Abstract
In one pot, the self-assembly of AgNO3 and 2-chloroquinoxaline (2Cl-quinox) in water–ethanol mixture afforded two novel crystalline Ag(I) complexes. The major product is the polymeric complex [Ag(2Cl-quinox)(NO3)]n; (1), while the minor product (2) comprises two molecules which are the monomeric [Ag(2Cl-quinox)2(NO3)]; (2a) and polymeric [Ag(2Cl-quinox)(NO3)]n; (2b) complexes. The single crystal X-ray structure revealed that 1 and 2b are made up of two-dimensional infinite sheets. In contrast, 2a is a monomeric complex which has a highly distorted tetrahedral geometry around Ag(I) center. In all cases, the 2Cl-quinox molecule acts as a terminal monodentate ligand. Complexes 1 and 2b have similar molecular structures and also have almost similar crystal packing. Using Hirshfeld surface analysis, the O…H hydrogen bonds and π–π stacking interactions contributed significantly to the molecular packing. Both complexes have broad-spectrum action towards multi drug-resistance bacteria. The most effective function of 2 is against Proteus morganii, with a MIC value of 8 μg/mL. Complex 2 (IC50 = 5.93 ± 0.52 μg/mL) has remarkably greater cytotoxic effect against lung carcinoma (A-549) than cis-platin (IC50 = 7.5 ± 0.69 μg/mL) and AgNO3 (IC50 = 14.7 ± 0.53 μg/mL). The higher Ag-content in 2 could be the main reason for its higher cytotoxicity than 1.
Similar content being viewed by others
Introduction
Microbial drug resistance motivates researchers to look for new materials to treat these harmful pathogenic microbes1. This search is particularly necessary in the light of the growing number of antibiotic-resistant strains and the greater challenges associated with infection treatment. Silver has been utilized as an antibacterial agent, whether in its metallic form or as different Ag(I) compounds2,3,4,5,6. Despite the fact that N-heterocyclic ligands demonstrate diverse biological actions against various pathogens7,8,9,10, their activity is enhanced when they are coordinated with Ag(I) ion11,12. The constituents of the silver(I) complexes are likely to work together in a synergistic manner. The significant antimicrobial activities of silver(I) complexes containing N-heterocycles were approved in the literature13,14,15,16,17,18,19,20,21,22,23. The antimicrobial properties of silver complexes are expected to be influenced primarily by the nature of the atoms coordinated to the silver center and the ease of ligand replacement. Silver(I) complexes with a higher likelihood of ligand replacement by biological ligands (sulfur-containing molecules) are powerful antimicrobial agents. Therefore, it makes sense that complexes with weak metal–ligand bonds, such as those with Ag–N and Ag–O, would exhibit a broader range of antimicrobial activity than those with Ag–S and Ag–P bonds whose silver complexes have weak or no activity against bacteria, molds, and yeast24,25,26,27. Furthermore, complexes of Ag(I) have attracted attention for cancer treatments in recent years. A set of review articles emphasized on Ag(I) complexes with N-heterocycles has targeted their uses as anticancer agents against a variety of cancer cell lines28. It has been found that several Ag(I) complexes have greater cytotoxicity than cis-platin with a relatively little poisonous effects to the healthy human cells29,30,31,32,33,34,35,36.
Quinoxaline derivatives have been established to have antifungal, antibacterial, and anticancer activities37,38,39,40,41,42,43,44,45,46. The combination of quinoxaline with Ag(I) ions results in the formation of complexes with fascinating biological characteristics, which could be an interesting alternatives to the current medications47. The current aim is to create a new synergic drug by combining bio-active metal ion such as Ag(I) with an effective organic ligand such as 2-chloroquinoxaline (Fig. 1). The syntheses, as well as the spectral and structural analyses of the target complexes were performed using single crystal X-ray diffraction, FTIR and 1H NMR spectroscopic methods. Furthermore, determining the antimicrobial potential of the complexes as zone of inhibition and MIC (minimum inhibitory concentration) allowed us to compare their action to that of commercially available antibiotics. Also, the anticancer activity of the novel silver(I) complexes is discussed.
Materials and methods
Chemicals and physicochemical characterizations
All of the chemicals were obtained from Sigma-Aldrich Company. The FTIR spectra were achieved at 4000–400 cm−1 using a Bruker Tensor 37 FTIR instrument in KBr pellets. The 1H NMR spectra of the studied complexes and the free ligand were collected on a JEOL JNM-ECA 500 MHz NMR spectrometer in DMSO-d6 as solvent. Perkin Elmer 2400 Elemental Analyzer was used for the CHN analysis. The amount of Ag content was measured using a Shimadzu atomic absorption spectrophotometer (AA-7000 series, Shimadzu, Ltd., Japan). Thermogravimetric analysis was carried out using a LINSEIS STA PT100 thermogravimetric analyzer in a platinum cell with a heating rate of 10 °C/min and under a nitrogen flow of 40 mL/min.
Syntheses of [Ag(2Cl-quinox)(NO3)]; (1) and [Ag3(2Cl-quinox)4(NO3)3]; (2)
At room temperature, an ethanolic solution (10 mL) of 2Cl-quinox (165 mg, 1 mmol) was added to a 10 mL aqueous solution of silver(I) nitrate (170 mg, 1 mmol). This mixture was left at room temperature and allowed to evaporate slowly. Complex 1 was obtained as colorless plate crystals after a few days. Near the dryness of the solution, complex 2 is obtained as needle crystals. The obtained crystals were found suitable for X-ray single crystal measurements. Schematic presentation for the synthesis of complexes 1 and 2 is shown in Fig. 2.
[Ag(2Cl-quinox)(NO3)]n; (1) (74% yield). Anal. Calc. C8H5AgClN3O3: C, 28.73; H, 1.51; N, 12.56; Ag, 32.25%. Found: C, 28.92; H, 1.46; N, 12.40; Ag, 32.01%. FTIR cm−1: 3519, 3446, 3056, 2395, 1540, 1489, 1383, 1155, 1093, 960, 760, 591, 454, 415. 2Cl-quinox: 3388, 3041, 2894, 1675, 1610, 1558, 1518, 1396, 1309, 1182, 763, 625, 580, 520, 466. (Figs. S1–S2, Supplementary data). 1H NMR (500 MHz, DMSO-d6) δH (ppm): 8.99 (s, 1H, C(3)H), 8.12 (d, 1H, C(8)H), 8.03 (d, 1H, C(5)H), 7.89 (t, 2H, C(6)H and C(7)H). Ligand (2Cl-quinox): 8.97 (s, 1H, C(3)H), 8.12 (d, 1H, C(8)H), 8.03 (d, 1H, C(5)H), 7.89 (t, 2H, C(6)H and C(7)H) (Figs. S3–S4, Supplementary data).
[Ag3(2Cl-quinox)4(NO3)3]; (2) Anal. Calc. C32H20Ag3Cl4N11O9: C, 32.91; H, 1.73; N, 13.19; Ag, 27.71%. Found: C, 32.76; H, 1.68; N, 13.02; Ag, 27.53%. FTIR cm−1: 3059, 2400, 1687, 1540, 1489, 1383, 1304, 1249, 1156, 1095, 961, 761, 591, 457, 416. (Fig. S5, Supplementary data). 1H NMR (500 MHz, DMSO-d6) δH (ppm): 8.94 (s, 3H, C(3)H), 8.12 (d, 3H, C(8)H), 8.03 (d, 3H, C(5)H), 7.90 (t, 6H, C(6)H and C(7)H). (Fig. S6, Supplementary data).
Crystal structure analysis
A Bruker APEX II diffractometer with graphite monochromated MoKα radiation was used to determine the crystal structures of the investigated complexes. Table S1 (Supplementary data) contains all information pertaining to the crystallographic measurements. SADABS was used for absorption corrections48 and all calculations were carried out using SHELXTL program package49.
Hirshfeld surface analysis
The Crystal Explorer Ver. 3.150 program package was used to analyze the Hirshfeld surface and draw the 2D fingerprint plots.
Biological studies
Determination of antimicrobial and cytotoxic activities
The antimicrobial evaluation of the Ag(I) complexes towards Gram-positive bacteria, Gram-negative bacteria, and harmful yeasts was determined11. More details are found in Method S1 (Supplementary data). The cytotoxic activity of the Ag(I) complexes against the lung carcinoma (A-549) and breast carcinoma (MCF-7) cell lines was determined using the procedure described in Method S2 (Supplementary data)51.
Results and discussion
X-ray crystal structure descriptions
Crystal structure of [Ag(2Cl-quinox)(NO3)]n; (1)
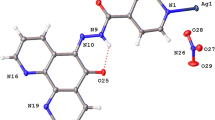
The complex [Ag(2Cl-quinox)(NO3)]n crystallizes in the monoclinic space group C2/c with Z = 8 and one [Ag(2Cl-quinox)(NO3)] monomeric unit as an asymmetric formula. Figure 3 shows the coordination geometry and the atom numbering scheme of this complex. The monomeric unit of this two-dimensional infinite coordination polymer includes one organic ligand per AgNO3. Selected interatomic distances and bond angles are presented in Table S2 (Supplementary data). The geometry around the Ag(I) ion in complex 1 could be considered as a distorted triangular planar augmented by strong argentophilic interaction with the symmetry related Ag1# atom (Symm. code: # 1/2 − x, 1/2 − y, 2 − z) which forms the tip of a triangular pyramid. The base of this triangular pyramid is constructed by the atoms: O1 and O2# (Symm. code: # 1/2 − x, 1/2 + y, 2.5 − z) of the nitrate anions and N1 of the quinoxaline ligand. The nitrate anion acts as a bridging ligand between the Ag(I) sites via Ag(1)–O(1) and Ag(1)–O(2) bonds with silver to oxygen distances of 2.5502(15) and 2.2602(14) Å, respectively. An additional weak Ag1…O3 interaction has a distance of 2.829 Å which is significantly long and could not be considered as a bond. Hence, the coordination number of the Ag(I) is 3.
As shown in Fig. 4, the crystal structure of 1 is made up of two-dimensional infinite sheets that expand perpendicular to the a-direction of the unit cell via strong Ag–O and Ag–Ag bonds. The alternating silver Ag1 and the oxygen atoms O1 and O2 of the bridging nitrate groups are connected in a wavy-like and coplanar pattern along the crystallographic b-direction, while the organic ligand units are arranged in a highly symmetric manner below and above the polymer array. These parallel polymeric chains are cross-linked by the argentophillic interactions along the c-direction in the unit cell where the Ag1−Ag1# (Symm.: #1/2 − x, 1/2 − y, 2 − z) bond distance is 3.1008(4) Å. This distance is significantly short, indicating significant argentophillic interactions52 which are responsible for the extension of the 2D polymer along the c-direction. As we note in the two units attached by the argentophilic interaction, the two quinoxaline ligands have an anti-configuration to one another.
In addition to the argentophilic interactions which play significant role in the polymer expansion along c-direction, there are many π–π stacking interactions between the quinoxaline rings which are found approximately parallel to each other. The shortest C…C contacts are C1…C6 (3.395 Å), C3…C4 (3.299 Å), C2…C6 (3.391 Å) and C2…C7 (3.242 Å) interactions. For better clarity, these π-π stacking interactions are displayed as yellow dotted lines in Fig. 5. Moreover, the distance between the ring centroids C3C4C5C6C7C8 and C1C2C3C4N1N2 is 3.528 Å which agrees with the reported values for the π–π stacking interactions in the aromatic six membered ring system53. Also, there are additional Ag…C interactions such as Ag1…C7 and Ag1…C8 contacts (Fig. 5). The corresponding interaction distances are 2.945 and 3.029 Å, respectively. Besides these interactions, the complex units are packed together via the hydrogen bridge bonds such as O…H (along the b-axis) and N…H (along the a-axis) contacts as given in Table S3 (Supplementary data) and displayed in Fig. 5.
Crystal structure of [Ag3(2Cl-quinox)4(NO3)3]; (2)
The crystal structure of complex 2 is presented in Fig. 6 while the crystal refinement data is listed in Table S1, Supplementary data. Also, it crystallizes in the monoclinic centrosymmetric space group C2/c. The parameters of the unit cell are a = 50.235(2) Å, b = 9.7041(4) Å, c = 7.3220(3) Å and β = 91.536(2)° while the unit cell volume is 3568.1(3) Å3. It consists of two different complex molecules: [Ag(2Cl-quinox)2(NO3)]; (2a) and [Ag(2Cl-quinox)(NO3)]n; (2b) which represent the monomeric and polymeric parts of this complex, respectively. The crystal structure of complex 2b is almost the same as that for complex 1, so the discussion of complex 2 will focus only on the monomer 2a. In this monomeric complex , the Ag(I) is coordinated with two 2Cl-quinox ligand units through the heterocyclic N-atom where the two ligand units are in syn configuration to one another, and two oxygen atoms belonging to a bidentate nitrate group (O1 and O1#, Symmetry code #: − x, y, 1/2 − z) indicating a highly distorted tetrahedral geometry around Ag(I) center. Because of the symmetry consideration, the two Ag–N bonds are equidistant (2.243(2) Å). The same is true for the two Ag–O bonds (2.613(2) Å) (Table S4, Supplementary data). It is clear that the two coordinating quinoxaline systems are nearly co-planar, where the angle between their ring planes is only 3.7° (Fig. S7, Supplementary data). Also, the mean plane passing through the two quinoxaline moieties adopts an angle of 61.31◦ to the plane constructed from the bidentate nitrate group (O1, O1#, O2, N3).
Displacement ellipsoids drawing (50% probability) of 2, showing the atom-numbering scheme. It consists of two complex units, 2a (left) and 2b (right). The Ag2–O3#1 and Ag2–Ag2#2 bonds in 2b, are shown with a broken line, because the O3#1 and Ag2#2 atoms belong to the neighboring monomer units, but still belong to the coordination sphere of the silver atom. Symmetry codes to generate O3#1 and Ag2#2 are 1/2 − x, − 1/2 + y, − 1/2 − z and 1/2 − z, 1/2 − y, − z, respectively.
Also, the packing of complex 2 include some π–π stacking contacts between the aromatic ring π-systems of 2a where the C2…C6 (3.231 Å) and C3…C7 (3.386 Å) are the most important π-π interactions. Also, the centroid–centroid distances are found to be in the range 3.647–3.851 Ǻ, which are generally larger than those found in 1 (Fig. 7). The 2a units in complex 2 are also packed together via Ag1…C6 (3.172 Å) and Ag1…C7 (3.236 A˚) intermolecular interactions as well as the O…H hydrogen bridge bonds. Details regarding the hydrogen bond parameters are given in Table S5 (Supplementary data). In addition, the two complexes 2a and 2b are interconnected in the crystal structure of complex 2 via N2…H12 (2.708 Å) and N5…H4 (2.503 Å) hydrogen bonds. For better clarity, these interactions are displayed as violet dotted lines in Fig. 7.
In comparison between complexes 1 and 2, the molecular packing of the former is simply described as a 2D coordination polymer via the nitrate ion as connector between Ag(I) sites which are further connected by Ag–Ag bonds (Fig. 4). On the other hand, the molecular packing of 2 is built from the alternative arrangement of the monomeric complex 2a and 2D polymer chain 2b (Fig. 8).
Unlike complexes 1 and 2, the previously reported X-ray structure of the structurally related [Ag(quinoxaline)]n(NO3)n complex revealed its polymeric nature through the two nitrogen atoms of quinoxaline as a connector ligand between the Ag(I) sites47. Generally, this bridging mode of the quinoxaline ligands is usual in its complexes54,55. Unexpectedly, the 2-chloroquinoxaline in the studied complexes acts as a terminal monodentate ligand rather than a connector where the coordination with Ag(I) occurs only through one nitrogen atom while the other nitrogen does not participate in coordination with Ag(I) as a consequence of the decrease in its electron density due to its proximity to the electron attracting group (chlorine atom). In contrast, the freely uncoordinated N-atoms contribute significantly to the molecular packing by connecting the parts 2a and 2b via N…H hydrogen bonding interactions.
FTIR spectra
The infrared spectra of complexes 1 and 2 compared to the ligand, 2Cl-quinox, have been measured and are shown in Figs. S1, S2 and S5 (Supplementary data). The IR spectrum of complex 1 is very similar to that of complex 2. The bands appeared in the range of 3041–3059 cm−1 in the FTIR spectra of the complexes, and their free ligand could be assigned to the ν(C–H) aromatic stretching vibrations. The bands appear at 1610 cm−1 for the ligand and 1540 cm−1 for both complexes can be attributed to the stretching vibrational frequency of the C=N bond. As we note, the observable shift of ν(C=N) band to a lower frequency in the spectra of both complexes indicates its involvement in the coordination to the silver metal. In the FTIR spectra of complexes 1 and 2, the presence of a sharp spectral band at 1383 cm−1 indicates the presence of the nitrate group, which doesn’t exist in the FTIR spectrum of the free ligand. Furthermore, in the regions of 591 and 414–457 cm−1, some new moderate-intensity bands are detected in the complexes spectra. These bands might be attributed to the vibrational modes of the Ag–O and Ag–N bonds, respectively56.
NMR spectra
The 1H NMR spectra of 2Cl-quinox compared to the complexes 1 and 2 are presented in Figs. S3, S4 and S6 (Supplementary data) while the chemical shifts and their assignments are summarized in Table S6 (Supplementary data) which shows no important shifts upon complexation of the free ligand. This is generally known for silver (I) complexes with N-donor ligands, which have relatively weak Ag–N and Ag–O interactions57. The spectral data is consistent with the structure of the organic part of complexes 1 and 2. The 1H NMR spectra of 2Cl-quinox and the corresponding Ag(I) complexes give four signals associated to the aromatic protons. Two doublet signals for the protons C(5)H and C(8)H, one triplet signal for C(6)H and C(7)H, and one singlet peak for C(3)H. The chemical shift values decrease in order C(3)H > C(8)H > C(5)H > C(6)H and C(7)H (Table S6, Supplementary data). The singlet peak for C(3)H has the largest chemical shift value as it is adjacent to the chlorine and nitrogen atoms. These NMR spectra give a good indication on the purity of the studied complexes.
Thermal analysis
Thermogravimetric (TG) and differential thermal analyses (DTA) curves for complexes 1 and 2 are displayed in Fig. S8 (Supplementary data), while the summary of their thermal decomposition is listed in Table S7 (Supplementary data). The TGA and DTA curves revealed that complexes 1 and 2 are thermally decomposed in two successive decomposition steps. The first one is an endothermic step, while the second one is exothermic. For 1, the first step represents the removal of the ligand with an estimated loss of 49.639% (theoretical loss = 49.211%) up to a temperature of 220 °C. The second step occurred within the temperature range 220–600 °C with an estimated loss of 18.175% (theoretical loss of about 18.539%), which is reasonably accounted for the successive liberation of [NO2 + 0.5O2], leaving a residue of metallic silver [Ag] of about 32.186% (theoretical mass about 32.251%). On the other hand, four molecules of 2Cl-quinox are lost in the first step of the thermal decomposition of complex 2 up to 240 °C, with an estimated loss of 55.423% (theoretical loss of about 56.367%). The second step started at a temperature of 240 °C, leaving a residue of 27.939% for the metallic silver with a theoretical mass percent 27.706% at the end of the thermal decomposition (800 °C) with an estimated loss of 15.638% (theoretical loss = 15.927%) which is reasonably accounted for the successive liberation of 3[NO2 + 0.5O2].
Analysis of Hirshfeld surface
Qualitative and quantitative analyses of the intermolecular contacts in the investigated Ag(I) complexes were carried out utilizing Hirshfeld surface analysis. In contrast to 1, the asymmetric unit of complex 2 consists of two different complex molecules, 2a and 2b, which represent the monomeric and polymeric parts of this complex, respectively (Fig. 6). Hence, the Hirshfeld surfaces of each molecule are calculated separately. The contributions of all intermolecular contacts to the total fingerprint plots of the crystalline structure of complexes 1, 2a, and 2b are depicted in Fig. 9. Hirshfeld analysis illuminated the importance of the O…H, H…Cl, N…H, C…H, H…H, C…C and C…N interactions on the packing of the crystal structure of the investigated compounds.
The dnorm maps for the most important contacts in the complexes under investigation are shown in Fig. 10. Interestingly, complexes 1 and 2b have slightly different packing and almost the same interaction patterns. The most significant interactions in 1 and 2b, which have almost similar structure, are Ag–O, Ag–Ag, N…H and Cl…Cl. In both cases, the Ag–O and Ag–Ag contacts occurred within the polymeric part of 1 and 2b. In contrast, the N…H and Cl…Cl interactions in complex 1 are different from those in 2b. In the former, the N…H and Cl…Cl interactions occur among the 2D polymer chains. The situation is totally different in complex 2, where the N…H and Cl…Cl interactions occur between the 2D polymer chains of 2b and monomeric units of 2a (Fig. 10).
The O…H interactions play a significant role in the crystal packing of the three complex units, as shown in Fig. 11. Their percentages to the overall fingerprint plots are 31.3, 20.5, and 31.9% for 1, 2a, and 2b, respectively (Fig. 9). The important role of these interactions in the packing was confirmed by the red spots in the decomposed dnorm maps and the sharp spikes pointing to the bottom left corner of the fingerprint plots presented in Fig. 11. These O…H contacts are owing to the interactions that took place between the O-atoms of the nitrate group and the C–H protons from the quinoxaline moiety. It’s worth noting that complexes, 1, 2a, and 2b also have a lot of intermolecular H…Cl interactions which contribute by 14.1, 18.2, and 14.2%, respectively (Fig. 9).
Another important feature of crystal packing is further revealed using Hirshfeld surface analysis which is the π–π stacking interactions. As shown in Fig. 12, all proofs for π–π interactions, including red zones in dnorm and red/blue triangles in the shape index with relatively flat areas on the curvedness map, were accomplished. Furthermore, there is a proper amount of C…C and C…N interactions in the molecular structure of the three complex units, as indicated by the fingerprint plots. The contributions of the C…C intermolecular interactions are 7.2, 8.2, and 5.9% of the overall fingerprint plots of 1, 2a, and 2b respectively. Also, the C…N percentages are 5.9, 6.4, and 4.5%, respectively (Fig. 9).
The polymeric nature of 1 and 2b was clearly identified by the intense red spots neighbouring the Ag atom in the corresponding dnorm maps, which are related to the Ag–O and Ag–Ag bonding with the adjacent complex units to construct the 2D coordination polymer, while the molecular compound 2a is a monomeric complex (Fig. 13).
Antimicrobial activity
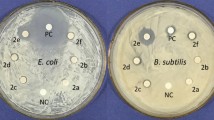
Ag(I) complexes based on various N-heterocyclic ligands have been approved to have significant biological properties11,58. In this regard, the antimicrobial activities of the studied complexes 1 and 2 were examined. The Minimum Inhibition Concentrations (MIC) of these complexes against MDR microbes were determined, and the results were compared with the activities of some commercial antibiotics. The bacteria used in this study are Gram-negative bacteria (Proteus morganii, Escherichia coli, and Serratia marcescens) and Gram-positive bacteria (Bacillus cereus, Micrococcus luteu, and Staphylococcus aureus). The results are presented in Table 1 and displayed graphically in Fig. S9 (Supplementary data).
In general, it can be seen from Table 1 that the free 2Cl-quinox ligand is inactive against all tested bacteria (MIC ≥ 256 µg/mL) except Staphylococcus aureus (MIC = 40 µg/mL). On the contrary, complexes 1 and 2 outperformed their free ligand, 2Cl-quinox, against all tested bacteria except Staphylococcus aureus and E. coli. Interestingly, complex 2 have better activity than AgNO3 against all tested bacteria. The same is true for complex 1 except against Staphylococcus aureus and E. coli. Complex 1 has good antibacterial potential against Micrococcus luteu, Bacillus cereus, Proteus morganii (MIC = 32 µg/mL) and Serratia marcescens (MIC = 40 µg/mL) which could be comparable to antibiotics such as Levofloxaciniii, Cefepimeiv, Cefoperazoneiii, Azithromycin, Cefuroximeii, and Gentamicin. Also, complex 2 exhibited excellent and remarkable efficacy towards all tested microbes, with MIC values ranging between 8 and 48 µg/mL. As a result, it has broad-spectrum activity against MDR (multi drug-resistance) bacteria. The most effective function of 2 is against Proteus morganii, with a MIC value of 8 μg/mL. As a result, it is superior to the best-performing antibiotics, Cefoperazoneiii (MIC = 32 µg/ml) and Cefepimeiv (MIC = 24 µg/ml). Also, Table 2 shows the diameters of the inhibition zones resulting from the treatment of these microbes by the studied compounds. They are often consistent with the MIC results, confirming the broad-spectrum activity of both complexes.
In addition, the investigated complexes have the same antifungal activity against Candida albicans as Ketoconazole as a standard antifungal medication (MIC = 312 µg/mL). On the other hand, both complexes have no antifungal action against Aspergillus fumigatus at the applied concentration (MIC = 1250 µg/mL). Based on literature, the antimicrobial properties of Ag(I)-complexes of weak metal–ligand bonds, such as those with Ag–N and Ag–O are expected to be exhibit a broader range of antimicrobial activity than those with Ag–S and Ag–P bonds24,25,26,27. The results presented in this work are found to be in accordance with the literature.
Cytotoxic activity
The MTT technique was applied to evaluate the in vitro cytotoxic activity of the studied Ag(I) complexes compared to the free ligand and silver nitrate against the two human lung (A-549) and breast (MCF-7) cancer cell lines. The results are also compared with the clinical anticancer drug, cis-platin, under the same experimental conditions. The results of the IC50 values in μg/mL are listed in Tables S8–S13 and visually presented in Fig. S10 (Supplementary data).
The studied silver(I) complexes demonstrated high levels cytotoxicity over their free ligand against the examined tumour cell lines. Complex 1 has potential activity against both cancerous cell lines but slightly less than cis-platin (Table 3). Interestingly, complex 2 has remarkable greater cytotoxic behaviour (IC50 = 5.93 ± 0.52 μg/mL) against lung carcinoma (A-549) than cis-platin (IC50 = 7.5 ± 0.69 μg/mL) and AgNO3 (IC50 = 14.70 ± 0.53 μg/mL). This confirms that, the antitumor potential of Ag(I) complexes is related to both the ligand and the metal ion12,20,33. In addition, complex 2 exhibits moderate activity (IC50 = 9.77 ± 0.74 μg/mL) against the MCF-7 cell line in comparison to cis-platin (IC50 = 4.59 ± 0.53 μg/mL) and AgNO3 (IC50 = 2.81 ± 0.97 μg/mL). Hence, AgNO3 has the most anticancer effect against the MCF-7 cell line. As we note from Table 3, the cytotoxicity of complex 2 towards both cancerous cell lines is greater than that of complex 1. This might be attributed to the higher content of silver in complex 2 than in 1. Similar to the previously reported Ag(I) complexes12,33 1 and 2 showed higher activity towards lung carcinoma (A-549) than breast carcinoma (MCF-7) (Table 3).
Conclusion
Two novel Ag(I) complexes were self-assembled from the reaction of 2-chloroquinoxaline and silver nitrate in one pot. The single crystal structure of complex 1 comprised a 2D polymer of [Ag(2Cl-quinox)(NO3)]n sheets via Ag–O and Ag–Ag bondings, while the crystal structure of complex 2 comprised alternating arrays of the [Ag(2Cl-quinox)(NO3)]n 2D polymer and the [Ag(2Cl-quinox)2(NO3)] monomer. Unusually, the 2-chloroquinoxaline ligand in the studied complexes acts as a terminal monodentate ligand and is not involved in the infrastructure of the 2D coordination polymer. Thermogravimetric analysis, FTIR, and NMR spectra are consistent with the complexes’ structures. Both complexes demonstrated broad-spectrum activity against multi drug-resistance (MDR) bacteria. Moreover, the antibacterial potency of complex 2 with a MIC value of 8 μg/mL is higher than that of reference drugs, Cefepimeiv and Cefoperazoneiii and AgNO3 in case of Proteus morganii. The cytotoxicity of complex 2 towards lung carcinoma A-549 and breast carcinoma MCF-7 cell lines is greater than that of complex 1. This might be attributed to the higher silver content in complex 2 than complex 1.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Sharkey, M. A. et al. Investigations into the antibacterial activity of the silver-based antibiotic drug candidate SBC3. Antibiotics 1, 25–28 (2012).
Silver, S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 27, 341–353 (2003).
Russell, A. D. & Hugo, W. B. 7 antimicrobial activity and action of silver. Prog. Med. Chem. 31, 351–370 (1994).
Chernousova, S. & Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chemie Int. Ed. 52, 1636–1653 (2013).
Simončič, B. & Klemenčič, D. Preparation and performance of silver as an antimicrobial agent for textiles: A review. Int. J. Text. Sci. 86, 210–223 (2015).
Kim, J. S. et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 3, 95–101 (2007).
Gavade, S. N., Markad, V. L., Kodam, K. M., Shingare, M. S. & Mane, D. V. Synthesis and biological evaluation of novel 2,4,6-triazine derivatives as antimicrobial agents. Bioorganic Med. Chem. Lett. 22, 5075–5077 (2012).
Jeong, L. S. et al. Synthesis and antitumor activity of fluorocyclopentenyl-pyrimidines. Nucleosides Nucleotides Nucl. Acids 26, 713–716 (2007).
Patel, N. B., Agravat, S. N. & Shaikh, F. M. Synthesis and antimicrobial activity of new pyridine derivatives-I. Med. Chem. Res. 20, 1033–1041 (2011).
Imanishi, M. et al. Sequential synthesis, olfactory properties, and biological activity of quinoxaline derivatives. ACS Omega 2, 1875–1885 (2017).
Abu-Youssef, M. A. M. et al. Synthesis, crystal structure, quantum chemical calculations, DNA interactions, and antimicrobial activity of [Ag(2-amino-3-methylpyridine)2]NO3 and [Ag(pyridine-2-carboxaldoxime)NO3]. Inorg. Chem. 49, 9788–9797 (2010).
El-Naggar, M. A. et al. New bioactive 1D Ag(I) coordination polymers with pyrazole and triazine ligands; Synthesis, X-ray structure, Hirshfeld analysis and DFT studies. Inorg. Chim. Acta 537, 120948 (2022).
Celik, S., Yurdakul, S. & Erdem, B. New silver(I) complex as antibiotic candidate: synthesis, spectral characterization, DFT, QTAIM and antibacterial investigations and docking properties. J. Mol. Struct. 1261, 132902 (2022).
Mather, J. C., Wyllie, J. A., Hamilton, A., da Soares Costa, T. P. & Barnard, P. J. Antibacterial silver and gold complexes of imidazole and 1,2,4-triazole derived N-heterocyclic carbenes. Dalt. Trans. 51, 12056–12070 (2022).
Bongoza, U., Zamisa, S. J., Munzeiwa, W. A. & Omondi, B. Silver(I) complexes of N, N′-diarylformamidine ligands: synthesis, crystal structures, and in vitro antibacterial studies. Appl. Organomet. Chem. 36, e6726 (2022).
Santos Oliveira, I. et al. Antibacterial and antifungal activities in vitro of a novel silver(I) complex with sulfadoxine-salicylaldehyde Schiff base. Polyhedron 225, 116073 (2022).
Munawar, A., Abubakar, M., Altaf, M. & Ashraf, R. S. Scientific investigation into silver(I) coordination complexes and their antibacterial studies with asima munawar. SSRN Electron. J. (2022).
Özdemir, I. et al. Synthesis, characterization and antimicrobial activity of new silver complexes with N-heterocyclic carbene ligands. Inorg. Chim. Acta 363, 3803–3808 (2010).
Vargová, Z. et al. New silver(I) pyridinecarboxylate complexes: synthesis, characterization, and antimicrobial therapeutic potential. J. Coord. Chem. 67, 1002–1021 (2014).
Medici, S. et al. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 327, 349–359 (2016).
Savić, N. D. et al. Silver(I) complexes with quinazoline and phthalazine: synthesis, structural characterization and evaluation of biological activities. Medchemcomm 7, 282–291 (2016).
El-Naggar, M. A. et al. Synthesis, X-ray structure, Hirshfeld, and antimicrobial studies of new Ag(I) complexes based on pyridine-type ligands. J. Mol. Struct. 1264, 133210 (2022).
Badr, A. M. A. et al. Structure, antimicrobial activity, hirshfeld analysis, and docking studies of three silver(I) complexes-based pyridine ligands. Appl. Sci. 10, 4853 (2020).
Nomiya, K., Tsuda, K., Sudoh, T. & Oda, M. Ag(I)-N bond-containing compound showing wide spectra in effective antimicrobial activities: Polymeric silver(I) imidazolate. J. Inorg. Biochem. 68, 39–44 (1997).
Nomiya, K., Noguchi, R. & Oda, M. Synthesis and crystal structure of coinage metal(I) complexes with tetrazole (Htetz) and triphenylphosphine ligands, and their antimicrobial activities. A helical polymer of silver(I) complex [Ag(tetz)(PPh3)2]n and a monomeric gold(I) complex [Au(tetz)(PPh3)]. Inorg. Chim. Acta 298, 24–32 (2000).
Nawaz, S. et al. Synthesis, characterization and antimicrobial studies of mixed ligand silver(I) complexes of triphenylphosphine and heterocyclic thiones: crystal structure of bis[{(μ2-diazinane-2-thione)(diazinane-2-thione)(triphenylphosphine)silver(I) nitrate}]. Polyhedron 30, 1502–1506 (2011).
Isab, A. A. et al. Synthesis, characterization and antimicrobial studies of mixed ligand silver(I) complexes of thioureas and triphenylphosphine; crystal structure of {[Ag(PPh3)(thiourea)(NO3)]2·[Ag(PPh3)(thiourea)]2(NO3)2}. Polyhedron 29, 1251–1256 (2010).
Raju, S. K. et al. Silver complexes as anticancer agents: A perspective review. Ger. J. Pharm. Biomater. 1, 6–28 (2022).
Medici, S. et al. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 284, 329–350 (2015).
Tan, S. J., Yan, Y. K., Lee, P. P. F. & Lim, K. H. Copper, gold and silver compounds as potential new anti-tumor metallodrugs. Future Med. Chem. 2, 1591–1608 (2010).
Zaki, M., Arjmand, F. & Tabassum, S. Current and future potential of metallo drugs: Revisiting DNA-binding of metal containing molecules and their diverse mechanism of action. Inorganica Chim. Acta 444, 1–22 (2016).
Banti, C. N. & Hadjikakou, S. K. Anti-proliferative and anti-tumor activity of silver(I) compounds. Metallomics 5, 569–596 (2013).
Altowyan, M. S. et al. Synthesis, X-ray structure, antimicrobial and anticancer activity of a novel [Ag(ethyl-3-quinolate)2(citrate)] complex. Crystal 12, 356 (2022).
Ali, K. A., Abd-Elzaher, M. M., Mahmoud, K. Synthesis and anticancer properties of silver(I) complexes containing 2,6-Bis(substituted)pyridine derivatives. Int. J. Med. Chem. 2013, (2013).
Tan, X. J. et al. Synthesis, characterization and in vitro cytotoxic properties of new silver(I) complexes of two novel Schiff bases derived from thiazole and pyrazine. Polyhedron 71, 119–132 (2014).
Puszyńska-Tuszkanow, M. et al. Silver(I) complexes with hydantoins and allantoin: Synthesis, crystal and molecular structure, cytotoxicity and pharmacokinetics. J. Inorg. Biochem. 105, 17–22 (2011).
Irfan, A. et al. Recent updates on the synthesis of bioactive quinoxaline-containing sulfonamides. Appl. Sci. 11, 5702 (2021).
Singh, D. P., Deivedi, S. K., Hashim, S. R. & Singhal, R. G. Synthesis and antimicrobial activity of some new quinoxaline derivatives. Pharmaceuticals 3, 2416–2425 (2010).
Tariq, S., Somakala, K. & Amir, M. Quinoxaline: An insight into the recent pharmacological advances. Eur. J. Med. Chem. 143, 542–557 (2018).
Kaushal, T., Srivastava, G., Sharma, A. & Singh Negi, A. An insight into medicinal chemistry of anticancer quinoxalines. Bioorg. Med. Chem. 27, 16–35 (2019).
Khatoon, H. & Abdulmalek, E. Novel synthetic routes to prepare biologically active quinoxalines and their derivatives: A synthetic review for the last two decades. Molecules 26, 1055 (2021).
Waring, M. J. et al. 2,3-bifunctionalized quinoxalines: Synthesis, DNA interactions and evaluation of anticancer, anti-tuberculosis and antifungal activity. Molecules 7, 641–656 (2002).
Carta, A., Sanna, P., Gherardini, L., Usai, D. & Zanetti, S. Novel functionalized pyrido[2,3-g]quinoxalinones as antibacterial, antifungal and anticancer agents. II Farmaco 56, 933–938 (2001).
Al-Marhabi, A. R., Abbas, H. A. S. & Ammar, Y. A. Synthesis, characterization and biological evaluation of some quinoxaline derivatives: A promising and potent new class of antitumor and antimicrobial agents. Molecules 20, 19805–19822 (2015).
Husain, A. & Madhesia, D. Recent advances in pharmacological activities of quinoxaline derivatives. J. Pharm. Res. 4, 924–929 (2011).
El-Sawy, E., Bassyouni, F., Abu-Bakr, S., Rady, H. & Abdlla, M. Synthesis and biological activity of some new 1-benzyl and 1-benzoyl-3-heterocyclic indole derivatives. Acta Pharm. 60, 55–71 (2010).
Abu-Youssef, M. A. M., Langer, V. & Öhrström, L. Synthesis, a case of isostructural packing, and antimicrobial activity of silver(I)quinoxaline nitrate, silver(I)(2,5-dimethylpyrazine) nitrate and two related silver aminopyridine compounds. Dalt. Trans. 4, 2542–2550 (2006).
Sheldrick, G. M. & SADABS,. Program for Empirical Absorption Correction of Area Detector Data (University of Gottingen, 1996).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. A. 71, 3 (2015).
Spackman, M. A. & Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 11, 19–32 (2009).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983).
der Bondi, A. V. Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964).
Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalt. Trans. 3885–3896 (2000).
Saha, S., Peña, B. & Dunbar, K. R. Partially solvated dinuclear ruthenium compounds bridged by quinoxaline-functionalized ligands as Ru(II) photocage architectures for low-energy light absorption. Inorg. Chem. 58, 14568–14576 (2019).
Steel, P. J. & Fitchett, C. M. Metallosupramolecular silver(I) assemblies based on pyrazine and related ligands. Coord. Chem. Rev. 252, 990–1006 (2008).
Masoud, M. S., Ali, A. E., Elasala, G. S. & Elwardany, R. E. Structural and thermal studies on some morpholine complexes. J. Mol. Struct. 1175, 648–662 (2019).
Khlobystov, A. N. et al. Supramolecular design of one-dimensional coordination polymers based on silver(I) complexes of aromatic nitrogen-donor ligands. Coord. Chem. Rev. 222, 155–192 (2001).
Abu-Youssef, M. A. M. et al. Synthesis and structure of silver complexes with nicotinate-type ligands having antibacterial activities against clinically isolated antibiotic resistant pathogens. Inorg. Chem. 46, 5893–5903 (2007).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A.E.: methodology, writing original draf, data curation, discussion and validation. M.M.S.: supervision. J.H.A.: writing. M.A.M.A.: supervision, review and editing. T.S.K..: supervision. S.M.S.: methodology, data curation, supervision, writing, review and editing. A.M.A.B.: writing review, editing and supervision. At the end, this manuscript has resulted by the collaboration of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Naggar, M.A., Sharaf, M.M., Albering, J.H. et al. One pot synthesis of two potent Ag(I) complexes with quinoxaline ligand, X-ray structure, Hirshfeld analysis, antimicrobial, and antitumor investigations. Sci Rep 12, 20881 (2022). https://doi.org/10.1038/s41598-022-24030-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24030-x
- Springer Nature Limited