Abstract
This study aims at identifying characteristics, risk factors and mortality of community-acquired (CAP) and health-care-associated pneumonia (HCAP) by Staphylococcus aureus (S. aureus). We retrieved adults with S. aureus CAP or HCAP diagnosed by blood or pleural effusion culture in 2.6 years, and compared with those of Streptococcus pneumoniae (S. pneumoniae) CAP or HCAP diagnosed by blood or respiratory culture, or urine antigen. We found 18 patients with CAP and 9 HCAP due to S. aureus (female 33%, 66.6 ± 12.4 years-old), and 48 patients with CAP and 15 HCAP due to S pneumoniae (female 41%, 69.5 ± 17.5 years). Diabetes mellitus (52% vs. 24%, p = 0.019), hemodialysis (11% vs. 0%, p = 0.046), skin lesions (44% vs. 0%, p < 0.001), cavitary nodules (37% vs. 1.6%, p < 0.001) and pleural effusions (48% vs. 18%, p = 0.007) were more common in staphylococcal than pneumococcal group. Three patients with staphylococcal pneumonia had acute myocardial infarction. Pneumonia severity index (139 ± 52 vs. 109 ± 43, p = 0.005) and 30-day mortality (41% vs. 9.5%, p = 0.001) were higher in staphylococcal group. Multivariate analysis showed underlying disease (especially cancer and cirrhosis), risk class 4/5, altered mentality, shock and bilateral pneumonia were risk factors for 30-day mortality.
Similar content being viewed by others
Introduction
Severe communityacquired pneumonia (CAP) caused by Staphylococcus aureus (S. aureus) has been reported since 2005, following the influenza season of 2003–2004 and 2006–2007 in the United States (USA)1,2,3. Among the initially reported cases many occurred in young adults without underlying diseases but with preceding influenza, and most were caused by methicillin-resistant S. aureus (MRSA). S. aureus was later reported to be an important cause of health-care-associated pneumonia (HCAP)4.
However, clinical features of CAP or HCAP caused by S. aureus varied in studies from different regions. The average age of patients with staphylococcal CAP was 45, 49, and 60 years in studies from Brazil5, Canada4, USA6, respectively. The mortality of S. aureus CAP was 0%, 13% and 46%, in reports from Canada, USA, and Australia, respectively4,6,7,8. Preceding influenza was a risk factor for primary staphylococcal pneumonia in an earlier series2, but not in a prospective study published later that identified chronic hemodialysis as a risk factor for MRSA CAP6. Therefore, clinical characteristics and risk factors for staphylococcal CAP/HCAP may differ among regions. To help first-line physicians diagnose this potentially fatal pneumonia, we conducted this study to investigate characteristics and risk factors of S. aureus CAP and HCAP in New Taipei City (a densely populated industrial city in Northern Taiwan) and compared them with those of CAP/HCAP caused by Streptococcus pneumoniae (S. pneumoniae).
Results
Patients number and diagnostic methods
During the period of 2 years and 7 months, we identified 27 adult patients with staphylococcal CAP (n = 18) or HCAP (n = 9). All had S. aureus isolated from blood (Table S1, supplementary materials). Two (7%, both having CAP) also had S. aureus cultured from pleural fluid, 11 (41%) sputum, 2 (7%) nasopharynx and 7 (26%) non-respiratory sites including wound and urine. All the 27 patients (except for one who was found comatose on roadside and sent to our ER) had respiratory symptoms at presentation, suggesting primary staphylococcal pneumonia. The one with initial coma had lobar pneumonia in the right lower and patchy consolidation in right upper lobe, liver cirrhosis, prolonged prothrombin time and cerebral hemorrhage. Thus all 27 patients were diagnosed to have primary staphylococcal pneumonia. Image study found that chest radiographs and computed tomography (CT, performed in 12 or 44%) of the 27 patients all showed patchy or lobar consolidation with or without nodules, compatible with primary staphylococcal pneumonia with or without secondary septic emboli.
We identified 63 adult patients with pneumococcal CAP (n = 48) or HCAP (n = 15) during the same period. Sixty one (97%) has blood culture performed at ER or within 48 h after admission. Only four (6%) had positive blood culture of S. pneumoniae (Table S2, supplementary materials), of whom one also had positive culture from sputum and another one cerebral spinal fluid (CSF). The other 59 cases were diagnosed on positive sputum culture and pneumococcal antigen (n = 2) or positive pneumococcal antigen alone (n = 57). All the 63 patients had respiratory symptoms at presentation, suggesting primary pneumococcal pneumonia. Chest radiographs and CT (performed in 16 or 25%) of the 63 patients all showed lobar or patchy consolidation with or without mottling shadows, compatible with primary pneumococcal pneumonia.
Clinical characteristics
Table 1 lists clinical characteristics. Patients with staphylococcal pneumonia were all middle aged or old. However, some patients with pneumococcal CAP were young and healthy (six or 9.5% < 40 year-old). Both staphylococcal and pneumococcal pneumonia occurred year-round, although more common in cool season. Co-detection of other pathogens, including influenza, was observed in 11% of staphylococcal and 44% of pneumococcal pneumonia (p = 0.435). MRSA accounted for 13 (48%) of staphylococcal pneumonia. Underlying diseases were present in 26 (96%) and 21 (67%) patients with staphylococcal and pneumococcal pneumonia, respectively (p = 0.007). Diabetes mellitus (DM) and chronic hemodialysis were more common in the staphylococcal than pneumococcal group (52% vs. 24%, p = 0.018 and 11% vs. 0%, p = 0.042, respectively). Among patients with staphylococcal CAP, two abused intravenous drugs.
The two groups had similar clinical manifestations. The presenting oxygen saturation was lower in the staphylococcal group. The most obvious differences in physical signs were skin lesions, including cellulitis, scabies, wound infection, herpes zoster, pressure sores, carbuncles or intravenous devices, at presentation in 11 of the staphylococcal, but none of the pneumococcal group (41% vs. 0%, p < 0.001). All those with skin lesions were diabetic.
Chest radiographs and CT scans revealed that pleural effusions (48% vs. 17%, p = 0.005) and multiple cavitary nodules (37% vs. 1.6%, p < 0.001, Fig. S1, supplementary materials) were more common in patients with staphylococcal than pneumococcal pneumonia.
Pneumonia severity index and mortality
Average PSI was significantly higher in the staphylococcal group. Totally 85% and 63% of the staphylococcal and pneumococcal group, respectively, presented with a PSI risk class of ≥ 4 (p = 0.067). Patients with staphylococcal pneumonia had significantly higher 30-day mortality than those with pneumococcal pneumonia (41% vs. 9.5%, p = 0.001). Patients with methicillin-susceptible S. aureus (MSSA) and those with MRSA pneumonia had similar PSI (148 ± 54 vs. 137 ± 45, p = 0.582) and 30-day mortality (50% vs. 31%, p = 0.555). Among 30-day survivors, the average admission duration was 24.2 and 12.8 days for those with staphylococcal and pneumococcal pneumonia, respectively (p = 0.001).
Complication
Table S3 lists major complications. Pyothorax that required catheter/chest tube/ thoracotomy occurred in two patients in each group; however, pyo-pneumothorax was observed only in two patients with staphylococcal pneumonia. Acute myocardial infarction (AMI) occurred in 3 patients with staphylococcal pneumonia (all men with MSSA, Table S4). Two of them had AMI at presentation with shock. The third one developed AMI simultaneously with cerebral artery emboli on the 27th day of admission. Two had underlying chronic kidney disease (CKD). All the three patients presented to ED with thrombocytopenia, prolonged activated thromboplastin time (APTT) and increased international normalized ratio (INR), suggestive of disseminated intravascular coagulation (DIC). All three died within 2 days of AMI.
Empirical antimicrobials
Empirical antimicrobials used at ED for patients with staphylococcal pneumonia were mostly β-lactam-β-lactamase inhibitor (BLBLI) combinations, including piperacillin tazobactam and cefoperazone sulbactam, second-generation cephalosporin, and combination of antimicrobials that often included vancomycin or teicoplanin (Table S5, supplementary materials). Seven (26%) did not receive effective antimicrobials within 48 h of presentation and had a higher 30-day mortaIity than the 20 who received effective antimicrobials within 48 h (57% vs. 35%, p = 0.567). By contrast, empirical antimicrobials used for patients with pneumococcal pneumonia were cefoperazone sulbactam, fluoroquinolone, piperacillin tazobactam and amoxicillin clavulanate, without vancomycin, teicoplanin, or any combinations including them. Seven (11%) did not receive antimicrobials at ED, but all were discharged uneventfully after 5–18 days.
Risk factors for mortality
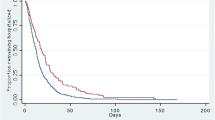
We analyzed risk factors associated with 30-day mortality among all the 90 patients with pneumonia (due to either S. aureus or S. pneumoniae) using multivariate regression analysis. First we discovered that all patients without co-morbidity (n = 22) survived. Therefore, THE presence of comorbidity is the most crucial prognostic factor for mortality (log-rank test p = 0.012; Table 2, Fig. 1A). After subtracting these 22 cases we analyzed risk factors among the remaining 68 patients with co-morbidity, and found that all the 13 patients with PSI risk classes 1–3 survived. Therefore, PSI risk class of 4 or 5 was the second most crucial prognostic factor for mortality (Table 2, Fig. 1B). After excluding 13 patients with risk classes of 1–3, we analyzed risk factors among the remaining 55 patients with co-morbidity and risk class of 4 or 5 using Cox regression backward selection model. Variables analyzed were pathogenic organisms, infection type (CAP or HCAP), age (≤ or > 65 years), sex, smoking status, blood culture result, DM, cancer, heart failure, chronic obstructive pulmonary disease (COPD), CKD with or without hemodialysis, cirrhosis, CVA, bilateral infiltrates, multiple cavitary nodules, shock, mental status, tachypnea, PaO2 (≥ or < 60 mm Hg), skin lesions, and effective antimicrobial use within 3 days. We identified five additional risk factors for 30-day mortality: cancer, cirrhosis, altered mental status, shock, and bilateral lung lesions (Table 3).
(A) Survival curves for patients with pneumonia caused by Staphylococcus aureus or Streptococcus pneumoniae with and without comorbidities (total: 90 cases, 22 without comorbidities [dotted line] and 68 with comorbidities [solid line]). (B) Survival curves for the patients with comorbidities (total: 68 cases, 13 with risk classes of 1–3 [dotted line] and 55 with risk classes of 4 or 5 [solid line]).
Discussion
Our study found that staphylococcal CAP and HCAP occurred throughout the year in our community, with a prevalence lower than pneumococcal pneumonia. Influenza virus has been identified as a potential predisposing factor for staphylococcal CAP1,2,3. Because of the retrospective nature of this study, influenza testing was not performed for all the patients. Nevertheless, the low rate of influenza co-infection among patients who underwent influenza testing (1/11, 9%) in our study was similar to that reported in a prospective study from USA (8.1%)6, indicating that staphylococcal pneumonia can occur with or without preceding influenza.
We reviewed only adults in this study. Our patients with staphylococcal pneumonia were middle-aged or old, mostly with underlying diseases, in contrast to a previous study in which half of the patients with staphylococcal CAP were < 18 year-old and healthy3. The age range of our staphylococcal patients also differed from that of the pneumococcal group, in which 10% were aged < 40 years and healthy. The absence of patients with no underlying disease in the staphylococcal group suggested that certain condition predisposed hosts to S. aureus pneumonia. DM, chronic hemodialysis and skin lesions were more common in the staphylococcal group, similar to previous studies from Brazil, USA and Australia5,6,7. Patients with these underlying diseases had higher risk of staphylococcal pneumonia because: (1) diabetic or chronic hemodialysis patients are prone to S. aureus colonization in nose and skin, which increases the risk of S. aureus infection9,10. (2) Skin sores, scabies and intravascular lines (present in 44% of our staphylococcal but none of pneumococcal group) were risk factors for invasive staphylococcal infection7. Three of the patients with staphylococcal pneumonia and DM (two were receiving hemodialysis) had scabies at presentation. Scabies is associated with overcrowding and poor hygiene11. In Taiwan it is associated with bed-ridden status, nursing home residence and catheters12. Because scabies can be treated with scabicides and by cleaning bedding and clothing, early diagnosis of scabies may reduce the risk of staphylococcal pneumonia.
Bilateral cavitary nodules (37%) and pleural effusions (48%) were more common among our patients with staphylococcal pneumonia, similar to previous studies4,13. The association of rapidly progressive cavitating pneumonia with a preceding influenza due to Panton–Valentine leukocidin (PVL) produced by MRSA or MSSA has been reported14. We did not perform PVL assays and could not verify this association and our patients were elderly with negative influenza antigen or PCR. Nevertheless, the mortality was equally high in our study.
Three of fatal patients with staphylococcal pneumonia had AMI. Laboratory data suggested DIC in two. Staphylococcus aureus septicemia has been known to induce platelet and coagulation activation, fibrinolysis, thrombosis and DIC15, but AMI has not been previously reported to be associated with staphylococcal pneumonia. Whether AMI is related to DIC with thromboembolism in the coronary system remains unclear, given that all the three patients had risk factors for coronary artery disease, including smoking, DM, and CKD.
The average PSI score and 30-day mortality of patients in the staphylococcal group were higher than the pneumococcal group, similar to previous report6. The mortality was even higher (57%) among those without effective antimicrobials in 48 h. For the pneumococcal group, the 30-day mortality was 9.5%, similar to data from a USA (12.5%)16 and an Australian study (11.1%)7, and the seven patients in pneumococcal group without antimicrobial treatment at ED survived. The observation suggests higher virulence of S. aureus than S. pneumoniae, and poorer host immunity of patients who contracted staphylococcal pneumonia. Another factor that may contribute to the lower PSI score and mortality in the pneumococcal group was the percentage of bacteremia, which was 100% (27/27) for the staphylococcal and 6% for the pneumococcal group. Fifty-nine (94%) of the 63 patients in the pneumococcal group was diagnosed by positive urinary antigen; among them 57 had negative blood culture. The low rate of positive culture in patients with pneumococcal pneumonia may be due to the tendency of S. pneumoniae to autolyze when reaching the stationary phase of growth and antibiotic treatment prior to specimen collection17. Nevertheless, previous studies revealed that patients with pneumococcal pneumonia and positive pneumococcal bacteremia had higher18 or similar19 30-day mortality than those without bacteremia. In our study patients with pneumococcal pneumonia and bacteremia had 30-day mortality rate (0/4) similar to non-bacteremic patients (6/59 or 10.2%, p = 0.834), and on multivariate analysis bacteremia was not a significant risk factor. We need to perform a prospective, larger study to see if bacteremia contributes to mortality.
Multivariate analysis showed risk factors for 30-day mortality were underlying diseases, risk class 4/5, cancer, cirrhosis, presenting with altered mental status, shock and bilateral pneumonia. A previous study found hemoptysis, erythroderma and leukopenia were associated with mortality in young patients (median age 14.5 years) with CAP due to PVL-producing S. aureus20. In a recent study of S. aureus CAP (age 29–67 years), MRSA and PVL were risk factors of mortality21. For pneumococcal pneumonia, age, smoking, alcohol abuse, solid tumor, liver and renal diseases were predictors of mortality22.
Our study has limitations: (1) Because of the retrospective nature, not all patients underwent testing for coexisting pathogens. Whether influenza was associated with staphylococcal or pneumococcal pneumonia was unclear. (2) We did not examine PVL that may help explain the virulence and complications associated with staphylococcal pneumonia. (3) Case number was small.
In conclusion, Staphylococcal CAP/HCAP in our community mainly affected elderly patients with DM or CKD. Many had skin lesions or intravenous catheters. AMI was a lethal complication. 30-day mortality in the staphylococcal group (41%) was higher than the pneumococcal group (9.5%), especially among those without effective antimicrobials in 48 h (57%). Risk factors for 30-day mortality for all patients with staphylococcal or pneumococcal pneumonia were underlying disease, risk class 4/5, cancer, cirrhosis, altered mentality, shock, and bilateral pneumonia.
Material and methods
Patients and methods
This study was conducted in Fu Jen Catholic University hospital, a primary-care hospital opened in October 2017 in New Taipei city with 460 beds currently. The study was approved by the ethics committee of Fu Jen Catholic University Hospital (FJUH108004), and the requirement of the informed consent was waived for the study. We defined S. aureus as the causative organism of pneumonia if it was isolated from blood or pleural fluid23. From the electronic database of our microbiology laboratory, we retrieved adult patients (> 17 years-old) with S. aureus isolated from blood or pleural fluid between October 2017 and April 2020, and reviewed their electronic records. We then identified all cases that fulfilled criteria for CAP or HCAP. Patients who presented to our emergency (ED) or outpatient department with at least 2 of the following criteria were defined as having CAP: fever, chest pain, cough, shortness of breath, in addition to having radiographic pneumonia, and without any criteria for HCAP23,24. Patients were determined to have HCAP if they had been hospitalized within 90 days; lived in nursing home; or had received intravenous antibiotics, chemotherapy, wound care, or hemodialysis in health-care facility within 30 days25. We calculated their pneumonia severity index (PSI) score and risk of death within 30 days26.
For comparison, we also retrieved adult patients with diagnosis of pneumococcal CAP or HCAP during the same period. We defined S pneumoniae as the causative organism of pneumonia if it was isolated from blood or pleural fluid, or if pneumococcal antigen was detected in urine23. We compared their clinical data and images with those of patients with staphylococcal CAP or HCAP.
Microbiological and viral testing
All patients had blood culture sampled at ED or within 48 h of admission, except for two patients with S. pneumoniae CAP. Blood culture was performed using Virtuo BacT/ALERT system (Biomerieux, Hazelwood, MO, USA). Staining and culturing of samples for common bacteria/fungi or Mycobacterium tuberculosis, urinary antigen tests for S. pneumoniae and Legionella pneumophila (BinaxNow, Alere, USA), nasopharyngeal swab for influenza antigen, influenza polymerase chain reaction (PCR) (Luminex ARIES system, Austin, TX, USA) or SARS-CoV-2 real-time PCR (Applied Biosystems, Waltham, MA, USA) were performed if deemed necessary by the attending physician. Bacterial identification and antimicrobial susceptibility were performed using VITEK 2 system (VITEK 2XL, Biomerieux, Duram, NC, USA).
Statistical analysis
Quantitative data were expressed as mean ± standard deviation, and differences between groups were analyzed by t-test. Percentages of cases with certain characteristics were compared using chi-square test. Associations between 30-day mortality and various factors were analyzed using chi-square test and multivariate logistic regression model. A two-sided p < 0.05 was considered significant. All analyses were performed using SAS (Version 9.2, SAS Institute Inc., Cary, NC).
Statement of ethics
We conducted the trial in accordance with good clinical practice guidelines and the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Fu Jen Catholic University Hospital, New Taipei City, Taiwan (FJUH108004). The informed consent was waived because of the retrospective nature of this study.
Data availability
Data collected and analyzed in the current study are available from the corresponding author on reasonable request.
References
Francis, J. S. et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the panton-valentine leukocidin genes. Clin. Infect. Dis. 40, 100–107. https://doi.org/10.1086/427148 (2005).
Hageman, J. C. et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg. Infect. Dis. 12, 894–899. https://doi.org/10.3201/eid1206.051141 (2006).
Kallen, A. J. et al. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann. Emerg. Med. 53, 358–365. https://doi.org/10.1016/j.annemergmed.2008.04.027 (2009).
Webster, D., Chui, L., Tyrrell, G. J. & Marrie, T. J. Health care-associated Staphylococcus aureus pneumonia. Can. J. Infect. Dis. Med. Microbiol. 18, 136796. https://doi.org/10.1155/2007/136796 (2007).
Santos, J. W. et al. Community-acquired Staphylococcal pneumonia. J. Bras. Pneumol. 34, 683–689 (2008).
Self, W. H. et al. Staphylococcus aureus community-acquired pneumonia: prevalence, clinical characteristics, and outcomes. Clin. Infect. Dis. 63, 300–309. https://doi.org/10.1093/cid/ciw300 (2016).
Skull, S. A., Krause, V., Coombs, G., Pearman, J. W. & Roberts, L. A. Investigation of a cluster of Staphylococcus aureus invasive infection in the top end of the Northern Territory. Aust. N. Z. J. Med. 29, 66–72. https://doi.org/10.1111/j.1445-5994.1999.tb01590.x (1999).
Turnidge, J. D. et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med. J. Aust. 191, 368–373. https://doi.org/10.5694/j.1326-5377.2009.tb02841.x (2009).
Kluytmans, J., van Belkum, A. & Verbrugh, H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10, 505–520. https://doi.org/10.1128/CMR.10.3.505 (1997).
Lipsky, B. A., Pecoraro, R. E., Chen, M. S. & Koepsell, T. D. Factors affecting Staphylococcal colonization among NIDDM outpatients. Diabetes Care 10, 483–486. https://doi.org/10.2337/diacare.10.4.483 (1987).
Heukelbach, J., Wilcke, T., Winter, B. & Feldmeier, H. Epidemiology and morbidity of scabies and pediculosis capitis in resource-poor communities in Brazil. Br. J. Dermatol. 153, 150–156. https://doi.org/10.1111/j.1365-2133.2005.06591.x (2005).
Wang, C.-H. et al. Risk factors for scabies in Taiwan. J. Microbiol. Immunol. Infect. 45, 276–280. https://doi.org/10.1016/j.jmii.2011.12.003 (2012).
Macfarlane, J. & Rose, D. Radiographic features of Staphylococcal pneumonia in adults and children. Thorax 51, 539–540. https://doi.org/10.1136/thx.51.5.539 (1996).
Löffler, B. et al. Pathogenesis of Staphylococcus aureus necrotizing pneumonia: the role of PVL and an influenza coinfection. Expert. Rev. Anti-Infect. Ther. 11, 1041–1051. https://doi.org/10.1586/14787210.2013.827891 (2013).
van der Poll, T. & Opal, S. M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 8, 32–43. https://doi.org/10.1016/S1473-3099(07)70265-7 (2008).
Jackson, M. L. et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin. Infect. Dis. 39, 1642–1650. https://doi.org/10.1086/425615 (2004).
Blaschke, A. J. Interpreting assays for the detection of Streptococcus pneumoniae. Clin. Infect. Dis. 52(Suppl 4), S331–S337. https://doi.org/10.1093/cid/cir048 (2011).
Capelastegui, A. et al. Pneumococcal pneumonia: differences according to blood culture results. BMC Pulm. Med. 14, 128. https://doi.org/10.1186/1471-2466-14-128 (2014).
Amaro Rodriguez, R. et al. Bacteremic vs non-bacteremic pneumococcal pneumonia in immunocompetent patients: predictive and prognostic factors. Eur. Respir. J. 48, PA604. https://doi.org/10.1183/13993003.congress-2016.PA604 (2016).
Gillet, Y. et al. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing panton-valentine leukocidin. Clin. Infect. Dis. 45, 315–321. https://doi.org/10.1086/519263 (2007).
Gillet, Y. et al. Prognostic factors of severe community-acquired staphylococcal pneumonia in France. Eur. Respir. J. 58, 2004445. https://doi.org/10.1183/13993003.04445-2020 (2021).
Naucler, P., Darenberg, J., Morfeldt, E., Örtqvist, A. & Henriques Normark, B. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax 68, 571. https://doi.org/10.1136/thoraxjnl-2012-203106 (2013).
Mandell, L. A. et al. Summary of Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can. J. Infect. Dis. Med. Microbiol. 11, 237–248. https://doi.org/10.1155/2000/457147 (2000).
Marrie, T. J. et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. JAMA 283, 749–755. https://doi.org/10.1001/jama.283.6.749 (2000).
American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171, 388–416. https://doi.org/10.1164/rccm.200405-644ST (2005).
Fine, M. J. et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336, 243–250. https://doi.org/10.1056/NEJM199701233360402 (1997).
Funding
The study was funded by Fu Jen Catholic University Hospital (PL-201908003-V). The funder is not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
L.N.L. conceptualization, methodology, formal analysis, investigation, resources, data curation, writing original and revised draft, visualization, project administration, funding acquisition; W.R.C. investigation, resources, project administration; J.Y.W. methodology, software, formal analysis, investigation, data curation, writing-review and editing, visualization; Y.L.K. investigation, resources, project administration; C.Y.C. investigation, resources, project administration; Y.C.L. investigation, resources, project administration; S.H.T. investigation, resources, project administration; W.C.T. methodology, investigation, data curation; K.Y.C. investigation, data curation, project administration; W.L.L. conceptualization, methodology, resources, writing-review and editing, project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, LN., Chou, WR., Wang, JY. et al. Characteristics and local risk factors of community-acquired and health-care-associated Staphylococcus aureus pneumonia. Sci Rep 12, 18670 (2022). https://doi.org/10.1038/s41598-022-23246-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23246-1
- Springer Nature Limited