Abstract
To develop a new tool to assess the global quality of care for post-partum hemorrhage (PPH)—the leading preventable cause of maternal mortality worldwide—and to identify characteristics of maternity units associated with inadequate PPH management. This is a secondary analysis of the EPIMOMS population-based study conducted in 2012–2013 in 119 french maternity units (182,309 women who gave birth). We included women with severe PPH. We first developed a score to quantify the quality of care for PPH. Then, we identified characteristics of the maternity units associated with “inadequate care” defined by a score below the 25th percentile, with multi-level logistic regression adjusted for individual characteristics. The score combined 8 key components of care and took into account delivery mode and PPH cause. For PPH after vaginal delivery, the risk of inadequate care was increased in low versus high-volume maternity units (< 1000 deliveries/year: aOR-2.20 [1.12–4.32], [1000–2000 [deliveries/year: aOR-1.90 [1.02–3.56] compared to ≥ 3500 deliveries/year), in private versus public units (aOR-1.72 [1.00–2.97]), and in low versus high-level of care units (aOR-2.04 [1.24–3.35]). For PPH after cesarean, the only characteristic associated with an increased risk of inadequate care was the absence of 24/24-onsite anesthesiologist (aOR-4.34 [1.41–13.31]). These results indicate where opportunities for improvement are the greatest.
Similar content being viewed by others
Introduction
Postpartum hemorrhage (PPH) complicates about 10% of deliveries and is a leading cause of maternal mortality and severe morbidity worldwide1,2,3. The prevention and initial management of PPH are determinant in limiting progression to a severe form. Certain inappropriate or missing practices are clearly associated with a risk of worsening of the initial hemorrhage4,5. Given the large preventabiliy6,7,8 of maternal mortality and severe morbidity due to PPH, involving inadequate care9,10,11,12, there has been a proliferation of initiatives to improve practices in the management of PPH. The World Health Organization (WHO) and numerous countries have issued evidence-based guidelines13,14,15 accompanied by operational initiatives to expedite their dissemination and application16,17,18. Despite these efforts, the implementation of the guidelines into clinical practice remains insufficient and there is a persistent high rate of nonoptimal management of PPH19,20,21, so it is important to further characterize opportunities for improvement.
To properly identify non optimal management of PPH, we first need to dispose of an appropriate measurement tool to assess the global quality of care for PPH. Indeed, tools have been proposed, but have limitations. Global qualitative assessment by experts is subjective and therefore of questionable reproducibility22. Measurement of the implementation of a particular recommended item of management is more objective, but does not allow assessment of management as a whole23,24,25. So, there is a pressing need for a tool enabling the objective assessment of the global quality of care for PPH. Such a tool could be used to monitor the impact of practice improvement interventions or to make comparisons between hospitals.
Available literature suggests that the organizational environment of delivery care, approached by maternity hospitals’ characteristics, may influence the quality of obstetrics care26. Better understanding the degree to which PPH guidelines are integrated into practice and the impact of maternity hospitals characteristics on this integration, using an appropriate assessment tool, would enable identification of the units where the scope for improvement is greatest.
Our aims were, first, to develop a new quantitative tool to assess the global quality of care for first line management of PPH and, second, to identify the characteristics of maternity units associated with inadequate care.
Materials and methods
Population
This was a secondary analysis of data from the EPIMOMS prospective population-based study designed to study severe maternal morbidity27,28. The source population comprised 182,309 women who gave birth in the 119 maternity units of 6 regions between May 2012 and November 2013, i.e. approximately one-fifth of births in France over this period. The characteristics of the women who gave birth and of the maternity units were similar to those of the national profile29. All women included gave their consents for the use of their medical records. Of these women, 2540 (1.4%) presented severe maternal morbidity and were prospectively identified by the health professionals using a multi-criteria definition previously standardized by the Delphi expert consensus method at a national level (Appendix S2). The most severe acute maternal morbidity events were defined as “near-misses” according to the WHO definition30. Data on the women’s characteristics were collected using a questionnaire completed from a manual review of medical files by research midwives. A specific section on the management of PPH was provided for the women who presented severe PPH. The questionnaire contained precise information on the procedures implemented, the treatments administered, and the different timings. Data on the characteristics and organization of the maternity units (general organization, equipment, human resources) were collected in a maternity unit-specific questionnaire completed by the head of each maternity unit.
For our analysis, we first selected from the EPIMOMS women with severe maternal morbidity those whose morbidity was caused by severe PPH (N = 1580). These were women who presented PPH with at least one criterion of major bleeding (blood loss ≥ 1500 mL or transfusion ≥ 4 RBC units, surgical procedure/embolization) or PPH that led to acute hematologic dysfunction (anemia ≤ 7 g/dL, thrombocytopenia ≤ 50,000 platelets/mm3, disseminated intravascular coagulation), any organ failure according to EPIMOMS criteria, admission to an intensive care unit, or maternal death. We excluded some cases of PPH whose management was specific and not addressed in the French guidelines for PPH31: surgical wound without associated atony (N = 132), abnormal placental insertion (N = 112), uterine rupture (N = 36), amniotic fluid embolism (N = 13), vaginal thrombus without associated atony (N = 12), uterine inversion (N = 2), secondary PPH (N = 47), and PPH occurring at home (N = 7).
Of the 1233 women eligible, 129 had missing data on the score criteria and could not be included, so our study population finally included 1104 women with severe PPH (Appendix S3 shows the characteristics of the 129 women with missing scores). The flow chart is presented in Appendix S4.
Outcome
We first developed a new quantitative tool to assess the global quality of care for PPH. The assessment score was based on key components of current guidelines selected by an independent and multidisciplinary panel of experts. All the experts had participated in the elaboration of the French guidelines for PPH31. Although those guidelines were edited in 2014 and thus posterior to the inclusion period, first line treatment for PPH management did not differ from the previous version in 200432. Key components of PPH care were discussed during a first meeting and then validated by consensus during a second meeting. The 8 criteria selected targeted prevention and first-line treatment, i.e. care administered before any invasive procedure. When relevant, the criteria were specific to a mode of delivery or PPH etiology. Each criterion was weighted as major (weight of 2) or minor (weight of 1) according to the consensus opinion of the experts. For each woman, the quality score was calculated by combining these weighted criteria into a single figure, as the ratio of the number of criteria met (numerator) over the number of expected criteria (denominator). The calculations are detailed in Table 1. This score therefore corresponds to the percentage of expected items of management that were implemented adequately. The score ranged between 0% for a woman in whom none of the expected items of care were implemented adequately to 100% for a woman in whom all the expected items of care were implemented adequately.
The outcome for the analysis of determinants was inadequate care, a binary variable defined by an quality score strictly below the 25th percentile of distribution in the study population.
Covariables
The characteristics of the maternity units considered were the level of care (level 1, no neonatal unit, to level 3, neonatal intensive care unit), hospital status (public university, other public, private), the annual number of deliveries (< 1000, [1000–2000], [2000–3500], ≥ 3500), and the 24/24 onsite presence of an obstetrician and of an anesthesiologist.
We explored as potential confounders individual covariables including sociodemographic characteristics of the women as well as characteristics of their pregnancy and delivery. We created a PPH risk profile composite variable for the women with at least one risk factor, among a history of PPH, a multiple pregnancy, pre-eclampsia, and delivering a baby of birth weight above 4000 g.
Analysis
We first described the characteristics of the women and of the maternity units where they gave birth, and then the prevalence of each criterion of quality of care as well as the overall distribution of the quality of care score.
The associations between the characteristics of the maternity units and inadequate care were studied by univariate and then multivariate multilevel logistic regression with a random intercept for the maternity unit. The choice of variables included in the multivariate models was guided by the available literature and by the results of the univariate analysis.
A first model included all the maternal variables selected. Then, we built separate models to estimate the association with quality of care for each hospital characteristic, adjusted for maternal characteristics. We assessed the relevance of combining several of these hospital characteristics in the same model, as those characteristics are very much interrelated. We did so based on the joint distribution of those characteristics in our sample and on the estimation of collinearity of these variables through the calculation of variance Inflation Factors (VIF).
We did a secondary analysis with stratification according to the mode of delivery, i.e. separately among the cases of PPH that occurred after vaginal delivery and among those that happened after cesarean delivery, because we hypothesized that the influence of the organizational environment on the quality of care may vary according to the mode of delivery. This analysis followed the same strategy as the analysis of the whole population.
The proportion of women with at least one missing value in the final multivariate model was 9.6%, principally for the variable “country of birth”. Characteristics of the women with full data were similar to those with missing data, which supported the missing at random hypothesis. We used multiple imputation chained equations according to Rubin’s rules to impute missing data (12 data sets imputed). The results of the univariate and multivariate analyses are presented with the imputed data. We also performed an analysis with the non-imputed data.
The statistical analyses were performed using Stata® software version 15.1. The threshold of significance was set at 0.05.
Accordance statement
The study was approved by the National Data Protection Authority (Commission Nationale de l’Informatique et des Libertés [CNIL] authorization no. 912210, Mar. 14, 2012). All methods were performed in accordance with the relevant guidelines and regulations. The study methods have been performed in accordance with the Declaration of Helsinki. All women included gave their written consents for the use of their medical records.
Ethical approval
All women included in this study gave their consents for the use of their medical records.
Results
Among the 1104 women with severe PPH included in the study, 364 (33%) presented very severe PPH qualified as a “near miss”. No PPH-related death occurred. There were 710 (64.3%) vaginal deliveries and 394 (35.7%) cesarean sections. In 771 (69.8%) of the cases of severe PPH, the cause was uterine atony. The description of the population is given in Table 2.
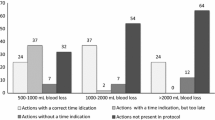
The proportion of compliant management varied from 91.7 to 35.7% depending on the item considered among the 8 selected (Table 3). With the composite score combining these different items, the overall care was fully adequate, i.e. the quality of care score was 100%, in 16% of the women. Twenty-five percent of the women received less than 53.8% of the expected quality of care items (i.e. 25th percentile of the distribution of the score defining inadequate care for the rest of the analysis) (distribution of the score in the study population in Fig. 1).
In the multivariate analysis, the only individual variable significantly associated with a decrease in the risk of inadequate care was maternal birthplace in sub-Saharan Africa (Appendix S5). After taking into account the maternal characteristics, all modes of delivery taken together, the only maternity unit characteristic associated with an increased risk of inadequate care was delivery in a type 1 maternity unit (aOR 1.68 [1.09–2.57] compared with a type 3 maternity unit) (Appendix S6).
Among women with PPH after vaginal delivery, delivery in a type 1 maternity unit was associated with a significant increase in the risk of inadequate care compared with a type 3 maternity unit (aOR 2.04 [1.24–3.35]), as was delivery in a private compared with a public university maternity hospital (aOR 1.72 [1.00–2.97]) or in a maternity unit with fewer than 2000 deliveries per year, compared with maternity units with more than 3500 deliveries per year (< 1000: aOR 2.20 [1.12–4.32]; 1000–2000: aOR 1.90 [1.02–3.56]) (Table 4). Among women with PPH after cesarean delivery, the only variable associated with a significant increase in the risk of inadequate care was delivery in a maternity unit where there was no 24/24 onsite anesthesiologist (aOR 4.34 [1.41–13.31]) (Table 5). The analysis with complete cases showed similar results (results not shown).
Discussion
Main findings
We developed a new synthetic assessment tool to quantify the global quality of care for PPH and applied it to a population-based sample of women with severe PPH. While the proportion of adequate care varied widely when considering each item of care separately, the use of our score allowed for an assessment of the global quality of care for PPH. This approach enabled us to explore the determinants of inadequate care and thus identify concrete areas for improvement. We found that some characteristics of the maternity units were associated with an increased risk of inadequate care and that these determinants differed by mode of delivery. Among the women with severe PPH after vaginal delivery, an increased risk of inadequate care was associated with delivery in a type 1 maternity unit, a private maternity unit, or a maternity unit with fewer than 2000 deliveries per year. Among the women with severe PPH after cesarean delivery, the only determinant associated with an increased risk of inadequate care was delivery in a maternity unit where there was no 24/24 onsite presence of an anesthesiologist.
Strengths and limitations
The main strength of our study was the creation and use of a composite score to evaluate the global quality of care for PPH. This score incorporates several dimensions of care and takes into account specificities related to the mode of delivery and to the etiology of PPH. This score is quantitative and easily reproducible so it can be used to evaluate practices and for monitoring, with a view to assessing the quality of care and disparities in quality over time or between maternity units.
A group of experts selected beforehand the components of care considered from French guidelines, which are very similar to international guidelines33. We chose to exclude some particular cases of PPH management (surgical wound without uterine atony, abnormal placental insertion, uterine rupture etc.) in which proper management of PPH require gesture specific to each etiology and circumstance, which are not standardized enough to be relevantly integrated in our score. This strategy allowed us to focus on a population in which PPH guidelines was duly applicable and in which our score was particularly appropriate.
The prospective identification of cases of severe PPH according to a standardized definition enabled exhaustive detection of cases in a source population comprising approximately one-fifth of all deliveries in France in a year. This strengthens the external validity of our results.
To study the determinants of inadequacy, we chose to perform the same analyses according to the mode of delivery, which was made possible by the construction of a quality of care score adaptable to each context. This approach seemed relevant to us because certain practices recommended in the case of PPH differ, thus raising questions regarding different aspects of the organization of care. The results in each of the two strata and the messages were in the end different.
In terms of the limitations of our study, we had to exclude 129 women, i.e. 10% of the population analyzed, because criteria of the score components were missing. However, there were few differences between the women excluded and the women included, which limited the impact of this potential selection bias. For the choice of quality of care criteria, some information on care provided was unavailable and so could not be included in the score, despite the wishes of the experts, notably the time taken to call the different health care professionals and their presence at the time of PPH, which are factors associated with the severity of PPH5. However, the fact that all the criteria of quality of care selected correspond to information easily available in the medical charts facilitates the practical use of the quality of care score. Lastly, the possibility of a measurement bias linked to the collection of data from medical charts cannot be eliminated, because a procedure performed but not noted or incompletely noted in the medical charts was considered as not done. However, failure to report clearly in the medical charts procedures that are deemed indispensable by the experts was considered to constitute inadequate care.
Interpretation
We found that an increased risk of inadequate care for PPH after vaginal delivery was associated with delivery in a private maternity unit, in a maternity unit with a low volume of deliveries, or in a type 1 (low level of care) maternity unit. In France, type 1 maternity units are generally private and small, while type 3 maternity units are usually public, associated with a university hospital, and large34. The risk factors for inadequate care revealed in our study therefore often correspond to the same institutions in which the overall care environment seems unfavorable to the proper application of guidelines. A first hypothesis is that the medical teams in institutions with a low volume of activity are less often faced with cases of severe PPH and so are less experienced in their management. Likewise, type 1 maternity units receive patients of low obstetrical risk and their medical teams are less used to managing complex obstetrical situations requiring coordination and team work. The culture of teamwork, particularly through the development of non-technical skills, is an important factor in the improvement of the quality of care for PPH, as shown by educational simulation training works35,36. In private maternity units, medical decisions concerning childbirth are taken individually by the referring obstetrician, which predisposes to variability of practices. Our results from real-life observations complete and strengthen those of a previous study which found more self-reported inadequate care in the management of the threat of premature delivery among practitioners from private maternity units26. In this study, the principal reported barriers to the application of guidelines were failure to question practitioners’ habits and poor team dialogue. A care environment that favors teamwork and harmonization of practices therefore seems to be important in reducing inadequate care for PPH. For instance, participation in a daily team meeting enabling discussion and debriefing regarding the previous day’s medical charts, as well as reviews of morbidity and mortality, help spread a culture of the evaluation of practices and of continuing training. These kinds of meetings are less frequent in type 1 maternity units and small maternity units34 which could therefore explain some of the disparities observed between maternity units11.
Finally, it is interesting to note that the determinants of inadequate care identified in our study align with those found in older population-based studies analyzing a specific item of PPH care or a subjective expert assessment22,23, but also with the determinants associated with the occurrence of severe complications of PPH9,37. These results strengthen the hypothesis of a continuum between inadequate care and worsening of PPH5. The use of our score to quantify the relationship between inadequate care and the severity of PPH could constitute an interesting avenue of research to support this hypothesis.
We found that, in PPH after cesarean, the only identified determinant of inadequate care was giving birth in a maternity unit where the anesthesiologist was not present on site 24/24. These results underscore the key role of the anesthesiologist in the management of PPH, as has been reported in several studies22,37. Because this factor was only identified in PPH after cesarean section, the post-surgical context seems particularly important in the pathway leading to inadequate care. One hypothesis is that in maternity units where the anesthesiologist is present onsite 24/24, post-cesarean monitoring is done in the recovery room and so is conducted by dedicated staff familiar with the post-operative context. This heightened monitoring could improve early detection of post-operative blood loss and so limit delays to care. Furthermore, the presence of a dedicated anesthesia team in the maternity unit facilitates collaboration between obstetricians and anesthesiologists by strengthening their involvement, for instance, in the implementation of protocols or in other aspects of the internal organization of care, like daily team meeting or reviews of morbidity and mortality. These organizational features, moreover, have been incorporated into multifaceted approaches to the implementation of guidelines on PPH care38,39 as well as into the “safety bundle” proposed by the American College of Obstetricians and Gynecologists17,40,41. Our results provide further support for their pivotal role in improving the quality of care.
In our study, the only maternal characteristic associated with a decrease in the risk of inadequate care was being born in sub-Saharan Africa. A first hypothesis is that these women are particularly represented in certain maternity units where, for reasons not explained by the variables studied in our models, the risk of inadequate care was reduced. In the French setting, migrant women often give birth in large urban public maternity units42. Another hypothesis is that medical teams are more attentive to these women, given their higher risk of severe obstetrical complications43.
Implications
Our results have implications for clinical practice as they provide an assessment of the quality of care for PPH and of the scope for improvement in this field. They are particularly relevant for the categories of maternity units shown to be at higher risk of inadequate care. Dissemination of these results may help local teams to involve in quality-of-care processes, and health care quality agencies to help them in this goal. Our score could be used to monitor quality of care in the same unit over time and between units.
Our results may guide future interventional research by identifying maternity units where opportunities for improvement are the greatest, which are privileged places to test the effectiveness of quality-of-care improvement interventions such as simulation training.
Finally, our results may also be of interest for health care users who look for more transparency on quality of care.
Conclusion
The development of a new quantitative assessment tool to quantify the global quality of care for PPH allowed the identification of some characteristics of maternity units associated with inadequate care, which indicate where opportunities for improvement are the greatest and where to focus practice improvement initiatives. The quality score developed in this study could be used to monitor the impact of such initiatives.
Data availability
The authors confirm that all data used in this study is available upon request from the Editorial Board Members.
References
Kassebaum, N. J. et al. Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1775–1812 (2016).
Calvert, C. et al. Identifying regional variation in the prevalence of postpartum haemorrhage: A systematic review and meta-analysis. PLoS ONE 7, e41114 (2012).
Carroli, G., Cuesta, C., Abalos, E. & Gulmezoglu, A. M. Epidemiology of postpartum haemorrhage: A systematic review. Best Pract. Res. Clin. Obstet. Gynaecol. 22, 999–1012 (2008).
Salati, J. A., Leathersich, S. J., Williams, M. J., Cuthbert, A. & Tolosa, J. E. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst. Rev. 4, CD001808 (2019).
Driessen, M. et al. Postpartum hemorrhage resulting from uterine atony after vaginal delivery: Factors associated with severity. Obstet. Gynecol. 117, 21–31 (2011).
Petersen, E. E. et al. Vital Signs: Pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb. Mortal. Wkly. Rep. 68, 423 (2019).
Mbrrace, U. Saving Lives, Improving Mothers’ Care. Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2015–17 2019 (National Perinatal Epidemiology Unit, University of Oxford Oxford, 2019).
Saucedo, M., Deneux-Tharaux, C., & Pour le Comité National d’Experts sur la Mortalité Maternelle. Maternal Mortality, Frequency, causes, women’s profile and preventability of deaths in France, 2013–2015. Gynecol. Obstet. Fertil. Senol. 49, 9–26 (2021).
Johansen, L. T., Braut, G. S., Acharya, G., Andresen, J. F. & Øian, P. How common is substandard obstetric care in adverse events of birth asphyxia, shoulder dystocia and postpartum hemorrhage? Findings from an external inspection of Norwegian maternity units. Acta Obstet. Gynecol. Scand. https://doi.org/10.1111/aogs.13959 (2020).
Dupont, C. et al. Severe postpartum haemorrhage after vaginal delivery: A statistical process control chart to report seven years of continuous quality improvement. Eur. J. Obstet. Gynecol. Reprod. Biol. 178, 169–175 (2014).
Rousseau, A., Rozenberg, P., Perrodeau, E., Deneux-Tharaux, C. & Ravaud, P. Staff and institutional factors associated with substandard care in the management of postpartum hemorrhage. PLoS One 11, e0151998 (2016).
Rousseau, A., Rozenberg, P., Perrodeau, E. & Ravaud, P. Variation in severe postpartum hemorrhage management: A national vignette-based study. PLoS ONE 13, e0209074 (2018).
World Health Organization & World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage (2012).
Vogel, J. P., Williams, M., Gallos, I., Althabe, F. & Oladapo, O. T. WHO recommendations on uterotonics for postpartum haemorrhage prevention: What works, and which one?. BMJ Glob. Health 4, e001466 (2019).
Dahlke, J. D. et al. Prevention and management of postpartum hemorrhage: A comparison of 4 national guidelines. Am. J. Obstet. Gynecol. 213(76), e1-76.e10 (2015).
Markow, C. & Main, E. K. Creating change at scale: Quality improvement strategies used by the California maternal quality care collaborative. Obstet. Gynecol. Clin. N. Am. 46, 317–328 (2019).
Althabe, F. et al. Postpartum hemorrhage care bundles to improve adherence to guidelines: A WHO technical consultation. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 148, 290–299 (2020).
Goffman, D. et al. The New York state safe motherhood initiative: Early impact of obstetric hemorrhage bundle implementation. Am. J. Perinatol. 36, 1344–1350 (2019).
Dupont, C. et al. Incidence and management of postpartum haemorrhage following the dissemination of guidelines in a network of 16 maternity units in France. Int. J. Obstet. Anesth. 18, 320–327 (2009).
Brace, V., Kernaghan, D. & Penney, G. Learning from adverse clinical outcomes: major obstetric haemorrhage in Scotland, 2003–05. BJOG Int. J. Obstet. Gynaecol. 114, 1388–1396 (2007).
Seacrist, M. J., VanOtterloo, L. R., Morton, C. H. & Main, E. K. Quality improvement opportunities identified through case review of pregnancy-related deaths from obstetric hemorrhage. J. Obstet. Gynecol. Neonatal Nurs. 48, 288–299 (2019).
Bouvier-Colle, M.-H. et al. Evaluation of the quality of care for severe obstetrical haemorrhage in three French regions. BJOG Int. J. Obstet. Gynaecol. 108, 898–903 (2001).
Schmitz, T. et al. Prostaglandin E2 analogue sulprostone for treatment of atonic postpartum hemorrhage. Obstet. Gynecol. 118, 257–265 (2011).
Woiski, M. D. et al. Guideline-based development of quality indicators for prevention and management of postpartum hemorrhage. Acta Obstet. Gynecol. Scand. 94, 1118–1127 (2015).
Talungchit, P., Liabsuetrakul, T. & Lindmark, G. Development and assessment of indicators for quality of care in severe preeclampsia/eclampsia and postpartum hemorrhage. J. Healthc. Qual. Off. Publ. Natl. Assoc. Healthc. Qual. 35, 22–34 (2013).
Rousseau, A., Azria, E., Baumann, S., Deneux-Tharaux, C. & Senat, M. V. Do obstetricians apply the national guidelines? A vignette-based study assessing practices for the prevention of preterm birth. BJOG Int. J. Obstet. Gynaecol. 127, 467–476 (2020).
Madar, H. et al. Severe acute maternal morbidity in twin compared with singleton pregnancies. Obstet. Gynecol. 133, 1141–1150 (2019).
Korb, D. et al. Risk of severe maternal morbidity associated with cesarean delivery and the role of maternal age: A population-based propensity score analysis. CMAJ. Can. Med. Assoc. J. J. Assoc. Med. Can. 191, E352–E360 (2019).
Blondel, B., Coulm, B., Bonnet, C., Goffinet, F. & Le Ray, C. Trends in perinatal health in metropolitan France from 1995 to 2016: Results from the French National Perinatal Surveys. J. Gynecol. Obstet. Hum. Reprod. 46, 701–713 (2017).
Say, L., Souza, J. P. & Pattinson, R. C. Maternal near miss—Towards a standard tool for monitoring quality of maternal health care. Best Pract. Res. Clin. Obstet. Gynaecol. 23, 287–296 (2009).
Sentilhes, L. et al. Postpartum hemorrhage: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 198, 12–21 (2016).
Goffinet, F. et al. Hémorragies du post-partum: recommandations du CNGOF pour la pratique clinique (décembre 2004). Gynécologie Obstétrique Fertil. 33, 268–274 (2005).
Sentilhes, L., Goffinet, F., Vayssière, C. & Deneux-Tharaux, C. Comparison of postpartum haemorrhage guidelines: Discrepancies underline our lack of knowledge. BJOG Int. J. Obstet. Gynaecol. 124, 718–722 (2017).
Fresson, J. et al. Les maternités en 2016. Premiers résultats de l’enquête nationale périnatale. (2017).
Brogaard, L. et al. The importance of non-technical performance for teams managing postpartum haemorrhage: Video review of 99 obstetric teams. BJOG Int. J. Obstet. Gynaecol. 126, 1015–1023 (2019).
Siassakos, D. et al. What makes maternity teams effective and safe? Lessons from a series of research on teamwork, leadership and team training. Acta Obstet. Gynecol. Scand. 92, 1239–1243 (2013).
Saucedo, M. et al. Delivery hospital characteristics and postpartum maternal mortality: A national case-control study in France. Anesth. Analg. 130, 52 (2020).
Audureau, E. et al. Practices for prevention, diagnosis and management of postpartum haemorrhage: Impact of a regional multifaceted intervention. BJOG Int. J. Obstet. Gynaecol. 116, 1325–1333 (2009).
de Visser, S. M. et al. Development of a tailored strategy to improve postpartum hemorrhage guideline adherence. BMC Pregnancy Childbirth 18, 49 (2018).
Burgansky, A., Montalto, D. & Siddiqui, N. A. The safe motherhood initiative: The development and implementation of standardized obstetric care bundles in New York. Semin. Perinatol. 40, 124–131 (2016).
Main, E. K. et al. Reduction of severe maternal morbidity from hemorrhage using a state perinatal quality collaborative. Am. J. Obstet. Gynecol. 216(298), e1-298.e11 (2017).
Azria, E., Stewart, Z., Gonthier, C., Estellat, C. & Deneux-Tharaux, C. Social inequalities in maternal health. Gynecol. Obstet. Fertil. 43, 676–682 (2015).
Urquia, M. L. et al. Disparities in pre-eclampsia and eclampsia among immigrant women giving birth in six industrialised countries. BJOG Int. J. Obstet. Gynaecol. 121, 1492–1500 (2014).
Acknowledgements
The authors thank the coordinators of the participating regional perinatal networks (Alsace, Aurore, Auvergne, Basse-Normandie, MYPA, NEF, Paris Nord, 92 nord, Lorraine) for their help in the coordination of women’s inclusion and data collection in their region; Chloé BARASINSKI, Sophie BEDEL, Aline CLIN D’AMOUR, Laurent GAUCHER, Isabelle LECREFF, Blandine MASSON, Carole RAMOUSSET, Mathias ROSSIGNOL, Zelda STEWART, Dalila TALAOURAR, Yacine TOURE, and Nicole WIRTH for their contribution to the implementation of the EPIMOMS study in their regions; the obstetricians, midwives, and anesthesiologists who contributed to case identification and documentation in their hospitals; and the research assistants who collected the data. The authors also thank the expert panel members (Marie-Pierre BONNET, Corinne DUPONT, Cyril HUISSOUD, Mathias ROSSIGNOL, Loïc SENTILHES, Véronique TESSIER, Christophe VAYSSIÈRE) for their contribution to the quality of care score. The authors thank David Marsh for his translation of this article.
Funding
The EPIMOMS study was funded by the National Research Agency (ANR) and the Ile de France Regional Health Agency (ARS). The first author received a training grant from the Ile-de-France Regional Health Agency. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
H.D. conducted the analyses and wrote the manuscript. C.D.-T. and F.G. initiated this study. They supervised the research work by planning and directing the realization of the analyses and correcting the writing of the manuscript. A.S. helped with the statistical analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Didelot, H., Goffinet, F., Seco, A. et al. Evaluating the quality of care for postpartum hemorrhage with a new quantitative tool: a population-based study. Sci Rep 12, 18626 (2022). https://doi.org/10.1038/s41598-022-23201-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23201-0
- Springer Nature Limited