Abstract
Podoplanin (PDPN) is intensely expressed on the podocyte membrane in an evolutionally conserved manner. CLEC-2, the endogenous ligand of PDPN, is highly expressed in platelets and also exists in a soluble form in plasma. Normally, podocytes are sequestered from CLEC-2, but when the glomerular barrier is injured, podocytes gain access to CLEC-2. We tested the effects of CLEC-2 in podocytes in vitro and in vivo. Cultured podocytes treated with Fc-CLEC-2 demonstrated that CLEC-2 induced the dephosphorylation of ezrin, radixin, and moesin (ERM) proteins. Podocytes treated with Fc-CLEC-2 also showed the dissociation of F-actin filaments from PDPN, F-actin degradation, detachment, and round morphology. Next, we perfused normal mouse kidney in vivo with FLAG-CLEC-2. CLEC-2 induced dephosphorylation of ERM and widening of the foot processes of podocytes. Platelets were detected by immunostaining for CD41 in the urine of mice with podocyte injury, indicating that podocytes can encounter platelets when glomeruli are injured. Collectively, these observations suggest that when platelets leak through the injured glomeruli, CLEC-2 from the platelets acts on PDPN in podocytes and induces morphological change and detachment, which may further aggravate podocyte injury. Thus, PDPN on podocytes may work as a leaked-platelet sensor.
Similar content being viewed by others
Introduction
Podoplanin (PDPN) is a membranous mucin-type O-glycosylated glycoprotein, which is negatively charged by abundant sialic acid. PDPN is expressed on the surface of various types of cells, including kidney podocytes, alveolar epithelial cells, lymphatic endothelial cells, stromal fibroblastic reticular cells (FRCs) of lymph nodes1, and tumors2. Among these, PDPN is most intensely expressed on podocytes and its expression is evolutionally conserved3.
PDPN was found to be the endogenous ligand of C-type lectin-like receptor 2 (CLEC-2) on platelets and is involved in platelet aggregation induced by tumor cells4, which facilitates invasion and metastasis of the tumor2,5,6. During developmental stages, CLEC-2 in platelets can interact with PDPN in lymphatic endothelial cells, and activated platelets facilitate blood-lymphatics vessel separation7. Platelet activation by PDPN also plays a critical role in the differentiation of alveolar duct myofibroblasts8.
CLEC-2–PDPN interaction mediates bidirectional signaling. PDPN works as a ligand for CLEC-2 as shown above. In addition, binding PDPN with CLEC-2 influences PDPN-expressing cells. Binding PDPN with CLEC-2 attenuates actomyosin contractility in FRCs in the lymph node9, inhibits migration of lymphatic endothelial cells10,11, stimulates CCL5 secretion in FRC-like cells in bone marrow12, stimulates IGF-1 secretion from stromal cells13, and attenuates inflammatory responses in a subset of Th17 cells14.
The role of PDPN in podocytes is not fully elucidated. Decreased expression of PDPN in podocytes is associated with foot process effacement, proteinuria, and decreased glomerular selective permeability in several animal models15,16,17. In biopsy specimens of patients with minimal change nephrotic syndrome, PDPN staining was decreased in the proteinuric state, and recovered when proteinuria was normalized18. These facts suggest that PDPN is important for maintenance of the normal function of podocytes. The cytoplasmic tail of PDPN interacts with ezrin, radixin, and moesin (ERM) proteins, which bind the actin cytoskeleton and regulate cell shape. It was reported that whole-body Pdpn-gene-disrupted mice showed no abnormal renal phenotypes19. Podocalyxin-NHERF2 complex and nephrin-ephrin-B1-NHERF2 complexes can bind and maintain ezrin-F-Actin complex20,21. This may be a reason for the lack of abnormal phenotype in podocytes of congenital Pdpn-deficient mice. However, suppression of PDPN by siRNA in cultured podocytes changed cell morphology from an elongated to a round shape along with a change in ezrin distribution18.
Normally, podocytes are sequestered from platelets, but when the glomerular barrier is injured, podocytes gain access to CLEC-2 on platelets. In addition, a soluble form of CLEC-2 with molecular weight 25 kDa exists in human plasma22. We speculated that CLEC-2, the known ligand for PDPN, may have some biological impact on podocytes. This hypothesis is supported by previous reports that injection of antibodies against specific epitopes of PDPN caused transient proteinuria and foot process effacement in rats23. In the present study, we examined the effect of CLEC-2 in podocytes in vitro and in vivo.
Results
Effects of CLEC-2 on in vitro podocytes
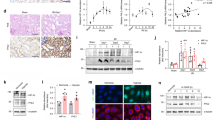
We first tested the effects of recombinant Fc-human CLEC-2 on cultured mouse podocytes. Although most proteins characteristic to podocytes, such as nephrin and podocin, are rapidly downregulated upon in vitro culture, PDPN staining was maintained in primary cultured podocytes with similar intensity to in vivo podocytes (Fig. 1A). As reported previously3, a pull-down experiment showed that Fc-human CLEC-2 bound to mouse PDPN (S.Fig. 1A). The majority of podocytes treated with Fc protein for 1 h showed an elongated morphology with sharp protrusions and had numerous F-actin filaments. In contrast, podocytes treated with Fc-CLEC-2 showed a round cell shape without sharp protrusions and a decrease in F-Actin (Fig. 1B). Furthermore, podocytes treated with Fc-CLEC-2 showed less adhesion to the collagen-1-coated plate within 1 h than the Fc control cells (59.6% vs. 65.3%) (Fig. 1C). Podocytes with Fc-CLEC-2 showed more migration than the Fc control (2.62 vs. 1.93 mm/24 h) (Fig. 1D).
In vitro study with Fc and Fc-CLEC-2. (A) Representative images of kidney sections and cultured podocytes, stained with podoplanin (PDPN). PDPN is intensely stained both in in vivo podocytes and cultured podocytes. Scale bar: 50 μm. (B) Representative images of cultured podocytes, stained with phalloidin after 1 h incubation at 37 °C with Fc or Fc-CLEC-2. Podocytes incubated with Fc-CLEC-2 showed a round shape with degradation of F-actin, while those with Fc showed an elongated morphology with numerous F-actin filaments. Scale bar: 100 μm. Podocytes with protrusions were significantly reduced by Fc-CLEC-2. (C) Adhesion assay. The percentage of attached podocytes was evaluated after 1 h incubation at 37 °C with Fc or Fc-CLEC-2. Podocytes incubated with Fc-CLEC-2 showed less attachment than those with Fc. (D) Migration assay. The distance of migration of podocytes was measured while incubated at 37 °C with Fc or Fc-CLEC-2 for 24 h. Podocytes incubated with Fc-CLEC-2 showed greater migration than those with Fc. (E). Western blot analysis for ERM and pERM after treatment of Fc or Fc-CLEC-2 at 37 °C for 1 h. The pERM/ERM ratio was 0.47-fold decreased in podocytes with Fc-CLEC-2, compared to those with Fc. The images of full-length blots are shown in Supplementary Figs. 2 and 3. Ezrin: 81 kDa, Moesin: 75 kDa, β-tubulin 55 kDa. (F) Representative images of cultured podocytes, stained with moesin after 1 h incubation at 37 °C with Fc or Fc-CLEC-2. Fc increased the number of protrusions that were positive for moesin staining, and Fc-CLEC-2 markedly decreased them. Scale bar: 50 μm. The podocyte shape was recognized by phase contrast imaging (shown in supplementary Fig. 5).
We next studied the phosphorylation status of ERM, which links PDPN with F-actin, to reveal the intracellular signaling induced by CLEC-2. Western blot analysis revealed that treatment with Fc-CLEC-2 reduced the pERM/ERM ratio in cultured podocytes, indicating that CLEC-2 induced dephosphorylation of ERM proteins (Fig. 1E, S.Figs. 2 and 3). Ezrin has been regarded as the major ERM protein in podocytes24. Using Western blot analysis and quantitative PCR, we found that ezrin was downregulated in cultured podocytes (S.Fig. 4A and B). Ezrin was not detected by immunostaining in cultured podocytes. Moesin was intensely stained in the protrusions of the Fc control podocytes, and incubation with Fc-CLEC-2 markedly decreased moesin staining (Fig. 1F, S.Fig. 5). These results collectively indicate that CLEC-2 induced the dephosphorylation of ERM proteins, which caused the dissociation of F-actin filaments from PDPN, F-actin degradation, and cell morphological changes.
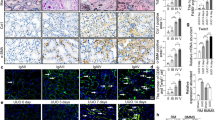
In vivo study with FLAG-CLEC-2 protein. (A) Double immunostaining for FLAG and nephrin in kidneys with or without infusion of FLAG-CLEC-2. After infusion of FLAG-CLEC-2, glomeruli were intensely stained for FLAG in nephrin-positive podocytes. Scale bar: 50 μm. (B) Internalization assay using cultured podocytes. Representative images of podocytes stained for FLAG, after 3 h incubation at 37 °C or 4 °C with or without subsequent washing with a stripping buffer. At either temperature, FLAG staining diminished after washing, indicating that FLAG-CLEC-2 was not internalized within the cells. Scale bar: 200 μm. (C) Serpine1 mRNA in the glomeruli (relative amount). Serpine1 mRNA expression was 2.45-fold increased in the mice with FLAG-CLEC-2 infusion. (D) Western blot analysis of glomerular lysate for ERM. Phosphorylation of ERM proteins was 0.65-fold decreased by FLAG-CLEC-2. The images of full-length blots are shown in Supplementary Figs. 7 and 8. Ezrin: 81 kDa, Moesin: 75 kDa, β-tubulin 55 kDa. (E) Dephosphorylation of ERM in podocytes by FLAG-CLEC-2 perfusion. Double immunostaining showed an intense signal for pERM (red) in podocytes labeled by podocalyxin staining (green) in control mice (upper panels). In the mice perfused with FLAG-CLEC-2, some podocytes lack pERM staining. Scale bar: 50 μm. (F) SEM images of the foot processes. Podocytes perfused with FLAG-CLEC-2 exhibited widening of foot processes (arrows) in 18.5% of visual fields. Original magnification: × 8000. Scale bar: 2 μm.
Detection of urinary platelets in podocyte injured mice. (A) Urinary protein and occult blood were detected by urine test strip in the urine 5 days after LMB2 injection [LMB2 (+)]. Those reactions were negative for LMB2-untreated mice [LMB2(−)]. Blood changes white paper to green, and protein changes yellow paper to green. Note that normal mice show 1 + proteinuria due to high concentration of small molecular weight proteins. (B) Confocal microscopy of Cy3-CD41 platelets in the urine. Immunostaining for CD41 showed platelets (arrows) were detected in the urine 5 days after LMB2 injection. Scale bar: 20 μm. (C) Confocal microscopy of DiI-platelets in the urine. DiI labeled platelets (arrows) were detected in the urine of mice injected with DiI platelets 5 days after LMB2 injection. Scale bar: 20 μm. Positive and negative control images for (B) and (C) are shown in supplementary Fig. 9.
The effects of CLEC-2 on in vivo podocytes
To test the effects of CLEC-2 on in vivo podocytes, we generated a new recombinant CLEC-2 protein with a smaller size, because the above Fc-CLEC-2 forms a tetramer with a size of about 240 kDa, which was not expected to reach podocytes through the normal glomerular barrier. The new mouse CLEC-2 protein, FLAG-CLEC-2, exists as a monomer in solution and the size is 30–35 kDa. FLAG-CLEC-2 bound to cultured podocytes, which was competed with Fc-CLEC-2, but not with Fc, confirming the specificity of the binding (S. Fig. 6).
We infused 5 μg/g body weight of FLAG-CLEC-2 into normal mice and excised the kidney 1 h later. Double immunostaining for FLAG and nephrin, a protein specific to podocytes, confirmed that FLAG-CLEC-2 bound to podocytes (Fig. 2A). However, when glomeruli were isolated from mice 1 h after injection of FLAG-CLEC-2, FLAG was not detected by Western blot in the glomerular lysate. This implies that FLAG-CLEC-2 was not internalized into podocytes and that FLAG-CLEC-2 was detached from glomeruli during the glomerular isolation procedure. To test this possibility, cultured podocytes were incubated with FLAG-CLEC-2 (10 μg/mL) at 37 °C and washed with an acidic buffer. FLAG staining in podocytes decreased after washing. When incubation was performed at 4 °C, FLAG was stained in podocytes with similar intensity to those incubated at 37 °C, and the staining was similarly decreased after washing with an acidic buffer (Fig. 2B). These observations indicate that FLAG-CLEC-2 is not internalized into podocytes.
Quantitative RT-PCR analysis of the glomerular RNA revealed that infusion of FLAG-CLEC-2 increased Serpine1 mRNA, a podocyte injury marker25, 2.45 (1.68–8.14)-fold compared to controls (Fig. 2C). The Western blot of glomerular lysate revealed that FLAG-CLEC-2 decreased the pERM/ERM ratio 0.65 (0.38–0.76)-fold (Fig. 2D, S.Figs. 7 and 8), indicating that CLEC-2 induced dephosphorylation of ERM proteins. This was also confirmed by double immunostaining of pERM and podocalyxin (Fig. 2E).
In scanning electron microscopy (SEM) images, widening of foot processes was observed in 18.5% of visual fields in the mice treated with FLAG-CLEC-2, contrasting with no such change in control mice, suggesting that CLEC-2 induced a change in the cytoskeleton in foot processes (Fig. 2F).
These findings indicate that CLEC-2 induced dephosphorylation of ERM and concomitant widening of the foot processes of podocytes.
Urinary platelets excreted by mice with podocyte injury
We tested the possibility that podocytes can encounter platelets when glomeruli are injured. For this purpose, we induced podocyte injury in NEP25 mice by injecting LMB2. Five days after the injection of LMB2 (5 ng/g body weight), NEP25 mice exhibited both urinary protein and urinary blood (Fig. 3A), indicating disruption of the glomerular barrier. Immunostaining of CD41 revealed that CD41 positive platelets were observed in the urinary sediments at this time point (Fig. 3B), but not in those from LMB2-untreated control mice (S.Fig. 9A). To further verify leakage of platelets, platelets were collected from wild-type mice, labeled with DiI, and injected into NEP25 mice 5 days after the injection of LMB2. DiI-labeled platelets were found in the urine collected from the injected mice (Fig. 3C). Thus, platelets in the bloodstream can make contact with podocytes when the glomerular barrier is injured.
Discussion
The present study revealed that recombinant CLEC-2, the ligand of PDPN, induced significant morphological change, attenuated adhesion, and promoted migration in cultured podocytes. CLEC-2 causes dephosphorylation of ERM and decomposition of PDPN-ERM-F-actin complex. A previous report indicated that PDPN binds to ezrin, whose phosphorylated form tightly connects with F-actin in podocytes18. A similar phenomenon has been reported in the FRCs of lymph nodes. CLEC-2 on dendritic cells acts on the PDPN of FRCs and induces dephosphorylation of ERM, disconnection of ERM from the plasma membrane, and a reduction in actomyosin contractility, which elongates FRCs26. In both FRCs and podocytes, binding with CLEC-2 attenuates the basal function of PDPN. Similarly, CLEC-2 inhibits the basal function of PDPN in keratinocytes and lymphatic endothelial cells11,27, but in these cases the net effect of CLEC-2 is the inhibition of cell migration, which is opposite to that in podocytes. The reason for the opposite direction is not clear, but differences in cell character may be involved. Podocytes are basically static cells and have unique thick actin bundles. In addition, a recent study showed that the stimulation of PDPN acts downstream of VEGF signaling, but not directly on ERM, in lymphatic endothelial cells28.
Transient exposure to recombinant FLAG-CLEC-2 protein in a short period (1 h) induced a significant morphological change in intact in vivo podocytes although the effect was modest compared to those in cultured podocytes. The modest effect may be caused by the monomeric feature of FLAG-CLEC-2. In injured glomeruli, polymeric CLEC-2 on platelets may bind to PDPN on podocytes and exert greater impacts. Moreover, in injured glomeruli, nephrin and podocalyxin are rapidly and remarkably downregulated29, which bind to NHERF2 and stabilize ezrin-F-Actin complex in intact podocytes20,21. Therefore, CLEC-2 may have a more significant impact on injured podocytes than those of the perfusion study.
CLEC-2 is a membrane-bound protein mainly expressed in platelets. In addition, CLEC-2 exists in the circulation as shed or microparticle-bound forms30. It was reported that the mean plasma concentration of these soluble forms of CLEC-2 was 59–100 pg/mL in healthy volunteers and was increased to 260–380 pg/ml in patients with platelet-activating diseases22. In some patients with thrombotic microangiopathy or disseminated intravascular coagulation, the concentration of soluble CLEC-2 exceeds 1000 pg/ml31,32,33. Because platelets are retained in inflamed glomerular capillaries34, the local concentration of soluble CLEC-2 may be higher in glomerular diseases. Nevertheless, these concentrations do not appear sufficient to induce morphological change in normal podocytes considering the high dose of the recombinant CLEC-2 (5 μg/g body weight) used in the present study.
Platelets are smaller than erythrocytes, therefore they can pass through the damaged glomerular barrier in glomerular diseases with hematuria. In fact, urinary platelets were detected in glomerular diseases35,36,37,38. We also demonstrated that platelets are excreted into urine in our podocyte injury mouse model. We speculate that platelets may pass through the glomerular barrier and act on PDPN in podocytes in kidney diseases. Although urinary platelets have received almost no attention, they may reflect a distinct disease condition.
Taken together, we propose that PDPN on podocytes works as a sensor of platelet CLEC-2, which is leaked through glomeruli after severe injury. Stimulation by CLEC-2 induces morphological change and detachment of podocytes, which appears to further aggravate podocyte injury. Considering that PDPN on podocytes and CLEC-2 on platelets are evolutionally conserved, this system may have some beneficial effects, such as facilitating the repair process. Further study is necessary to establish the role of PDPN on podocytes.
Methods
Animal ethics
All animal experiments were approved by the Animal Experimentation Committee of Tokai University School of Medicine. All animal experiments were performed in accordance with relevant guideline and regulations, and the study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Recombinant CLEC-2 proteins
Fc-CLEC-2, a fusion of the Fc tag and C-terminal extracellular domain of human CLEC-2 (51–229), was prepared as previously reported39. Fc-CLEC-2 expression plasmid was transiently transfected in HEK293 cells using X-tremeGENE 9 DNA (Roche), and Fc-CLEC-2 protein was purified by protein A affinity chromatography (KANEKA KanCapA, Wako). HEK293 cells were kindly provided by Dr. Takehito Sato at Tokai University and used in a previous study39. SDS-PAGE and Coomassie Brilliant Blue (CBB) stain confirmed the expected size of bands (Fc; 30 kDa, Fc-CLEC-2; 60 kDa) (S.Fig. 1B). Pull-down assay confirmed that mouse PDPN can bind Fc-CLEC-2 (S.Fig. 1A).
StepTagII-3FLAG-CLEC-2, a fusion of StrepTactin FLAG double tags and the C-terminal extracellular domain of mouse CLEC-2 (51–229), was generated in HEK293 cells transiently transfected with the expression plasmid using polyethyleneimine Max reagent (Polysciences, Inc.). The supernatant of cell medium was added with biotin blocking solution (Biolock, iba Life Science), and StepTagII-FLAG-CLEC-2 protein (hereafter designated as FLAG-CLEC-2) was purified by a StrepTactin Sepharose column (StrepTrap HP, GE healthcare Life Sciences). Mass spectrometric analysis by the LCMS-IT-TOF (Shimadzu) confirmed that the purified protein contained peptides specific to mouse CLEC-2. SDS-PAGE and CBB stain showed double bands around 30-35 kDa (S.Fig. 1B). The deglycosylation by PNGase F (New England Biolabs) changed the two bands to a single band (S.Fig. 1C). Pull-down assay confirmed that mouse PDPN can bind FLAG-CLEC-2 (S.Fig. 1B).
Blue Native PAGE showed that Fc and Fc-CLEC-2 exist as tetramers, and FLAG-CLEC-2 exists as a monomer in aqueous solution (S.Fig. 1D).
Western blot analysis
Cells were lysed in a lysis buffer containing 1% Triton X-100, 2 mM CaCl2, 0.5 mM PMSF, protease inhibitor cocktail (cOmplete, Roche), and 50 mM Tris/HCl (pH7.4). For analysis of phosphorylated protein, 10 mM NaF, 1 mM Na3VO4, and 5 mM Na4P2O7 were added. The homogenates were centrifuged at 15,000 rpm to remove the insoluble fraction. Each protein sample was separated by SDS-PAGE and transferred onto a PVDF membrane. The protein-blotted membranes were incubated with 1:1000 diluted primary antibodies overnight at 4 °C and then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. The density of the positive bands was quantified by image analysis with CS Analyzer 3.0 (ATTO). The following primary antibodies were used: pERM (Cell Signaling, #3726), ERM (Cell Signaling, #3142), and β-tubulin (Cell Signaling, #2128).
The antigens of anti-ERM (#3142) and anti-pERM (#3726) antibodies are commonly shared by ezrin, radixin, and moesin.
Pull-down assay
Podocyte lysate (1.5 g/L, 50 µL) containing 1% Triton X-100 was mixed with 3 μg of Fc-CLEC-2 or FLAG-CLEC-2 for 1 h at 4 °C, and the samples were incubated with KANEKA KanCapA (Wako) or Strep-Tactin Superflow plus (Qiagen), respectively, for 1 h at 4 °C. After removing the supernatant, the beads were washed three times with cell lysis buffer and then incubated in SDS sample buffer. The supernatants were subjected to SDS-PAGE followed by Western blot with anti-PDPN antibody.
Isolation of glomeruli and primary podocyte culture
Glomeruli were harvested using the bead method as previously reported29. They were cultured on a collagen-1-coated dish for 7 days in DMEM/F12 medium containing 5% FCS and 0.5% ITS-A. Outgrowing cells were detached and passaged after removing residual beads and glomeruli. Cells were used for in vitro experiments 1 to 7 days after the first to third passages. Cultured podocytes were treated with Fc or Fc-CLEC-2 (10 µg/mL).
Isolation of in vivo podocyte mRNA
We utilized RiboTag mice, which express the ribosomal protein, Rlp22, which is tagged with hemagglutinin (HA) only in cells expressing Cre recombinase40. The RiboTag mice were crossed with podocyte-specific Cre-expressing mice (Nphs1-Cre mice). Podocyte RNA was purified from podocyte polysomes obtained by immunoprecipitation with anti-HA antibody of glomerular lysate.
Adhesion assay
Primary cultured podocytes were seeded at a density of 30,000/cm2 in collagen-1-coated 96-well plates. After 1 of hour incubation at 37 °C with Fc or Fc-CLEC-2 (10 µg/mL) in 0.5% FCS medium, all wells were washed several times with PBS. The number of attached cells was quantified by Cell Counting Kit-8 (Dojindo Laboratories).
Migration assay
Primary cultured podocytes were seeded at a density of 30,000/cm2 within O-rings on collagen-1-coated dishes and cultured to reach 90–100% confluency. After the O-rings were removed, the cells were allowed to migrate for 24 h at 37 °C in 5% FCS medium with Fc or Fc-CLEC-2 (10 µg/mL). The longest migration length was measured.
Perfusion of mouse kidney with recombinant CLEC-2 protein
C57BL/6 mice (4–6 months of age, approximately 12-19 g body weight) were used for the experiments. Under anesthesia with pentobarbital (50 mg/kg, i.p.) and buprenorphine (0.05 mg/kg, s.c.), the celiac and superior mesenteric arteries were transiently occluded with clips. The kidneys were perfused with 300 μl of PBS or PBS containing 5 μg/g body weight of FLAG-CLEC-2 through a catheter placed in the abdominal aorta at a distal site and then the clips were removed. After 1 h, kidneys were perfused with PBS, harvested, and analyzed by electron microscopy and immunohistochemistry. In some experiments, glomeruli were collected and used for PCR and Western blot analyses.
Quantitative RT-PCR
Total RNA was extracted from isolated glomeruli with an RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions. Single-stranded cDNA was prepared from 100 ng of RNA using TaqMan Reverse Transcription Reagents (ThermoFisher). A TaqMan primer probe set (Thermo Fisher) was used for Gapdh. For other genes, the following primers were used: Serpine1, 5’-AGGATCGAGGTAAACGAGAGC-3’ and 5’-GCGGGCTGAGATGACAAA-3’; Moesin, 5’-TCTTATGCCGTCCAGTCTAAGT-3’ and 5’-GGTCCTTGTTGAGTTTGTGCT -3’; Ezrin, 5’-CAATCAACGTCCGGGTGAC-3’ and 5’-GCCAATCGTCTTTACCACCTGA-3’. Relative amounts of mRNA were determined using the delta-delta CT method.
Immunostaining and F-actin staining
Primary cultured podocytes were seeded at a density of 5000/cm2 on glass-based dishes 1–2 days before staining. For PDPN and moesin staining, cells were fixed in acetone. For FLAG* and F-actin staining, cells were fixed in 4% paraformaldehyde (PFA) and permeabilized in 0.1% Triton X-100/PBS. After blocking, they were incubated with Alexa Fluor 594-phalloidin (Invitrogen, diluted at 1/100) for F-actin staining. For double staining of FLAG** and nephrin, frozen kidney sections were fixed in acetone. For double staining of pERM and podocalyxin, kidneys were fixed in trichloroacetic acid (TCA) for 1 h, followed by 4% PFA/PBS for 1 h before preparing frozen blocks with OCT to preserve the phosphorylation of ERM. Information regarding antibodies is shown in Table 1. Can Get Signal solution (TOYOBO) was used for FLAG** and nephrin staining of frozen kidney sections.
Electron microscopy
Kidneys were fixed by perfusion with 4% PFA and then immersed in 2.5% glutaraldehyde for 30 min. Subsequent preparation of SEM was performed using standard methods. We randomly selected 8–10 glomeruli in each mouse and 3 images at X8000 magnification were captured for evaluation of foot processes. The number of images containing foot processes that were more than twice as thick as normal ones were counted.
Urinary platelets in mice with podocyte injury
As a podocyte injury model, we used NEP25 mouse line41, which expresses human (h) CD25 selectively in podocytes. Injection of an hCD25-targeted immunotoxin, LMB2, induces podocyte injury dose-dependently. In this study, NEP25 mice were injected with 5 ng/g body weight of LMB2. Five days after the LMB2 injection, urinary protein and blood were detected by Uropaper III UHAGKSpH 7S (Eiken), and then urine was collected and centrifuged at 500 g. The sediment was washed several times with PBS containing 0.1% BSA, EGTA 1 mM and PGE1 0.25 μM and stained with anti-CD41 antibody (Biolegend, #133,9011:100), and then inspected with confocal microscopy (ZEISS, LSM-880).
Injection of DiI-labeled platelet solution into NEP25 mice
Platelets were isolated from the blood of C57BL/6 mice42 and stained with Vybrant DiI (Thermo Fisher scientific). A separate set of NEP25 mice were injected with LMB2. Five days later, DiI-labeled platelets were injected. The urine was collected and centrifuged at 180 g. Thereafter, the supernatant was centrifuged at 1300 g, according to the platelets isolation protocol42. The pellets were washed several times with washing buffer containing BSA, EGTA and PGE1, and then inspected with confocal microscopy (ZEISS, LSM-880).
Statistical analyses
The results are expressed as the median and interquartile range (IQR). P values of < 0.05 were considered to indicate statistical significance. For Fig. 1B, C and F, differences between groups were analyzed using Paired sampled t-test. For Fig. 1E and 2D, ratio of Fc-CLEC-2/Fc or FLAG-GLEC-2/Control was obtained in each experiment, and the experiments were repeated 4 and 5 times, respectively. A one-sample t test was used to determine whether the mean ratio was different from 1. For other analyses, differences between groups were analyzed using the Mann–Whitney U‐test for continuous data. Statistical analyses were performed using the JMP software program (version 11, SAS Institute Inc.; Cary, NC, USA).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Suzuki-Inoue, K., Osada, M. & Ozaki, Y. Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: Partners from in utero to adulthood. J. Thromb. Haemost. 15, 219–229. https://doi.org/10.1111/jth.13590 (2017).
Wicki, A. et al. Tumor invasion in the absence of epithelial-mesenchymal transition: Podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9, 261–272. https://doi.org/10.1016/j.ccr.2006.03.010 (2006).
Wang, L. et al. Structural and functional conservation of CLEC-2 with the species-specific regulation of transcript expression in evolution. Glycoconj. J. 29, 335–345. https://doi.org/10.1007/s10719-012-9415-0 (2012).
Suzuki-Inoue, K. et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J. Biol. Chem. 282, 25993–26001. https://doi.org/10.1074/jbc.M702327200 (2007).
Tsuruo, T. & Fujita, N. Platelet aggregation in the formation of tumor metastasis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 84, 189–198. https://doi.org/10.2183/pjab.84.189 (2008).
Kato, Y. et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 99, 54–61. https://doi.org/10.1111/j.1349-7006.2007.00634.x (2008).
Bertozzi, C. C. et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116, 661–670. https://doi.org/10.1182/blood-2010-02-270876 (2010).
Tsukiji, N. et al. Platelets play an essential role in murine lung development through Clec-2/podoplanin interaction. Blood 132, 1167–1179. https://doi.org/10.1182/blood-2017-12-823369 (2018).
Acton, S. E. et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature 514, 498–502. https://doi.org/10.1038/nature13814 (2014).
Finney, B. A. et al. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood 119, 1747–1756. https://doi.org/10.1182/blood-2011-09-380709 (2012).
Osada, M. et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem 287, 22241–22252. https://doi.org/10.1074/jbc.M111.329987 (2012).
Tamura, S. et al. Podoplanin-positive periarteriolar stromal cells promote megakaryocyte growth and proplatelet formation in mice by CLEC-2. Blood 127, 1701–1710. https://doi.org/10.1182/blood-2015-08-663708 (2016).
Otake, S. et al. CLEC-2 stimulates IGF-1 secretion from podoplanin-positive stromal cells and positively regulates erythropoiesis in mice. J. Thromb. Haemost. 19, 1572–1584. https://doi.org/10.1111/jth.15317 (2021).
Nylander, A. N. et al. Podoplanin is a negative regulator of Th17 inflammation. JCI Insight https://doi.org/10.1172/jci.insight.92321 (2017).
Breiteneder-Geleff, S. et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 151, 1141–1152 (1997).
Ijpelaar, D. H. et al. Glomerular hypertrophy precedes albuminuria and segmental loss of podoplanin in podocytes in Munich-Wistar-Fromter rats. Am. J. Physiol. Renal. Physiol. 294, F758-767. https://doi.org/10.1152/ajprenal.00457.2007 (2008).
Koop, K. et al. Selective loss of podoplanin protein expression accompanies proteinuria and precedes alterations in podocyte morphology in a spontaneous proteinuric rat model. Am. J. Pathol. 173, 315–326. https://doi.org/10.2353/ajpath.2008.080063 (2008).
Suzuki, K. et al. Alteration in the podoplanin-ezrin-cytoskeleton linkage is an important initiation event of the podocyte injury in puromycin aminonucleoside nephropathy, a mimic of minimal change nephrotic syndrome. Cell Tissue Res. 362, 201–213. https://doi.org/10.1007/s00441-015-2178-8 (2015).
Takara, K. et al. Morphological study of tooth development in podoplanin-deficient mice. PLoS ONE 12, e0171912. https://doi.org/10.1371/journal.pone.0171912 (2017).
Kerjaschki, D., Sharkey, D. J. & Farquhar, M. G. Identification and characterization of podocalyxin–the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98, 1591–1596. https://doi.org/10.1083/jcb.98.4.1591 (1984).
Kerjaschki, D. Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J. Clin. Investig. 108, 1583–1587. https://doi.org/10.1172/JCI14629 (2001).
Kazama, F. et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets 26, 711–719. https://doi.org/10.3109/09537104.2015.1021319 (2015).
Matsui, K., Breiteneder-Geleff, S. & Kerjaschki, D. Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J. Am. Soc. Nephrol. 9, 2013–2026. https://doi.org/10.1681/ASN.V9112013 (1998).
Hugo, C. et al. The plasma membrane-actin linking protein, ezrin, is a glomerular epithelial cell marker in glomerulogenesis, in the adult kidney and in glomerular injury. Kidney Int. 54, 1934–1944. https://doi.org/10.1046/j.1523-1755.1998.00195.x (1998).
Philippe, A. et al. A missense mutation in podocin leads to early and severe renal disease in mice. Kidney Int. 73, 1038–1047. https://doi.org/10.1038/ki.2008.27 (2008).
Astarita, J. L. et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 16, 75–84. https://doi.org/10.1038/ni.3035 (2015).
Asai, J. et al. Platelets regulate the migration of keratinocytes via podoplanin/CLEC-2 signaling during cutaneous wound healing in mice. Am. J. Pathol. 186, 101–108. https://doi.org/10.1016/j.ajpath.2015.09.007 (2016).
Langan, S. A., Navarro-Nunez, L., Watson, S. P. & Nash, G. B. Modulation of VEGF-induced migration and network formation by lymphatic endothelial cells: Roles of platelets and podoplanin. Platelets 29, 486–495. https://doi.org/10.1080/09537104.2017.1336210 (2018).
Okabe, M. et al. Global polysome analysis of normal and injured podocytes. Am. J. Physiol. Renal. Physiol. 316, F241–F252. https://doi.org/10.1152/ajprenal.00115.2018 (2019).
Gitz, E. et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 124, 2262–2270. https://doi.org/10.1182/blood-2014-05-572818 (2014).
Yamashita, Y. et al. Elevated plasma levels of soluble C-type lectin-like receptor 2 (CLEC2) in patients with thrombotic microangiopathy. Thromb. Res. 178, 54–58. https://doi.org/10.1016/j.thromres.2019.03.018 (2019).
Yamamoto, A. et al. Soluble C-type lectin-like receptor 2 is a biomarker for disseminated intravascular coagulation. J. Clin. Med. https://doi.org/10.3390/jcm10132860 (2021).
Ishikura, H. et al. Early recognition of sepsis-induced coagulopathy using the C2PAC index: A ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets https://doi.org/10.1080/09537104.2021.2019694 (2022).
Finsterbusch, M., Norman, M. U., Hall, P., Kitching, A. R. & Hickey, M. J. Platelet retention in inflamed glomeruli occurs via selective prolongation of interactions with immune cells. Kidney Int. 95, 363–374. https://doi.org/10.1016/j.kint.2018.08.042 (2019).
Shirato, I., Tomino, Y. & Koide, H. Detection of “activated platelets” in the urinary sediments using a scanning electron microscope in patients with IgA nephropathy. Am. J. Nephrol. 10, 186–190. https://doi.org/10.1159/000168079 (1990).
Tomino, Y. et al. Detection of activated platelets in urinary sediments by immunofluorescence using monoclonal antibody to human platelet GMP-140 in patients with IgA nephropathy. J. Clin. Lab. Anal. 7, 329–333. https://doi.org/10.1002/jcla.1860070606 (1993).
Kamitsuji, H., Nakajima, M., Kawahara, S. & Nishimura, T. Detection of activated platelets in urine by double immunofluorescence in children with IgA nephropathy. Nephron 78, 162–167. https://doi.org/10.1159/000044905 (1998).
Taira, K., Hewitson, T. D. & Kincaid-Smith, P. Urinary platelet factor four (Pf4) levels in mesangial IgA glomerulonephritis and thin basement membrane disease. Clin. Nephrol. 37, 8–13 (1992).
Watanabe, N. et al. A pull-down and slot blot-based screening system for inhibitor compounds of the podoplanin-CLEC-2 interaction. PLoS ONE 14, e0222331. https://doi.org/10.1371/journal.pone.0222331 (2019).
Sanz, E. et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. U S A 106, 13939–13944. https://doi.org/10.1073/pnas.0907143106 (2009).
Matsusaka, T. et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J. Am. Soc. Nephrol. 16, 1013–1023. https://doi.org/10.1681/ASN.2004080720 (2005).
Im, J. H. & Muschel, R. J. Protocol for murine/mouse platelets isolation and their reintroduction in vivo. Bio Protoc. 7, e2132. https://doi.org/10.21769/BioProtoc.2132 (2017).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP20K17257. We acknowledge Ms. Shiho Imai, Ms. Chie Sakurai and the Support Center for Medical Research and Education of Tokai University for excellent technical assistance, Ms. Yukiko Tanaka for administrative assistance and Dr. Shunya Nakayama for confocal imaging.
Author information
Authors and Affiliations
Contributions
K.T. and T.M. were involved in the conception and design of the experiments. K.T. performed the experiments. M.T., N.W., and M.I. were contributed to generating recombinant proteins. IP provided the LMB2 and related insights. K.T., M.K. and T.M. analyzed the data and interpreted the results. K.T. prepared the figures and drafted the manuscript, and T.M. critically revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, K., Tanaka, M., Watanabe, N. et al. C-type lectin-like receptor (CLEC)-2, the ligand of podoplanin, induces morphological changes in podocytes. Sci Rep 12, 22356 (2022). https://doi.org/10.1038/s41598-022-26456-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26456-9
- Springer Nature Limited