Abstract
Despite various intraoperative thermal strategies, core heat loss is considerable during liver transplantation and hypothermia is common. We tested whether forced-air prewarming prevents hypothermia during liver transplantation. Adult patients undergoing living donor liver transplantation were randomly assigned to non-prewarming group (n = 20) or prewarming group (n = 20). Patients in prewarming group underwent 30-min forced-air warming before anesthetic induction. During surgery, core temperature was measured in the pulmonary artery. The primary outcome was intraoperative hypothermia (< 36.0 °C). The secondary outcomes included plasma lactate concentration. Intraoperative hypothermia risk was significantly lower in prewarming group than in non-prewarming group (60.0% vs. 95.0%, P = 0.020). The difference in hypothermia incidence between groups was greater in the post-induction phase (20.0% vs. 85.0%, P < 0.001) than in the anhepatic or post-reperfusion phase, suggesting that prewarming mainly acts on preventing post-induction core-to-peripheral heat redistribution. Hypothermia duration was significantly shorter in prewarming group (60 [0–221] min vs. 383 [108–426] min, P = 0.001). Lactate concentration decreased during 3 h after graft reperfusion in prewarming group, whereas it continuously increased in non-prewarming group (− 0.19 [− 0.48 to 0.13] mmol/L vs. 1.17 [3.31–0.77] mmol/L, P = 0.034). In conclusion, forced-air prewarming decreases the incidence and duration of intraoperative hypothermia with potential clinical benefit while mainly acting by preventing the core-to-peripheral heat redistribution.
Clinical trial registration: Registered at the Clinical Research Information Service (https://cris.nih.go.kr, [KCT0003230]) on 01/10/2018.
Similar content being viewed by others
Introduction
Liver transplantation is attended with various intraoperative metabolic disturbances. Significant decrease in core temperature is one of them and results from decreased baseline functional reserve, extended surgical procedures, large exposure of intra-abdominal tissues, and the absence of hepatic heat production along with the insertion of cooled liver during the anhepatic phase1. Normally, core temperature is strictly controlled ranging from 36.5 to 37.5 °C to offer optimal thermal environment for various cells2. In contrast, hypothermic environment prevents optimal cell function and results in complications such as coagulopathy, immunomodulation, arrhythmia, and cardiac dysfunction as well as decreased tissue recovery from damage3,4,5,6,7,8. In particular, the newly transplanted liver graft is more vulnerable to any damages since it suffers from overload initiating rapid liver regeneration while performing full metabolic functions9,10,11. In this situation, hypothermia is thought to prevent from triggering various signals for active liver regeneration and increase the risk of graft failure12. Accordingly, intraoperative thermal managements have long been an issue in liver transplantation, and various thermal strategies have been introduced13,14,15. However, hypothermia is still not rare and the optimal thermal strategy remains unresolved.
Intraoperative core temperature drops dominantly immediately after the start of general anesthesia when the heat of the core compartment translates into the peripheral compartment due to dilatation of the peripheral vascular system which acts as the thermal barrier between the two compartments and controls the balance between core heat and peripheral heat16. This is not different in liver transplantation15; thus, it is important to prevent the core-to-peripheral heat redistribution during the post-induction phase to maintain intraoperative normothermia during liver transplantation. In this regard, previous studies in various surgical settings have suggested that active warming of peripheral tissues prior to anesthetic induction, which is the so-called prewarming, decreases the amount of the core-to-peripheral heat redistribution and prevents intraoperative hypothermia18–19 while forced-air prewarming has been widely accepted for its efficacy and safety20.
Because liver is the major thermoregulatory organ, liver transplant recipients may be more vulnerable to hypothermia induced by the core-to-peripheral heat distribution. Also, the vascular barrier to preserve core heat may be disturbed with severely decreased vascular resistance21,22. Nonetheless, the effects of prewarming have never been evaluated in liver transplantation while previous studies mainly focused on intraoperative warming after significant core heat loss already occured13,14,15. Thus, we hypothesized that prewarming decreases the amount of the core-to-peripheral heat redistribution and the risk of intraoperative hypothermia for liver transplant recipients. In this study, we tested whether forced-air prewarming prevents intraoperative hypothermia during liver transplantation.
Materials and methods
Subjects
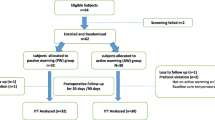
As shown in Fig. 1, 40 adult patients (18–80 yr old) undergoing an elective living donor liver transplantation were enrolled from October 2018 to April 2019. Patients with preoperative fever (> 38.0 °C) or hypothermia (< 36.0 °C), previous transplant history, septic condition, encephalopathy, autonomic neuropathy, thyroid dysfunction, model for end-stage liver disease score > 30, and the risk of malignant hyperthermia were excluded from the study. This prospective, parallel-group, assessor-blind randomized controlled trial was approved by the Samsung Medical Center Institutional Review Board on July 04 2018 (SMC 2018-05-061-005) and registered at the Clinical Research Information Service on October 01 2018 (https://cris.nih.go.kr/, Identifier: KCT0003230). Written informed consent was obtained from the recipients or their legal authorized representatives and all methods were performed in accordance with the relevant guidelines and regulations.
Intervention
Eligible patients were randomized 1:1 to either prewarming group or non-prewarming group using computer-generated numbers by a statistician not involved in patient screening or enrolment. Before entering the operating room, patients stayed at the preoperative waiting room for the intervention (prewarming vs. non-prewarming). The ambient temperature of the preoperative waiting room was thermostatically controlled being set at 25.0 °C at least 30 min before the patients' entrance. During the stay at the preoperative waiting room, non-invasive blood pressure, pulse oxymetry, and electrocardiography were monitored. Patients in prewarming group were covered from the neck to the feet by a disposable full-body forced-air blanket (Model 30000; 3M patient warming blanket, Eden Prairie, MN, USA). The blanket was tucked underneath the body to prevent direct leaking of forced-air to the ambient environment. They underwent forced-air warming during 30 min with the temperature of the forced-air being 43.0 °C using a dedicated warming device (Model 775; 3M Bair Hugger, St. Paul, MN, USA). Patients in non-prewarming group were covered by the same full-body forced-air blanket in the same way and a cotton blanket was additionally applied over it to prevent possible heat loss during the stay at the preoperative waiting room. They did not undergo active warming while the forced-air warming device was powered off. After the 30-min intervention, patients were immediately transferred to the operating room. The thermal comfort was assessed before and after the intervention in both groups with 11-point scale (0, worst imaginable cold; 5, identified thermal comfort; 10, worst imaginable hot)17. The thermal comfort change is the difference between the thermal comfort value before and after prewarming intervention.
Standard thermal care
The ambient temperature of the operating room was thermostatically controlled being set at 24.0 °C at least 30 min before the patients’ entrance. A circulating water mattress (Blanketrol II, Clininnati Sub-Zero Products, Inc, Ohio) was placed on the operative table underneath the patient body being set at 37.0 °C. The patient’s body surface exposure was minimized with cotton blankets and surgical drapes throughout the anesthesia and surgery without intraoperative forced-air warming23. The upper extremities were additionally wrapped in vinyl to be separated from fluids outflowed from the surgical field. Active airway warming was performed using a heated wire breathing system (VentiMyst®, Flexicare Medical Limited, Mountain Ash, UK)15. A rapid fluid infusion device (Level 1® H-1200, Smiths medical, Dublin, OH, USA) was used to prevent hypothermia for all crystalloids, colloids, red blood cells, and fresh frozen plasma, while cryoprecipitate, platelets, and albumin products were infused at room temperature24. Warmed fluid was used to irrigate and wash the surgical field. When the patient’s core temperature reached to < 35.5 °C, the set temperature of operating room was changed to 26.0 °C and the set temperature of the circulating water mattress was changed to 40.0 °C. In contrast, when the patient’s core temperature reached to > 37.5 °C, the circulating water mattress and rapid fluid warming device were turned off.
Monitoring and anesthetic induction
Anesthetic managements were performed according to the standardized institutional protocol, as described previously25,26. After initiating standard monitoring (pulse oximetry, 5-lead electrocardiography, and non-invasive arterial blood pressure measurements), anesthesia was induced with thiopental sodium (5 mg/kg) and maintained with isoflurane titrated to a bispectral index of 40–60. Mechanical ventilation was delivered at a tidal volume of 8 mL/kg (ideal body weight) and positive end-expiratory pressure of 6 mmHg using a mixture of medical air and oxygen at a fresh gas flow rate of 2 L/min, and the respiratory rate was adjusted as needed to maintain normocapnea. Direct arterial blood pressure monitoring was performed via the right radial artery and the right femoral artery. Central venous pressure was monitored through the right internal jugular vein and the right femoral vein. A large-bore 9-Fr catheter was placed in the right internal jugular vein in combination with a pulmonary arterial catheter (Swan-Ganz CCOmboV, Edward Lifesciences, LLC, Irvine, CA). The tip of the pulmonary arterial catheter was located at 1 cm proximal from the point where pulmonary artery occlusion occurred. During the arterial/venous line insertion, sterile drapes covered the body with minimal exposure of the skin to the ambient environment.
The routine intraoperative laboratory measurements included arterial blood gas analysis (including lactate), cell blood count, and coagulation profile (prothrombin time [PT], activated partial thromboplastin time [aPTT], and fibrinogen). They were regularly checked at the following time points: start of dissection phase (skin incision), start of the anhepatic phase, and 5/60/180 min after graft reperfusion, as described previously11.
Data acquisition
In the preoperative operating room, core temperature was measured via the tympanic membrane using an infrared thermometer (Thermoscan 5, Braun, Kronberg, Germany) immediately before and after the intervention. In the operating room, core temperature was measured once via the tympanic membrane with an infrared thermometer (Thermoscan 5 IRT 4520, Braun, Kronberg, Germany) before the anesthesia was induced. After placing the pulmonary artery catheter, core temperature was continuously measured in the pulmonary artery and automatically recorded every 5 min into our electronic anesthesia record. The pulmonary catheter was removed immediately before transferring the patient to intensive care unit based on our center policy.
Other data were derived from electronic medical record system. Hemodynamic variables and ventilation-related variables were automatically recorded every 5 min during surgery into our electronic anesthesia record. The amount of intraoperative blood loss was calculated by using a formula designed for liver transplantation27. All perioperative laboratory findings were automatically recorded in the electronic medical record system.
Variables and statistics
The primary outcomes were the incidence and duration of intraoperative hypothermia. Hypothermia was defined when core temperature was < 36.0 °C28. The sample size was calculated based on the previous study, demonstrating that 30-min prewarming before surgery reduced the intraoperative hypothermia incidence by 91.3%29. Given that liver transplant patients might be affected by relatively more thermal factors, we assumed that prewarming decreases intraoperative hypothermia by 85% and a minimum of 20 patients was required in each group (β = 0.8, α = 0.05, and 10% dropout rate). The secondary outcomes were the degree of core temperature change, blood lactate concentration, prothrombin time (PT [INR]), activated partial thromboplastin time (aPTT), and blood fibrinogen level as well as postoperative outcomes including 1-yr graft failure/mortality, acute kidney injury (AKI), major complication (the Clavien-Dindo grade was at least 2)30, and early graft dysfunction. Acute kidney injury was defined using the International Club of Ascites new classification for patients with cirrhosis31. Early graft dysfunction was defined as the presence of one or more of the followings: bilirubin ≥ 10 mg/dL on day 7, international normalized ratio ≥ 1.6 on day 7, and alanine or aspartate aminotransferases > 2000 IU/L within the first 7 days32. Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range), being analyzed using student t-test or Mann–Whitney U test. Distribution normality was tested by Kolmogorov–Smirnov test. Paired data, such as core temperature before and after prewarming was analyzed using paired t-test. Repeatedly measured variables such as core temperature, prothrombin time, activated partial thromboplastin time, and fibrinogen level were analyzed using repeated measure ANOVA. Categorical variables are described as frequency (%), being analyzed using chi-square test or the Fisher’s exact test. A two-sided P value < 0.05 was considered statistically significant. All analyses were performed using SPSS 25.0 (IBM Corp., Chicago, IL, USA).
Prior presentation
This study was presented in part at the 8th Annual Conference of the Korean Society of Transplantation Anesthesiologists, 20/03/2021.
Results
The two groups were not significantly different regarding the baseline characteristics, anesthetic factors, and surgical factors (Table 1). Core temperature at the preoperative waiting room immediately before the intervention was not significantly different between the two groups (36.6 °C [36.5 °C, 36.8 °C] in non-prewarming group vs. 36.5 °C [36.4 °C, 36.7 °C] in prewarming group, P = 0.201). During the intervention, core temperature was not significantly changed in non-prewarming group (36.7 °C [36.4 °C, 36.8 °C] after the intervention, P = 0.741), whereas it was significantly increased in prewarming group (36.8 °C [36.6 °C, 37.0 °C] after the intervention, P = 0.018). Although, the degree of thermal comfort changed significantly greater in prewarming group than non-prewarming group (3 [1, 4] vs. 0 [0, 2], P < 0.001, Table 1), no patients requested adjustment in warming temperature or experienced thermal injury.
As shown in Fig. 2, intraoperative hypothermia incidence was significantly greater in non-prewarming group than in prewarming group (95.0% vs. 60.0%, odds ratio [OR] = 2.30 [1.39, 3.78], P = 0.020) and the duration of hypothermia was significantly longer in non-prewarming group (383 [108, 426] min vs. 60 [0, 221] min, P = 0.001). The difference between the initial core temperature at the entrance of operating room and the lowest intraoperative core temperature was significantly greater in non-prewarming group (1.4 °C [1.1 °C, 1.5 °C] vs. 0.9 °C [0.7 °C, 1.4 °C], P = 0.040).
As shown in Fig. 3, core temperature dropped significantly during the post-induction phase (from anesthesia induction to the skin incision) in both groups (36.7 °C [36.4 °C, 36.8 °C] to 35.8 °C [35.6 °C, 35.9 °C], P < 0.001, in non-prewarming group; 36.9 °C [36.6 °C, 37.0 °C] to 36.2 °C [36.0 °C, 36.4 °C], P < 0.001, in prewarming group). The rate of core temperature drop during the post-induction phase was 0.8 (0.7, 1.0) °C/hr in non-prewarming group and 0.6 (0.3, 0.8) °C/hr in prewarming group (P = 0.019) while the duration of the post-induction phase was 57 (49, 66) min in non-prewarming group and 59 (52, 71) min in prewarming group. During the post-induction phase, hypothermia risk was significantly lower in prewarming group (20.0% vs. 85.0%, P < 0.001, Fig. 2). In contrast, the degree of change in core temperature was not significantly different between non-prewarming group and prewarming group during the dissection phase (0 °C [− 0.1 °C, 0.1 °C] vs. 0 °C [− 0.2 °C, 0.1 °C], P = 0.883), anhepatic phase (− 0.1 °C [− 0.2 °C, 0 °C] vs. − 0.1 °C [− 0.2 °C, 0 °C], P = 0.862), or post-reperfusion phase (0.2 °C [0.2 °C, 0.3 °C] vs. 0.2 °C [0.1 °C, 0.3 °C], P = 0.369) (Table 2), suggesting the particular impact of forced-air prewarming on the core-to-peripheral heat redistribution following anesthesia induction. Core temperatures repeatedly measured throughout the surgery were significantly higher in prewarming group (P = 0.027).
Serial change in intraoperative core temperature. Prewarming group showed higher core temperature at all analyzed times from the start of anesthetic induction to the end of surgery. The start of anesthetic induction [I0], start of dissection phase [D0], start of anhepatic phase [A0], 10 min before graft reperfusion [R(-10)], 5 min after reperfusion [R05], 30 min after reperfusion [R30], 60 min after reperfusion [R60], and at the end of surgery [E].
Blood lactate concentration continuously increased in non-prewarming group, whereas it decreased in prewarming group after graft reperfusion; accordingly, the degree of change in blood lactate concentration during the first 3 h after graft reperfusion was significantly different between the two groups (1.17 [3.31, 0.77] mmol/L in non-prewarming group and − 0.19 [0.13, − 0.48] mmol/L in prewarming group, P = 0.034, Fig. 4). Although statistical significance was not found, coagulation profile showed trend of recovery after graft reperfusion in prewarming group, whereas it did not recover in non-prewarming group (P = 0.192 in prothrombin time [INR], P = 0.323 in activated partial thromboplastin time, and P = 0.246 in Fibrinogen, Fig. 4). The two groups were not significantly different regarding blood loss (2000 [850, 2500] mL in non-prewarming group and 1350 [900, 3600] mL in prewarming group, P = 0.820) and red blood cell transfusion (1 [0, 3] units and 0 [0, 2] units, P = 0.698).
Changes of lactate and coagulation profile of non-prewarming group (straight line) and prewarming group (dotted line). The start of the dissection phase [D0], start of the anhepatic phase [A0], 5 min after graft reperfusion [R05], 60 min after graft reperfusion [R60], and 180 min after graft reperfusion [R180]. *Statistically significant (P < 0.05) between groups.
For postoperative clinical outcomes, the incidence of acute kidney injury (5.0% in non-prewarming group vs. 10.0% in prewarming group, P > 0.99), major complications (10.0% in both groups, P > 0.99), and early graft dysfunction (10.0% in both groups, P > 0.99) within first 7 days of liver transplantation were not significantly different between the two groups. Also, 1-yr graft failure risk (0 patients vs. 2 [10.0%] patients, P = 0.487) and 1-yr mortality (1 [5.0%] patients vs. 3 [15.0%] patients, P = 0.605) were not significantly different between non-prewarming group and prewarming group.
Discussion
This is the first to test the effects of forced-air prewarming on intraoperative core temperature of liver transplant recipients who are at high risk of hypothermia and its complications. In this randomized clinical trial, 30-min forced-air prewarming significantly reduced the incidence and duration of intraoperative hypothermia. The thermic benefit of forced-air prewarming was mainly found during the post-induction phase before the skin incision when significant core-to-peripheral heat redistribution occurs. Forced-air prewarming increased core temperature already before the start of anesthesia, which was thought to be due to the increase in total body heat content, and also decreased the degree of core temperature drop immediately after the start of general anesthesia, suggesting the decrease in the amount of core-to-peripheral heat redistribution. Also, we found that patients undergoing forced-air prewarming experienced better biochemical courses after graft reperfusion (e.g. lactate and coagulation profile), indicating a better metabolic function of the newly implanted liver graft9,10,12. Although clinical impact of intraoperative hypothermia and the benefits of normothermia are well developed in various surgical settings4,5,8, they have not been well demonstrated in liver transplantation. Our findings add an important evidence on clinical benefits of intraoperative normothermia during liver transplantation and suggests incorporating forced-air prewarming into thermic strategy for liver transplantation.
In agreement with previous studies of other surgical settings18,19, we found that preoperatively delivered heat content using forced-air warming effectively prevents intraoperative hypothermia during liver transplantation. Prewarming increases heat content mainly in the peripheral compartment and decreases the core-to-peripheral temperature gradient29,33. The reduced temperature gradient between peripheral heat content and central heat content decreases core-to-peripheral heat flow following vasodilatation after anesthetic induction33. In our study, prewarming was conducted by forced-air warming device with a disposable full-body forced-air blanket8. In particular, full-body forced-air blanket is known to deliver about 95 watts of convection heat across the patient’s body surface when the forced-air warming device is set to 43.0 °C for 30 min34.
Generally, core temperature increases progressively after the post-induction core-to-peripheral heat redistribution35. However, disturbed hepatic thermogenesis with the end-stage liver disease, absence of hepatic heat production during the anhepatic phase, and the cold graft contributes to continual core heat loss until graft reperfusion as shown in the previous and current studies, making it hard to recover from hypothermia once it occurs1,13. Thus, it is more important to prevent the post-induction hypothermia which may be difficult to overcome until graft reperfusion when core heat can be regained following active hepatic heat production. Moreover, previous studies have demonstrated that the core-to-peripheral heat redistribution occurs regardless of intraoperative active warming4,7. Of importance, the effects of the alteration in the laminar air flow during surgery resulted from forced and warmed air on liver transplant patients, who are considered at high risk of immunomodulation, has not been evaluated23. Thus, forced-air prewarming should be considered as an important part of thermal strategy in liver transplantation regarding its efficacy and safety.
Clinical significance of intraoperative hypothermia has not been evaluated in liver transplantation. To our knowledge, there was only one study showing the relationship between intraoperative core temperature and post-transplant cytomegalovirus infection36, a surrogate indicator for immune dysfunction37. Thus, we evaluated clinical indicators, such as lactate and coagulation profiles, which represent sequential liver function during transplantation (particularly after graft reperfusion)38,39. About 70% of lactate clearance is dependent to the liver, which converts lactate to pyruvate through lactate dehydrogenoase40. Accordingly, impaired graft function or delayed liver regeneration after graft reperfusion decreases lactate clearance38. In our study, plasma lactate concentration started to decrease after graft reperfusion in prewarming group, whereas it continuously increased until 3 h after graft reperfusion in non-prewarming group. As mentioned above, liver graft performs vigorous quantitative/qualitative regeneration along with full metabolic functions; thus, the efforts to provide better thermal environment for liver cells are important for the critical time window9,10,12. Moreover, this effect would be more evident in patients who are with marginal grafts that has greater risk to fail to meet the recipient’s metabolic demands. Regarding coagulation profiles, it is well known that even mild hypothermia can result in coagulopathy by impairing the function of enzymes involved in the coagulation cascade3,41. That is, even mild hypothermia can increase the amount of intraoperative bleeding3,6,7.
In the current study, patients without prewarming did not recover blood fibrinogen level even after 3 h after graft reperfusion. Fibrinogen is synthesized in the liver and it is known that the newly transplanted liver is the source of ≥ 98% of the circulation fibrinogen after graft reperfusion42, indicating plasma fibrinogen as an indicator for early graft function and recovery.
Although the importance of thermal management is widely accepted, the reluctance to use prewarming in patients undergoing liver transplantation may be at least in part due to the lack of evidence of the effective and safe duration of prewarming. In our study, we demonstrated that prewarming only for 30 min with a widely used forced-air warming device effectively decreases the core-to-peripheral heat redistribution and prevents intraoperative hypothermia without side effects, being in line with studies of other surgical populations20. Moreover, the effects of prewarming was questioned in liver transplant recipients because their peripheral vascular heat barrier could be thought to be already disturbed based on hyper-dynamic circulation and decreased systemic vascular resistance21,22,25. The current study is the first to prove the efficacy and safety of forced-air prewarming even for liver transplant recipient with end-stage liver disease or cirrhotic circulatory changes.
This study has limitations. First, patients who underwent prewarming might have been aware of the intervention. However, it is unlikely that this influenced the results because patients cannot control core heat content or core temperature irrespective of the awareness. Also, data collection was done by blinded assessors. Second, the forced-air prewarming strategy used in the current study could not be implicated in deceased donor liver transplantation at which most patients are critically ill, and many body parts should be directly observed and easily approached. In such situations, forced-air warming using a cover type blanket is not feasible although an underbody type blanket could be an alternate. Third, there was possibility of type II error for secondary outcomes. Further research with sufficient sample size is warranted to analyze the effects of prewarming on clinical outcomes.
In the current study, we found that 30-min forced-air prewarming is effective to reduce post-induction core heat loss, prevent intraoperative hypothermia, and decrease the duration of intraoperative hypothermia in living donor liver transplantation. Also, lactate clearance early after graft reperfusion was improved in relation to the use of forced-air prewarming and consequent greater core temperature during the reperfusion phase. No clinically significant side effects were found with forced-air prewarming. Therefore, we concluded that forced-air prewarming is an effective and safe method to prevent intraoperative hypothermia with potential clinical benefits in living donor liver transplantation.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Han, S. et al. Risk factors for inadvertent hypothermia during adult living-donor liver transplantation. Transpl. Proc. 46, 705–708. https://doi.org/10.1016/j.transproceed.2013.11.091 (2014).
Romanovsky, A. A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R37–R46. https://doi.org/10.1152/ajpregu.00668.2006 (2007).
Rohrer, M. J. & Natale, A. M. Effect of hypothermia on the coagulation cascade. Crit. Care Med. 20, 1402–1405. https://doi.org/10.1097/00003246-199210000-00007 (1992).
Kurz, A., Sessler, D. I. & Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 334, 1209–1215. https://doi.org/10.1056/NEJM199605093341901 (1996).
Beilin, B. et al. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology 89, 1133–1140 (1998).
Rajagopalan, S., Mascha, E., Na, J. & Sessler, Daniel I. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 108, 71–77. https://doi.org/10.1097/01.anes.0000296719.73450.52 (2008).
Sun, Z. et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology 122, 276–285. https://doi.org/10.1097/aln.0000000000000551 (2015).
Madrid, E. et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst. Rev. 4, CD009016. https://doi.org/10.1002/14651858.CD009016.pub2 (2016).
Michalopoulos, G. K. Liver regeneration. Science 276, 60–66. https://doi.org/10.1126/science.276.5309.60 (1997).
Clavien, P. A. Liver regeneration: A spotlight on the novel role of platelets and serotonin. Swiss Med. Wkly. 138, 361–370 (2008).
Han, S. et al. Association between intraoperative platelet transfusion and early graft regeneration in living donor liver transplantation. Ann. Surg. 264, 1065–1072. https://doi.org/10.1097/SLA.0000000000001526 (2016).
Munoz, S. J. Hypothermia may impair hepatic regeneration in acute liver failure. Gastroenterology 128, 1143–1144 (2005) (author reply 1144–1145).
Russell, S. H. & Freeman, J. W. Prevention of hypothermia during orthotopic liver transplantation: Comparison of three different intraoperative warming methods. Br. J. Anaesth. 74, 415–418 (1995).
Muller, C. M. et al. Forced-air warming maintains normothermia during orthotopic liver transplantation. Anaesthesia 50, 229–232 (1995).
Han, S. B. et al. Effect of active airway warming on body core temperature during adult liver transplantation. Transpl. Proc. 45, 251–254. https://doi.org/10.1016/j.transproceed.2012.05.088 (2013).
Sessler, D. I. Perioperative thermoregulation and heat balance. Lancet 387, 2655–2664. https://doi.org/10.1016/s0140-6736(15)00981-2 (2016).
Sessler, D. I., Schroeder, M., Merrifield, B., Matsukawa, T. & Cheng, C. Optimal duration and temperature of prewarming. Anesthesiology 82, 674–681 (1995).
Andrzejowski, J., Hoyle, J., Eapen, G. & Turnbull, D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br. J. Anaesth. 101, 627–631. https://doi.org/10.1093/bja/aen272 (2008).
Lau, A. et al. Effect of preoperative warming on intraoperative hypothermia: A randomized-controlled trial. Can. J. Anesth. 65, 1029–1040. https://doi.org/10.1007/s12630-018-1161-8 (2018).
Connelly, L. et al. The optimal time and method for surgical prewarming: A comprehensive review of the literature. J. Perianesth. Nurs. 32, 199–209. https://doi.org/10.1016/j.jopan.2015.11.010 (2017).
Newby, D. E. & Hayes, P. C. Hyperdynamic circulation in liver cirrhosis: Not peripheral vasodilatation but “splanchnic steal”. QJM Mon. J. Assoc. Physicians 95, 827–830 (2002).
Moller, S. & Henriksen, J. H. Cardiovascular complications of cirrhosis. Gut 57, 268–278. https://doi.org/10.1136/gut.2006.112177 (2008).
Wood, A. M., Moss, C., Keenan, A., Reed, M. R. & Leaper, D. J. Infection control hazards associated with the use of forced-air warming in operating theatres. J. Hosp. Infect. 88, 132–140. https://doi.org/10.1016/j.jhin.2014.07.010 (2014).
Han, S. et al. Comparison of two fluid warming devices for maintaining body core temperature during living donor liver transplantation: Level 1 H-1000 vs Fluid Management System 2000. Korean J. Anesthesiol. 67, 264–269. https://doi.org/10.4097/kjae.2014.67.4.264 (2014).
Han, S. et al. Bioreactance is not interchangeable with thermodilution for measuring cardiac output during adult liver transplantation. PLoS ONE 10, e0127981. https://doi.org/10.1371/journal.pone.0127981 (2015).
Kwon, J. H. et al. Blood Salvage and Autotransfusion With Single Leukoreduction Does Not Increase the Risk of Tumor Recurrence After Liver Transplantation for Advanced Hepatocellular Carcinoma. Ann. Surg. https://doi.org/10.1097/SLA.0000000000004866 (2022).
Bang, S. R. et al. Predictors of high intraoperative blood loss derived by simple and objective method in adult living donor liver transplantation. Transpl. Proc. 42, 4148–4150. https://doi.org/10.1016/j.transproceed.2010.10.017 (2010).
Sessler, D. I. Mild perioperative hypothermia. N. Engl. J. Med. 336, 1730–1737. https://doi.org/10.1056/nejm199706123362407 (1997).
Horn, E. P. et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia 67, 612–617. https://doi.org/10.1111/j.1365-2044.2012.07073.x (2012).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae (2004).
Hilmi, I. A. et al. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 114, 919–926. https://doi.org/10.1093/bja/aeu556 (2015).
Olthoff, K. M. et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 16, 943–949. https://doi.org/10.1002/lt.22091 (2010).
Matsukawa, T. et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology 82, 662–673. https://doi.org/10.1097/00000542-199503000-00008 (1995).
Sessler, D. I. & Moayeri, A. Skin-surface warming: Heat flux and central temperature. Anesthesiology 73, 218–224 (1990).
Kurz, A., Sessler, D. I., Christensen, R. & Dechert, M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology 83, 491–499. https://doi.org/10.1097/00000542-199509000-00007 (1995).
Paterson, D. L. et al. Intraoperative hypothermia is an independent risk factor for early cytomegalovirus infection in liver transplant recipients. Transplantation 67, 1151–1155. https://doi.org/10.1097/00007890-199904270-00011 (1999).
Kang, R. et al. Postoperative hyperglycemia may negatively impact cytomegalovirus infection in seropositive liver transplant recipients: A retrospective cohort study. Transpl. Int. 33, 68–75. https://doi.org/10.1111/tri.13496 (2020).
Golse, N. et al. Arterial lactate concentration at the end of liver transplantation is an early predictor of primary graft dysfunction. Ann. Surg. 270, 131–138. https://doi.org/10.1097/sla.0000000000002726 (2019).
Kang, Y. G. et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth. Analg. 64, 888–896 (1985).
Phypers, B. & Pierce, J. T. Lactate physiology in health and disease. Contin. Educ. Anaesth. Crit. Care Pain 6, 128–132 (2006).
Reed, R. L. II., Bracey, A. W. Jr., Hudson, J. D., Miller, T. A. & Fischer, R. P. Hypothermia and blood coagulation: Dissociation between enzyme activity and clotting factor levels. Circ. Shock 32, 141–152 (1990).
Tennent, G. A. et al. Human plasma fibrinogen is synthesized in the liver. Blood 109, 1971–1974. https://doi.org/10.1182/blood-2006-08-040956 (2007).
Funding
This study was supported by a grant provided by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and Information and Communication Technology (2021R1F1A106323712).
Author information
Authors and Affiliations
Contributions
Conception of the idea: S.H. Study design: E.J.O. and S.H. Data collection: E.J.O., S.H., S.L., J.S.K., M.S.G., and G.S.K. Data and statistical analysis: E.J.O., S.H., S.L., E.A.C. Drafting the manuscript: E.J.O., S.H., J.S.K., M.S.G., and G.S.K. Revising the manuscript: E.J.O., S.H., S.L., J.S.K., M.S.G., and G.S.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. The study subjects were liver transplant recipients undergoing living donor liver transplantation (LDLT). All recipients in the current study received a graft harvested in our hospital. The living donor selection process was rigorously performed according to our national and institutional medical and ethical protocols. In South Korea, all living donors should obtain official approval from a national institute named Korean Network for Organ Sharing (KONOS) prior to donation and one of the key national policies is that organ donation is allowed for offspring, spouse, and parents in principle. All of the 40 recipients included in the current study were related to the donors. Informed consent for liver donation was obtained for all living donors prior to donation hepatectomy and LT.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oh, E.J., Han, S., Lee, S. et al. Forced-air prewarming prevents hypothermia during living donor liver transplantation: a randomized controlled trial. Sci Rep 13, 3713 (2023). https://doi.org/10.1038/s41598-022-23930-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23930-2
- Springer Nature Limited