ALP-AGS was less regular and contained structures resembling crystalline outgrowths. An irregular and rough surface might indicate strong cross-links with Ca2+34 and can be also attributed to water evaporation and surface shrinking48.
Elemental composition
SEM–EDX analysis revealed 9 elements on the ALG surface, and the major constituents (expressed in mass %) were C (11.03 ± 0.08), O (67.76 ± 4.20), Ca (12.53 ± 0.04), Na (3.95 ± 0.11), and Cl (2.97 ± 0.15) (Fig. S2). On the ALP-AGS surface, 12 elements were identified, with lower contents of C (5.48 ± 2.28), O (58.74 ± 1.70), and Na (0.96 ± 0.21) than ALG, and higher contents of Ca (13.04 ± 2.88), Al (1.87 ± 0.19), S (1.29 ± 0.25), P (4.04 ± 0.30), and Cl (6.31 ± 0.24). In contrast to ALG, ALP-AGS also contained N (7.47 ± 0.23) and trace amounts of Fe (0.16%) and Cu (0.30%). For comparison, Isik et al.49 showed that calcium ALG beads were composed of C (9 mass %), O (19 mass %), Na (6 mass %), Cl (34 mass %), and Ca (32 mass %). The presence of C and O may correspond to functional groups distributed on the surface of the adsorbents50. The presence of Cl in alginate sorbents is often due to incomplete washing of the adsorbent with water at the end of gelation with CaCl2. The presence of S and Al in ALG beads can be due to algae components or impurities associated with alginate extraction47. Finally, the nitrogen and phosphorous in the ALP-AGS indicate that these nutrients were transferred from AGS during ALP isolation.
Adsorption of Cd2+ onto ALG and ALP-AGS
Cd2+ adsorption onto the tested adsorbents was optimized in terms of adsorbent dosage, pH, initial Cd2+ concentration, and sorption time.
Adsorbent dosage
At the lowest adsorbent dosage (0.7 g d.m./L), the process efficiency was higher for ALG (91%) than for ALP-AGS (75%) (Fig. 5a). This may be connected with the lower number of active sites on ALP-AGS. As the dosage of ALP-AGS was increased from 0.7 to 7.9 g d.m./L, the efficiency increased from 75 to 93%, which can be attributed to the presence of a greater number of adsorbent sites at a higher adsorbent dosage50. With adsorbent dosages in the range of 7.9–15.9 g d.m./L, the average Cd2+ adsorption efficiency, at an initial metal concentration of 10 mg/L, was 94.3 ± 0.2% for ALG and 94.4 ± 0.9% for ALP-AGS. Based on the residual concentration of Cd2+ in the solution after adsorption and the Cd2+ removal efficiency, an ALP-AGS dosage of 7.9 g d.m./L was selected as optimum.
pH
The efficiency of Cd2+ removal by ALG and ALP-AGS depended on the solution pH (Fig. 5b). The pH is known to be important for controlling metal adsorption, as it affects both the chemical properties of surface functional groups and the speciation of metal ions51. The best sorption effects with both sorbents were obtained with pH values in the range of 4–8; at these values, the average adsorption efficiency was 88.7 ± 0.9% for ALP-AGS and 97.0 ± 0.2% for ALG. Adsorption efficiency and adsorption capacity were lowest at pH 2–3. Kuczajowska-Zadrożna et al.52 found that Cd2+ adsorption onto ALG beads reached 91% at pH values ranging from 5.0 to 9.0, and that reducing the pH to 2.0 significantly decreased the adsorption efficiency to 23%. Mahmood et al.53 reported that pH 6.0 was optimum for Cd2+ adsorption onto ALG.
Changes in the pH can affect the charge of functional groups on the surface of the adsorbent, which, in turn, affects its capacity to adsorb metals. ALG sorbents consist mainly of guluronic and mannuronic acid units containing carboxyl and hydroxyl groups54,55. The pKa values of the carboxyl groups of the mannuronic and guluronic acid units in ALG are 3.38 and 3.65, respectively54,55. Although the individual constituents of the ALP-AGS were not analyzed in the present study, EPS that was recovered from AGS contained mainly proteins (≈ 87%), polysaccharides (≈10%), and humic acids (2.3%)56. Proteins are the main source of carboxyl, hydroxyl, and amine groups, while polysaccharides and humic acids are the sources of hydroxyl groups56. Although the content of humic acids in the EPS from AGS was lower than that of proteins, humic acids are high in carboxylic acids and phenols, implying that they might be used as chelating agents. Humic acids and proteins can form complexes with cationic metals that are beneficial for metal adsorption57. The negative surface charge of EPS at a pH range of 3 to 10 is related to the deprotonation of carboxyl (pKa ≈ 3.0), phosphoryl (pKa ≈ 6.5), amine (pKa ≈ 8.4), and hydroxyl (pKa ≈ 10.2) groups56. In the EPS from the granular sludge, carboxyl and hydroxyl groups were most abundant and were mainly responsible for Pb2+, Cd2+, and Zn2+ adsorption56.
In the present study, all FTIR spectra (Fig. 6) contained absorption bands that indicated the presence of hydroxyl, ether, and carboxylic functional groups. From 3600 to 3000/cm, stretching vibrations of O–H bonds appeared, which are typical of polysaccharides46, and also N–H stretching vibrations of amino groups at ~ 3283/cm in the ALG-AGS spectra, which could confirm the presence of proteins27,58,59. At 2952–2852/cm in ALP-AGS spectra, stretching vibrations of aliphatic C–H were observed. These bands can be assigned to fatty acids60, and their intensity was greater in the ALP-AGS spectra than in the ALG spectra (Fig. 6b). An additional band at ~ 3087/cm in the ALP-AGS spectra indicates the presence of aromatic structures (also the band at ~ 1540/cm). The latter band may also have a contribution from the vibrations of amide II groups, i.e., N–H bending (also visible at 1515/cm) and C–N stretching in proteins60. The presence of C–N bonds is, in turn, confirmed by the band at 1378/cm58. The presence of hydrophobic components does not affect the adsorbent’s capacity for metal adsorption, but it can indirectly affect the location of polar hydrophilic groups responsible for metal adsorption47. The peaks at ~ 1730/cm are characteristic of C=O symmetric stretching in carboxylic acids. After Cd2+ adsorption, this band is no longer visible in the ALG spectrum (Fig. 6a), which may indicate that Cd2+ bonded with these acid groups. The bands at ~ 1590/cm (Fig. 6a) indicate the presence of ionic carboxylate salts.
From 1652 to 1630/cm in the ALP-AGS spectra, bands are visible that correspond to amide C=O and C–N stretching, N–H bending, C=C stretching in proteins, hydroxyl O–H stretching of polysaccharides27,58,60, and/or COO– groups (Fig. 6b), which may indicate that these groups were more covalent in character. After Cd2+ adsorption, the intensity of these bands in the ALP-AGS spectra decreased (Fig. 6b), which may indicate the participation of carboxylate groups in the formation of complexes with Cd2+. The bands at 1100–1000/cm correspond to the glycosidic bonds in the polysaccharide (C–O–C stretching)56,61,62.
The presence of bands at ~ 1410/cm may result from the overlapping of C–H group vibrations and COO– group stretching61. This band can be assigned to the stretching vibration of C=O and the deformation vibration of –OH in carboxylate, alcohol, or phenol structures56,63, and its enhancement after adsorption is explained by the rearrangement of molecular bonds and therefore the formation of new bands. After Cd2+ adsorption, both the ALG and ALP-AGS spectra have additional bands at ~ 1350/cm, and the bands at ~ 1410/cm have higher intensity, which may indicate both complexation of Cd2+ and the presence of nitrates (the source of the Cd2+ in the aqueous solution).
Thus, the high efficiency of Cd2+ removal at pH values over 4.0 was related to metal complexation with negatively charged functional groups (especially COOH and OH groups) on the ALG and ALP-AGS surfaces according to these reactions: 2ALG-COO– + Cd2+ → (ALG-COO)2Cd2+ and 2ALP-AGS-COO– + Cd2+ → (ALP-AGS-COO)2Cd2+64. The presence of monovalent species of Cd, which is due to the pH, can promote its complexation65. At pH < 6.0, it is present mainly as Cd2+. At pH > 6.0, the Cd2+ content gradually decreases and other Cd-containing species appear, e.g., CdOH+, Cd2OH+3, and Cd(OH)2(s)51,66. Due to the amphiphilic character of EPS from AGS and the presence of abundant negatively-charged functional groups, metals can also be adsorbed via electrostatic attraction, ion exchange, or surface precipitation56,67.
Contact time and adsorption kinetics
The effect of contact time on Cd2+ adsorption onto ALG and ALP-AGS is shown in Fig. 7. The amount of Cd2+ adsorbed at a specific adsorption time (qt) indicates that, as the contact time was increased from 5 to 60 min, Cd2+ adsorption increased, and then between 60 and 180 min, the adsorption curve was flat. Similarly, Liu et al.56 observed very quick metal (Pb2+, Cd2+, Zn2+) adsorption onto EPS recovered from AGS cultivated in a lab-scale sequencing batch reactor. The metal adsorption sharply increased within the first 10 min, and progressively slowed until saturation after about 60 min. The fast adsorption onto EPS could be related to metal interactions with functional groups of proteins. The R2 and SSE values indicate that Cd2+ adsorption onto both types of adsorbents at different contact times was well described by a pseudo-second-order kinetics model (Fig. 7)54.
The good fit of the model to the data indicates that the rate-limiting step in Cd2+ adsorption is chemisorption, which in the case of ALG and ALP-AGS, was due to complexation and electrostatic attraction between Cd2+ and negatively charged groups, as well as ion exchange between Cd2+ and other cations on the adsorbents’ surfaces, e.g., Ca2+. During the initial stage of adsorption, Cd2+ removal was rapid, as shown by the initial rate of adsorption (r), which was higher for ALP-AGS than for ALG. As time elapsed, the adsorption process slowed, due to the saturation of the available surface-active sites. For both adsorbents, equilibrium conditions for Cd2+ removal were reached within 60 min (Fig. 7).
Initial Cd2+ concentration and adsorption isotherms
The effects of the initial Cd2+ concentration (5–200 mg/L) on the metal removal efficiency and the adsorption capacity of the ALG and ALP-AGS are shown in Fig. 8a–d respectively.
The efficiency of Cd2+ adsorption onto ALG and ALP-AGS depending on initial Cd2+ concentration (a, b) and isotherms of Cd2+ adsorption (c, d) (pH 5.0; 2 h; adsorbent dosage 7.9 g d.m./L) (n = 2). For ALP-AGS, the adsorption isotherm of the Langmuir model (blue line) coincides with the Sips model isotherm (red line).
The initial Cd2+ concentration affected the efficiency of adsorption with ALG and ALP-AGS. At initial Cd2+ concentrations in the range of 5–15 mg/L, the average Cd2+ removal efficiency slightly increased from 96.7 to 98.9% for ALG and from 92.1 to 94.6% for ALP-AGS. However, at initial concentrations above 15 mg/L, the efficiency gradually decreased to 73.5% (ALG) and 79.9% (ALP-AGS) (Fig. 8c, d). This means that the presence of available adsorption sites onto the adsorbents was high up to the relevant Cd2+ concentration.
The relationship between the amount of Cd2+ adsorbed by ALG and ALP-AGS and the concentration of Cd2+ remaining in the solution was evaluated with the Freundlich, Langmuir, and Sips models. Of these three adsorption models, the Sips model had the highest R2 values and the lowest SSE values (Table 2), indicating that it was the most suitable for describing Cd2+ adsorption onto tested adsorbents. The qmax is one of the principal criteria for the suitability of a particular adsorbent for metal removal. According to the Sips model, the qmax values were 29.3 mg/g d.m. for ALG and 29.5 mg/g d.m. for ALP-AGS. With comparable qmax, a lower value for energy of adsorption (b) was for ALP-AGS. With the Langmuir model, the qmax value for ALP-AGS was similar to this calculated from the Sips model. Thus, ALP-AGS has the potential to serve as a substitute for commercial ALG. These qmax values are similar to those reported from other studies of Cd2+ adsorption with ALG adsorbents, e.g., ALG–calcium carbonate beads: qmax, Langmuir = 10.2 mg/g53, qmax, Langmuir = 37.8–68.9 mg/g68, qmax, Langmuir = 38.05 mg/g and qmax, Sips = 38.65 mg/g47. Liu et al.56 found that EPS extracted from AGS exhibited a high adsorption capacity for Cd2+ (1470 mg/g) that was much higher than for ALP-AGS and conventional biosorbents. The differences in adsorption capacities can be related to the methods used for the biopolymers’ recovery from the sludge matrix and their purification, which can provide more efficient exposure to the negatively charged sites. In the present study, ALP recovered from AGS was not purified.
The Sips model being a combination of Langmuir and Freundlich isotherm models allows for predicting the heterogeneous adsorption systems. This model adjusts to low and high concentrations of metal ions where the interactions of the metal with the adsorbent are different69. Based on the values of heterogeneity index (n), adsorption of Cd2+ by ALP-AGS seems to occur onto more uniform and homogenous active sites compared to ALG69. A degree of homogeneity/heterogeneity of the adsorption sites can depend on the number of functional groups with the same capacity for adsorption. Cd2+ with low bonding strength is mainly adsorbed by carboxyl groups in the ALG molecule. Due to the conformational changes in the saccharide chains of G and M blocks, all carboxyl groups are not easily available, which affects the adsorption process52. Within the egg-box structure of ALG, the G-block carboxyl groups are less readily available to the metal ions, whereas the M-block carboxyl groups can more easily interact with Cd2+70. In contrast to ALG, active sites on ALP-AGS, come not only from polysaccharides but also from proteins or humic acids71. The investigations of metal adsorption onto individual EPS components (proteins, humic acids, and polysaccharides) recovered from different sludges have demonstrated that proteins exhibited the highest adsorption capacity for Cd2+ removal, while the polysaccharides were less efficient71. Thus, it might suggest that some irregularities in active sites onto polysaccharides in ALP-AGS can be compensated by active sites from other polymers co-existing in ALP.
Chemical composition of alginate adsorbents after Cd2+ adsorption
SEM–EDX analysis of adsorbents after Cd2+ adsorption revealed that their composition had undergone some changes during the adsorption (Figs. 8, S2) process. Sorption of Cd2+ caused a decrease in the share of most elements analyzed with SEM–EDX, especially C (which may be related to changes in the carbon structure of alginate), as well as Ca (which may be related to the ion exchange mechanism of Cd2+ adsorption)72. Alkali metals play the role of ion exchange in the process of metal adsorption. Among them, Ca ions play an important role in the ion exchange of metal cations under medium acidic conditions73,74. In the present study, the experiment on Cd adsorption was performed at pH 5.0, and Ca release was observed from both ALG and ALP-AGS. Similarly, Ablough et al.75 found that after the adsorption of Pb on hybrid beads of chitosan and sodium alginate, the Pb peak appeared in the EDX spectra, while the Ca peak disappeared, indicating that Ca was completely removed from the adsorbent and ion exchange was the main mechanism of Pb adsorption. Bée et al.76 related the binding of Pb to Ca-alginate beads to the release of Ca and found that the amount of Ca remaining in the biopolymer bead was very small when the sorbent was saturated with Pb. Some divalent heavy metal ions with high reactivity can exchange Ca ions while maintaining the structure of the crosslinked adsorbent55 without unraveling the polymer.
The surface EDX mapping of the ALG and ALP-AGS proved the realization of the Cd2+ adsorption process, with comparable mass % of Cd2+ onto ALP-AGS (3.92 ± 1.25 mass %) and ALG surfaces (3.20 ± 0.43 mass %). These results correspond well with the maximum adsorption capacities estimated from Langmuir and Sips models (Table 2). The images obtained through mapping elements in SEM–EDX for Cd2+ exhibit uniform metal distribution on the surface of both adsorbents (Fig. 9).
Changes in the chemical composition of ALG (a) and ALP-AGS (b). Δ mass percent indicates the difference in the content of an individual element before and after Cd2+ adsorption (a positive value = an increase of a given element, a negative value = a decrease of a given element). The images show the elemental mapping of Cd after adsorption on ALG and ALP-AGS surface (pH 5.0, adsorbent dosage 7.9 g d.m./L, 200 mg Cd2+/L, 2 h).
Conclusions
This study showed that AGS from a full-scale WWTP is a rich source of ALP, which can be used for the effective adsorption of Cd2+. ALP content in the AGS from the full-scale facility increased in the transition period between winter and spring, reaching over 150 mg/g MLSS. In the batch reactor cycle, ALP content was highest 1 h after the start of aeration, about 2 times higher than at the end of the cycle. ALP-AGS has a low total pore volume and surface area, and its sorption properties were determined by the presence of carboxylic and hydroxyl groups. The Cd2+ removal mechanism was governed by chemisorption and a monolayer of Cd2+ on ALP-AGS surface was created. The most efficient Cd2+ removal was observed at an adsorbent dosage of 7.9 g d.m./L. ALP-AGS, pH ranging from 4 to 8, and 60 min. Under these conditions, the mass percentage of Cd2+ adsorbed onto ALP-AGS was 3.92%, while that adsorbed onto commercial ALG was 3.20%. ALP-AGS can serve as an attractive substitute for commercial ALG in the elimination of toxic Cd2+ from the environment, and sustainable ALP recovery from waste AGS can be implemented as part of comprehensive strategies for utilizing wastewater in a circular economy.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Świątczak, P. & Cydzik-Kwiatkowska, A. Performance and microbial characteristics of biomass in a full-scale aerobic granular sludge wastewater treatment plant. Environ. Sci. Pollut. Res. 25(2), 1655–1669 (2018).
Allesen-Holm, M. et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59(4), 1114–1128 (2006).
Liu, X. et al. Functional analysis of extracellular polymeric substances (EPS) during the granulation of aerobic sludge: Relationship among EPS, granulation and nutrients removal. Environ. Res. 2022, 112692 (2022).
Liu, H. & Fang, H. H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 95(3), 249–256 (2002).
Guibaud, G. et al. Cd (II) and Pb (II) sorption by extracellular polymeric substances (EPS) extracted from anaerobic granular biofilms: Evidence of a pH sorption-edge. J. Taiwan Inst. Chem. Eng. 43(3), 444–449 (2012).
Zeng, J. et al. Composition and aggregation of extracellular polymeric substances (EPS) in hyperhaline and municipal wastewater treatment plants. Sci. Rep. 6(1), 1–9 (2016).
Hogendoorn, A. Enhanced digestion and bio-ALE-like exopolysaccharides extraction from Nereda sludge. Master’s Thesis, University of Delft, Delft, The Netherlands (2013), (accessed 29 Aug 2022); https://repository.tudelft.nl/islandora/object/uuid:b1c86da0-3786-4fd7-b2c6-1cf0d3da8865?collection=education.
Kavitha, S., Jayashree, C., Kumar, S. A., Yeom, I. T. & Banu, J. R. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour. Technol. 168, 159–166 (2014).
Schambeck, C. M. et al. Chemical and physical properties of alginate-like exopolymers of aerobic granules and flocs produced from different wastewaters. Bioresour. Technol. 312, 123632 (2020).
Yang, G., Lin, J., Zeng, E. Y. & Zhuang, L. Extraction and characterization of stratified extracellular polymeric substances in Geobacter biofilms. Bioresour. Technol. 276, 119–126 (2019).
Chen, X. et al. A review on recovery of extracellular biopolymers from flocculent and granular activated sludges: Cognition, key influencing factors, applications, and challenges. Bioresour. Technol. 363, 127854 (2022).
Zahra, S. A., Abdullah, N., Iwamoto, K., Yuzir, A. & Mohamad, S. E. Alginate-like exopolysaccharides in aerobic granular sludge: A review. Mater. Today: Proc. 65(7), 3046–3053 (2022).
Felz, S., Al-Zuhairy, S., Aarstad, O. A., van Loosdrecht, M. C. & Lin, Y. M. Extraction of structural extracellular polymeric substances from aerobic granular sludge. J. Vis. Exp. 115, e54534 (2016).
dos Santos, A. F. et al. Carbon source affects the resource recovery in aerobic granular sludge systems treating wastewater. Bioresour. Technol. 357, 127355 (2022).
Rollemberg, S. L. D. S., Dos Santos, A. F., Ferreira, T. J. T., Firmino, P. I. M. & Dos Santos, A. B. Evaluation of the production of alginate-like exopolysaccharides (ALE) and tryptophan in aerobic granular sludge systems. Bioprocess Biosyst. Eng. 44(2), 259–270 (2021).
de Carvalho, C. D. A. et al. Resource recovery in aerobic granular sludge systems: Is it feasible or still a long way to go?. Chemosphere 274, 129881 (2021).
Lin, Y., de Kreuk, M., Van Loosdrecht, M. C. M. & Adin, A. Characterization of alginate-like exopolysaccharides isolated from aerobic granular sludge in pilot-plant. Water Res. 44(11), 3355–3364 (2010).
Tan, X. et al. High value-added biomaterials recovery from granular sludge based wastewater treatment process. Resour. Conserv. Recycl. 169, 105481 (2021).
Lin, Y. M., Nierop, K. G. J., Girbal-Neuhauser, E., Adriaanse, M. & Van Loosdrecht, M. C. M. Sustainable polysaccharide-based biomaterial recovered from waste aerobic granular sludge as a surface coating material. SM&T 4, 24–29 (2015).
Go, L. C., Holmes, W., Depan, D. & Hernandez, R. Evaluation of extracellular polymeric substances extracted from waste activated sludge as a renewable corrosion inhibitor. PeerJ 7, e7193 (2019).
Kim, N. K., Lin, R., Bhattacharyya, D., van Loosdrecht, M. C. & Lin, Y. Insight on how biopolymers recovered from aerobic granular wastewater sludge can reduce the flammability of synthetic polymers. Sci. Total Environ. 805, 150434 (2022).
Campo, R., Carretti, E., Lubello, C. & Lotti, T. Recovery of structural extracellular polymeric substances (sEPS) from aerobic granular sludge: Insights on biopolymers characterization and hydrogel properties for potential applications. J. Environ. Manage. 324, 116247 (2022).
de Melo, B. A. G., Motta, F. L. & Santana, M. H. A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 62, 967–974 (2016).
Ladnorg, S. et al. Alginate-like exopolysaccharide extracted from aerobic granular sludge as biosorbent for methylene blue: Thermodynamic, kinetic and isotherm studies. J. Environ. Chem. Eng. 7(3), 103081 (2019).
Dall’Agnol, P. et al. A comparative study of phosphorus removal using biopolymer from aerobic granular sludge: A factorial experimental evaluation. J. Environ. Chem. Eng. 8(2), 103541 (2020).
Pagliaccia, B. et al. Heavy metal biosorption by extracellular polymeric substances (EPS) recovered from anammox granular sludge. J. Hazard. Mater. 424, 126661 (2022).
Li, N. et al. Comparative study of the role of extracellular polymeric substances in biosorption of Ni (II) onto aerobic/anaerobic granular sludge. J. Colloid Interface Sci. 490, 754–761 (2017).
Zhang, Z. & Zhang, J. Characteristics of Cadmium (II) adsorbed by the extracellular polymeric substance extracted from waste-activated sludge after short-time aerobic digestion. Water Air Soil Pollut. 225(2), 1–10 (2014).
Lin, Y. M., Sharma, P. K. & Van Loosdrecht, M. C. M. The chemical and mechanical differences between alginate-like exopolysaccharides isolated from aerobic flocculent sludge and aerobic granular sludge. Water Res. 47(1), 57–65 (2013).
Cydzik-Kwiatkowska, A., Nosek, D., Wojnowska-Baryła, I. & Mikulski, A. Efficient dewatering of polymer-rich aerobic granular sludge with cationic polymer containing hydrocarbons. Int. J. Environ. Sci. Technol. 17(1), 361–370 (2020).
Ali, P. et al. A glacier bacterium produces high yield of cryoprotective exopolysaccharide. Front. Microbiol. 10, 3096 (2020).
Bengoa, A. A. et al. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 69, 212–218 (2018).
Yang, Y. C., Liu, X., Wan, C., Sun, S. & Lee, D. J. Accelerated aerobic granulation using alternating feed loadings: Alginate-like exopolysaccharides. Bioresour. Technol. 171, 360–366 (2014).
Schambeck, C. M. et al. Biopolymers recovery: dynamics and characterization of alginate-like exopolymers in an aerobic granular sludge system treating municipal wastewater without sludge inoculum. J. Environ. Manage. 263, 110394 (2020).
Huang, J. L., Wang, H. H., Alam, F. & Cui, Y. W. Granulation of halophilic sludge inoculated with estuarine sediments for saline wastewater treatment. Sci. Total Environ. 682, 532–540 (2019).
Hoskisson, P. A. & Hobbs, G. Continuous culture–making a comeback?. Microbiology 151(10), 3153–3159 (2005).
Kıvılcımdan Moral, Ç. & Yıldız, M. Alginate production from alternative carbon sources and use of polymer based adsorbent in heavy metal removal. Int. J. Polym. Sci. 2016, 7109825 (2016).
Cydzik-Kwiatkowska, A., De Jonge, N., Poulsen, J. S. & Nielsen, J. L. Unravelling gradient layers of microbial communities, proteins, and chemical structure in aerobic granules. Sci. Total Environ. 829, 154253 (2022).
Sabra, W., Zeng, A. P., Lünsdorf, H. & Deckwer, W. D. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl. Environ. Microbiol. 66(9), 4037–4044 (2000).
Clementi, F., Fantozzi, P., Mancini, F. & Moresi, M. Optimal conditions for alginate production by Azotobacter vinelandii. Enzyme Microb. Technol. 17(11), 983–988 (1995).
Gimmestad, M. et al. Characterization of three new Azotobacter vinelandii alginate lyases, one of which is involved in cyst germination. J. Bacteriol. 191(15), 4845–4853 (2009).
Pena, C., Trujillo-Roldán, M. A. & Galindo, E. Influence of dissolved oxygen tension and agitation speed on alginate production and its molecular weight in cultures of Azotobacter vinelandii☆. Enzyme Microb. Technol. 27(6), 390–398 (2000).
Trujillo-Roldán, M. A., Moreno, S., Espín, G. & Galindo, E. The roles of oxygen and alginate-lyase in determining the molecular weight of alginate produced by Azotobacter vinelandii. Appl. Microbiol. Biotechnol. 63(6), 742–747 (2004).
Ohemeng-Boahen, G., Sewu, D. D. & Woo, S. H. Preparation and characterization of alginate-kelp biochar composite hydrogel bead for dye removal. Environ. Sci. Pollut. Res. 26(32), 33030–33042 (2019).
Aziz, F. et al. Composites with alginate beads: A novel design of nano-adsorbents impregnation for large-scale continuous flow wastewater treatment pilots. Saudi J. Biol. Sci. 27(10), 2499–2508 (2020).
Sam, S. B. & Dulekgurgen, E. Characterization of exopolysaccharides from floccular and aerobic granular activated sludge as alginate-like-exoPS. Desalin. Water Treat. 57(6), 2534–2545 (2016).
Torres-Caban, R., Vega-Olivencia, C. A. & Mina-Camilde, N. Adsorption of Ni2+ and Cd2+ from water by calcium alginate/spent coffee grounds composite beads. Appl. Sci. 9(21), 4531 (2019).
Minh, V. X., Dung, K. T. T., Lan, P. T., Hanh, L. T. M. & Dung, N. T. Study on Ni (II) adsorption by calcium alginate beads. Vietnam J. Chem. 58(3), 358–363 (2020).
Isik, Z., Saleh, M. & Dizge, N. Adsorption studies of ammonia and phosphate ions onto calcium alginate beads. Surf. Interfaces 26, 101330 (2021).
Eablouh, E. H., Hanani, Z., Eladlani, N., Rhazi, M. & Taourirte, M. Chitosan microspheres/sodium alginate hybrid beads: an efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Environ. Res. 29(1), 1–11 (2019).
Bian, Y. et al. Adsorption of cadmium ions from aqueous solutions by activated carbon with oxygen-containing functional groups. Chin. J. Chem. Eng. 23(10), 1705–1711 (2015).
Kuczajowska-Zadrożna, M., Filipkowska, U. & Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 8(4), 103878 (2020).
Mahmood, Z. et al. Adsorption studies of cadmium ions on alginate–calcium carbonate composite beads. Appl. Water Sci. 7(2), 915–921 (2017).
Benettayeb, A., Guibal, E., Morsli, A. & Kessas, R. Chemical modification of alginate for enhanced sorption of Cd (II), Cu (II) and Pb (II). Chem. Eng. J. 316, 704–714 (2017).
Gao, X. et al. Adsorption of heavy metal ions by sodium alginate based adsorbent—a review and new perspectives. Int. J. Biol. Macromol. 164, 4423–4434 (2020).
Liu, W., Zhang, J., Jin, Y., Zhao, X. & Cai, Z. Adsorption of Pb (II), Cd (II) and Zn (II) by extracellular polymeric substances extracted from aerobic granular sludge: Efficiency of protein. J. Environ. Chem. Eng. 3(2), 1223–1232 (2015).
Felz, S., Kleikamp, H., Zlopasa, J., van Loosdrecht, M. C. & Lin, Y. Impact of metal ions on structural EPS hydrogels from aerobic granular sludge. Biofilm 2, 100011 (2020).
Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts (Wiley, 2001).
Wei, D. et al. Extracellular polymeric substances for Zn (II) binding during its sorption process onto aerobic granular sludge. J. Hazard. Mater. 301, 407–415 (2016).
Oliveira, A. S. et al. Variability in the composition of extracellular polymeric substances from a full-scale aerobic granular sludge reactor treating urban wastewater. J. Environ. Chem. Eng. 8(5), 104156 (2020).
Kavita, K., Mishra, A. & Jha, B. Isolation and physico-chemical characterisation of extracellular polymeric substances produced by the marine bacterium Vibrio parahaemolyticus. Biofouling 27(3), 309–317 (2011).
Huang, H., Grun, I. U., Ellersieck, M. & Clarke, A. D. Measurement of total sodium alginate in restructured fish products using fourier transform infrared spectroscopy. Semant. Sch. 11, 33–45 (2017).
Lu, W. B., Shi, J. J., Wang, C. H. & Chang, J. S. Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp. J1 possessing high heavy-metal resistance. J. Hazard. Mater. 134(1–3), 80–86 (2006).
Hashem, A. & Elhmmali, M. M. Modification of sodium alginate for the removal of Cd (II) from aqueous solutions. Polym. Plast. Technol. Eng. 45(6), 707–712 (2006).
Soco, E. & Kalembkiewicz, J. Comparison of adsorption of Cd (II) and Pb (II) ions on pure and chemically modified fly ashes. Chem. Process. Eng. 37(2), 215–234 (2016).
Chen, T. et al. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 134, 286–293 (2015).
Yan, P. et al. Thermodynamics of binding interactions between extracellular polymeric substances and heavy metals by isothermal titration microcalorimetry. Bioresour. Technol. 232, 354–363 (2017).
Kwiatkowska-Marks, S. & Wójcik, M. Removal of cadmium (II) from aqueous solutions by calcium alginate beads. Sep. Sci. Technol. 49(14), 2204–2211 (2014).
Guarín-Romero, J. R., Rodríguez-Estupiñán, P., Giraldo, L. & Moreno-Piraján, J. C. Simple and competitive adsorption study of nickel (II) and chromium (III) on the surface of the brown algae Durvillaea antarctica biomass. ACS Omega 4(19), 18147–18158 (2019).
Papageorgiou, S. K. et al. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 137(3), 1765–1772 (2006).
Wei, L. et al. Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: Sorption properties and mechanisms. Bioresour. Technol. 291, 121868 (2019).
Gusiatin, Z. M. et al. A mineral by-product from gasification of poultry feathers for removing Cd from highly contaminated synthetic wastewater. Minerals 10(12), 1048 (2020).
Benettayeb, A., Guibal, E., Morsli, A. & Kessas, R. Chemical modification of alginate for enhanced sorption of Cd (II), Cu (II) and Pb (II). Chem. Eng. J 316, 704–714 (2017).
Mo, G., Xiao, J. & Gao, X. To enhance the Cd2+ adsorption capacity on coconut shell-derived biochar by chitosan modifying: Performance and mechanism. Biomass Convers. Biorefin. 2022, 1–16 (2022).
Ablouh, E. H., Hanani, Z., Eladlani, N., Rhazi, M. & Taourirte, M. Chitosan microspheres/sodium alginate hybrid beads: An efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Environ. Res. 29(1), 1–11 (2019).
Bée, A., Talbot, D., Abramson, S. & Dupuis, V. Magnetic alginate beads for Pb (II) ions removal from wastewater. J. Colloid Interface Sci. 362(2), 486–492 (2011).
Acknowledgements
This study was supported by the National Science Centre, Poland (grant number 2016/21/B/NZ9/03627). The authors gratefully acknowledge Sylwia Pasieczna-Patkowska from the University of Maria Skłodowska-Curie in Lublin, Poland for providing scientific support during FTIR analyses.
Funding
Language correction supported by the Minister of Education and Science under the program entitled "Regional Initiative of Excellence" for the years 2019–2023, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Author information
Authors and Affiliations
Contributions
A.C.-K.: writing—original draft, conceptualization, data curation, methodology, investigation, supervision, funding acquisition M.Z.G.: writing—original draft, conceptualization, visualization, data curation, methodology, investigation M.Z.: writing—review & editing, data curation, investigation I.W.-B.: writing—review & editing, formal analysis D.K.: writing—review & editing, data curation K.B.: writing—review & editing, data curation
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cydzik-Kwiatkowska, A., Gusiatin, M.Z., Zielińska, M. et al. Alginate-like polymers from full-scale aerobic granular sludge: content, recovery, characterization, and application for cadmium adsorption. Sci Rep 12, 22260 (2022). https://doi.org/10.1038/s41598-022-26743-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26743-5
- Springer Nature Limited