Abstract
The objective of this study was to determine the relationship between maternal pre-pregnancy body mass index (BMI) and child hyperactivity-inattention symptoms (HIS) at 5 years, including preterm and term-born children, and to determine whether this association varied with gestational age. Maternal pre-pregnancy BMI and offspring HIS were assessed in 10,898 participants born ≥ 33 weeks of gestation from the ELFE cohort and 2646 children born between 23 and 34 weeks from the EPIPAGE 2 cohort. Reported pre-pregnancy weight (kg) and measured height (m) were collected from mothers at inclusion and used to classify BMI (kg/m2). Child HIS were evaluated using the Strengths and Difficulties Questionnaire around 5 years of age. Logistic regression estimated odds ratios (OR) of a high HIS score (≥ 90th percentile) in the ELFE cohort and generalized estimated equations were used in EPIPAGE 2 to account for non-independence of multiple births. As a negative control, paternal BMI was also considered as an exposure of interest in sensitivity analyses. Maternal pre-pregnancy obesity and overweight were associated with child HIS at 5 years in ELFE (adjusted OR [aOR] for obesity 1.27 [1.06, 1.53]; overweight aOR 1.16 [1.00, 1.36]) and pre-pregnancy obesity was associated with high HIS scores in preterm infants of EPIPAGE 2 (aOR 1.48 [1.06, 2.08]). In ELFE, the magnitude of the association increased with decreasing gestational age (interaction p = 0.02). High maternal pre-pregnancy BMI is associated with greater likelihood of high HIS scores in both at-term and preterm children at 5 years of age.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Despite the adverse consequences for both the mother and the child accompanying high maternal body mass index (BMI) before and during pregnancy, rates of overweight and obesity in pregnancy continue to rise globally, with estimated 10-year changes in some countries higher than 90%1. This leads to increasing concern as high BMI before pregnancy has been associated with numerous obstetric complications including: gestational diabetes, pre-eclampsia, congenital anomalies, and stillbirth2. Maternal pre-pregnancy obesity is also associated with several long-term adverse effects for the offspring such as increased risk for obesity, cardiovascular disease and type 2 diabetes3. High maternal pre-pregnancy BMI has also been linked to neurodevelopmental and psychiatric disorders in the child such as attention-deficit hyperactivity disorder (ADHD), cognitive impairment, autism spectrum disorder, anxiety, depression, schizophrenia and eating disorders4. ADHD is one of the most common neurodevelopmental disorders in childhood and can have negative long-term effects on the children and their families5. Children with ADHD have lower educational and professional attainment and poorer mental health6,7. Families suffer from the strain of the condition and ADHD contributes to marital dysfunction6. Symptoms also frequently persist into adulthood and more than half of these children have other comorbid conditions6, making the underlying etiology of ADHD an important avenue of investigation.
Animal models support a causal link between maternal obesity and offspring hyperactivity8. Several large cohort studies of children born at term have similarly observed an association between high maternal pre-pregnancy BMI and ADHD or hyperactivity-inattention symptoms (HIS), which can often accurately estimate an ADHD diagnosis outside of a clinical setting9. In a previous study conducted in a cohort of French children born at term from the general population, we also reported an association between maternal pre-pregnancy obesity and high HIS score trajectories in children from 3 to 8 years10. This association remained robust even after adjustment for numerous factors such as maternal lifestyle, childcare, child sleeping habits and home stimulation. However, the association between maternal obesity and HIS in premature children has been little explored. To our knowledge, there exists only one study limited to children born extremely preterm (< 28 weeks)11 despite the fact that children born preterm are already at increased risk of HIS/ADHD12 and the risk of neurodevelopmental deficits appear to increase in a step-wise manner with decreasing gestational age13. Compared to term-born children, premature children are also at increased risk for infant mortality, neonatal morbidity and numerous neurodevelopmental deficits13 that persist into adulthood as long-term disabilities and affect their educational attainment and incomes14. As maternal obesity is a state of chronic low-grade inflammation and inflammation has been shown to have adverse effects on the developing brain4,15, it is possible that infants born premature, both more functionally and immunologically immature than infants born at term, may be more sensitive to the insults triggered by maternal obesity due to the heightened or dysregulated inflammatory state they may be exposed to both in utero and after birth16. Given that maternal obesity is also a major risk factor for preterm labour17 and that this population is already highly vulnerable, it is imperative to identify and understand the consequences of modifiable risk factors, such as maternal pre-pregnancy obesity, that can influence a healthy birth and childhood.
Thus, the primary objective of this study was to determine if high maternal pre-pregnancy BMI is associated with child hyperactivity-inattention symptoms in both preterm and at-term infants. Secondarily, we aimed to determine whether this association varied in conjunction with gestational age.
Methods
Study population
ELFE
The ELFE cohort (French Longitudinal Study since Childhood) was launched in 2011 to study the determinants, from birth and throughout the life course, of child health, development and socialization18. Children were recruited at birth from 320 randomly selected maternity units throughout metropolitan France who agreed to participate. This recruitment spanned 25 days in four periods throughout the year. Eligibility criteria included: births ≥ 33 weeks of gestation, mothers ≥ 18 years old, and no plan to leave metropolitan France in the following 3 years. Information and consent documents were available in French, English, Arabic and Turkish and written informed consent was obtained from parents or the mother alone at enrollment. If not present at enrollment, the father was informed of his right to deny participation.
In total, 18,040 mothers agreed to participate in ELFE and gave birth to 18,329 infants (Fig. 1A). For 59 women, baseline data were withdrawn at her demand, resulting in an initial study population of 18,270 children. Data were collected from telephone interviews and internet or paper questionnaires with one or both parents.
Epipage 2
EPIPAGE 2 is a large population-based French cohort of preterm infants designed to study the short and long-term outcomes in preterm infants, the determinants of preterm birth, and to examine the impact of the changes in medical care and practices on preterm infants19. Live born, still born babies, and terminations of pregnancy (TOP) between 22 and 34 weeks of gestation in all maternities in 25 of 26 French regions were eligible. The recruitment period varied by gestational age group (extremely preterm [22–26 weeks of gestation], very preterm [27–31 weeks], moderately preterm [32–34 weeks]), with longer recruitment periods for the smaller gestational age groups. Between March 2011 and December 2011, 7804 participants were recruited into EPIPAGE 2 (Fig. 1B). A total of 4467 infants were discharged alive from neonatal care and 177 subsequently withdrew (family refused or child died), resulting in a total of 4290 infants eligible for follow-up.
At birth and in the neonatal period, data were extracted from medical records. Follow-up information was collected by questionnaires completed by parents, physicians, and assessments of health status and development at regional exam centres.
Exposure assessment
Information about maternal pre-pregnancy height and weight were extracted from medical records (EPIPAGE 2) or collected through face-to-face interviews by survey technicians at the time of delivery (ELFE). Maternal pre-pregnancy BMI (kg/m2) was subsequently categorized using the World Health Organization classification: underweight (< 18.5), normal weight (18.5–24.9), overweight (25.0–29.9) and obese (≥ 30).
Outcome assessment
HIS were assessed using the Strengths and Difficulties Questionnaire (SDQ) at 5.5 years of age. In ELFE, the questionnaire was administered through a phone interview with one of the parents (usually the mother), while in EPIPAGE 2 parents responded through a self-administered questionnaire. The SDQ is a validated tool to detect problem behaviours and emotions in children20. It includes 5 domains (hyperactivity-inattention, emotional symptoms, conduct problems, peer problems and prosocial behaviour), each comprised of 5 items with responses ranging from “Not true (0 points)” to “A little true (1 point)” to “Very true (2 points)”. The resulting score from each domain can range from 0 to 10. Data on HIS were collected for 11,247 children in ELFE and 2646 in EPIPAGE 2. A score ≥ 90th percentile in ELFE was considered “High”, which corresponded to a score of 7. This cut-off is in agreement with both the traditional SDQ threshold (which predicts a substantially increased probability of an independently diagnosed psychiatric disorder) and the French-specific threshold for “Abnormal”20,21. The same threshold was used in EPIPAGE 2.
Covariates
At baseline, covariates collected included maternal and paternal: age, country of birth, level of education, employment status, region of residence, household income and cohabitation status. Factors collected or calculated related to the pregnancy were: maternity unit, parity, folic acid intake before conception/from 0 to 12 weeks/ > 12 weeks (ELFE only), folic acid supplements around conception (EPIPAGE 2), gestational weight gain (ELFE), type 1 or 2 diabetes history, gestational diabetes, hypertension during pregnancy, pre-eclampsia, any psychiatric disorder before or during pregnancy, mode of delivery, cause of prematurity (EPIPAGE 2), gestational age, birth weight, birth length and maternal anxiety score (EPIPAGE 2). Variables concerning maternal lifestyle: smoking during pregnancy, alcohol consumption during pregnancy (ELFE), diet quality during pregnancy (score of adherence to the National Nutrition guidelines for pregnant women; ELFE)22 and physical activity during pregnancy (score = metabolic equivalents (METS)activity × time (hours) × 7 (days); ELFE). Information concerning current paternal anthropometrics (height, weight) was collected in the questionnaire 2 months postpartum (ELFE) or at discharge from the hospital (EPIPAGE 2). In ELFE, the question was directed to the father but could be responded to by the mother if the father did not respond. In EPIPAGE 2, the question was directed to the mother. Lastly, variables related to the child included: sex, duration of breastfeeding, childcare from birth to 5 years, sleep duration at 2 years, difficulties falling asleep at 2 years, duration of daily screentime at 2 years (ELFE), and the HOME-SF (Home Observation Measurement of the Environment-Short Form) score, a measurement of the quality and quantity of stimulation in the child’s home23 at 5.5 years (EPIPAGE 2).
Statistical analysis
Missing data
In the complete ELFE cohort (n = 18,329), missing data ranged from 0.3 to 46.3% from birth to 5.5 years (Supplementary Table S1a), while missing data ranged from 0 to 65% in EPIPAGE 2 (n = 4467) (Supplementary Table S1b). Multiple imputation with chained equations was used to impute missing data and generate 45 imputed datasets in ELFE and 65 in EPIPAGE 2. All variables listed above were used in the multiple imputation, with the discriminant function method for categorical variables and predictive mean matching for quantitative variables. To validate the imputed datasets, the means and frequencies for each imputation were compared to the original dataset to determine if they differed significantly24. The imputed datasets did not significantly differ from the original dataset (results not shown).
Weighting
After excluding those for which baseline data could not be imputed in ELFE (n = 59), inverse probability weighting (IPW) was used to attenuate the bias due to attrition in both ELFE and EPIPAGE 2. Models were fit for baseline data associated with dropout before the 5-year follow-up (p < 0.20). In ELFE, logistic regression models were used while in EPIPAGE 2 generalized estimating equations (GEE) models allowed us to take into account the large number of multiple births. Stabilized weights were calculated from these models and subsequently applied to further analyses. In general, attrition was more likely in younger parents who were not born in France, with lower socioeconomic status, and mothers with more health problems or an unhealthier lifestyle (Supplementary Table S2).
Data analysis
In ELFE, we excluded the small number of twins (n = 349) and those missing HIS data at 5.5 years (n = 7023), the final study population included 10,898 children (Fig. 1A). The final study population in EPIPAGE 2 was 2646 children after excluding those with no HIS data at 5.5 years (Fig. 1B). Descriptive analyses were performed on both populations to describe the overall distribution of characteristics. In EPIPAGE 2, the descriptive statistics were weighted to account for the recruitment scheme. Characteristics of participants with low and high (≥ 90th percentile) HIS scores were compared using ANOVA or by chi2 test, as appropriate. As high pre-pregnancy BMIs have been associated with greater risk of perinatal death25, we also performed descriptive analyses in the EPIPAGE 2 population between covariates and vital status (termination of pregnancy; stillbirth; death in delivery room; death in neonatology; alive at discharge) to better understand the competing risks in this vulnerable population.
An extensive literature review allowed the identification of potential confounding factors. We performed logistic regression in ELFE and GEE models in EPIPAGE 2 to account for the non-independence of multiple births. Univariate models were performed on all potential confounders and those associated with a high HIS score were considered for inclusion in the multivariate model (p < 0.20). Variables were retained in the multivariate model if they were considered essential based on the literature or if they changed the beta coefficients for the BMI categories by more than 10%. We tested for interactions with sex, gestational weight gain (ELFE only), gestational diabetes, hypertension and gestational age. All statistical analyses were performed on SAS 9.4 (SAS Institute Inc, Cary, NC).
Sensitivity analyses
Sensitivity analyses were conducted: (1) using only complete cases to evaluate the robustness of our findings with respect to the selection bias due to missing data; (2) Using the continuous HIS score; (3) Adjusting for paternal BMI, using it as a negative control of unmeasured familial or genetic confounding; (4) Additionally adjusting for gestational weight gain (ELFE only), as the role of gestational weight gain in the relationship between maternal obesity and child neurodevelopment is not clear; (5) Testing for interactions with sex, gestational weight gain and gestational age (continuous); (6) using the same adjustment factors for models in both ELFE and EPIPAGE 2 (and excluding potential moderators or mediators of the relationship).
Ethics approval
Both ELFE and EPIPAGE 2 received ethics approval for data collection from the French National Commission on Informatics and Liberty (CNIL), the Advisory Committee on Information Processing in Material Research in the Field of Health (CCTIRS), and the Committee for Protection of People (CPP).
Results
Descriptive
The descriptive characteristics of the ELFE study population are summarized in Table 1. Children with higher HIS scores were more likely to have slightly younger mothers with lower education, income, and no previous children. Parents of children with higher HIS scores were more likely to be obese or underweight, and mothers were more likely during pregnancy to smoke, have a psychiatric disorder, and have a less healthy diet. Children with higher HIS scores were more likely cared for by parents rather than in childcare centres, had more difficulties falling asleep at 2 years, longer screentime durations at 2 years and were slightly younger at evaluation.
Table 2 displays the descriptive characteristics of the EPIPAGE 2 study population by HIS scores. Children with higher HIS scores were more likely to have younger parents and in very and moderately preterm children, these mothers were more likely from a lower SES class and the children were more likely male. Very preterm children with high HIS were more likely born to mothers from France, with higher BMI, more symptoms of anxiety, and who were less likely to smoke during pregnancy. Very preterm children with HIS scores ≥ 7 were also born with lower birth weight, breastfed for shorter periods of time, had a less stimulating home environment at 5.5 years and suffered more frequent night waking at 2 years.
Vital status by maternal BMI in EPIPAGE 2
Mothers with pre-pregnancy obesity were over-represented in the vital status categories: stillborn, died in delivery room and died in neonatology (Supplementary Table S3). With regards to stillborns and infants that died in neonatology, women who were underweight before pregnancy were also more prevalent.
Maternal BMI and hyperactivity-inattention symptoms in offspring
In bivariate logistic regression in ELFE, maternal pre-pregnancy obesity, overweight and underweight were significantly associated with increased odds of a high HIS score in children at 5 years (unadjusted odds ratio [OR] 1.42 [95% CI 1.19, 1.69]; OR 1.28 [1.10, 1.49]; OR 1.27 [1.03, 1.56]; for obesity, overweight, and underweight, respectively) (Table 3). Adjustment for confounding factors attenuated the relationship between maternal obesity and high offspring HIS scores slightly (adjusted OR [aOR] 1.27 [1.06, 1.53]) and those for maternal overweight to the threshold of statistical significance (aOR 1.16 [1.00, 1.36]). After adjustment maternal underweight was no longer statistically significant, (aOR 1.14 [0.92, 1.41]).
In univariate analyses in EPIPAGE 2, maternal obesity was associated with increased odds of a high HIS score (OR 1.39 [1.02, 1.89]), while overweight and underweight were not. After adjustment for covariates, the magnitude of the relationship became slightly stronger (aOR 1.48 [1.06, 2.08]) (Table 3).
Sensitivity analyses
Complete case analyses
In complete case analyses in ELFE, we observed similar, though somewhat stronger in magnitude, relationships between maternal pre-pregnancy obesity and overweight with high HIS scores. Univariate models including 10,640 participants without missing data found comparable estimates to the imputed and weighted datasets (Supplementary Table S4a). After adjustment for confounding factors, the odds ratios were attenuated but remained significantly associated with a high HIS score in a population of 7185 children. Univariate complete case analyses in EPIPAGE 2 included 2465 participants (Supplementary Table S4b). Maternal pre-pregnancy obesity was associated with 1.5× increased odds of a high HIS score in offspring. After adjustment for covariates and the population decreased to 1232, the estimates were attenuated to 1.4x.
Linear regression analyses
In adjusted linear models for ELFE, both pre-pregnancy obesity and overweight were strongly associated with HIS scores as a continuous variable (p < 0.001 for obese mothers; p < 0.001 for overweight mothers) (Supplementary Table S5). Pre-pregnancy underweight was not associated with HIS scores in offspring at 5 years (p = 0.35). In adjusted linear models in EPIPAGE 2, only maternal obesity was associated with higher HIS scores (p < 0.001).
Paternal BMI
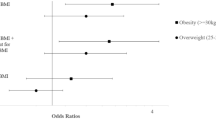
In ELFE, when paternal BMI was added to the model with maternal BMI, though the relationship with maternal obesity with offspring HIS did not change (aOR 1.24 [1.02, 1.49]), paternal obesity was also significantly associated with offspring HIS, with similar magnitude (aOR 1.30 [1.02, 1.64]) (Fig. 2). In EPIPAGE 2, the association between maternal obesity and a high HIS score did not significantly change when the model was adjusted for paternal BMI (aOR 1.53 [1.04, 2.24]) and paternal obesity was not associated with HIS symptoms (aOR 1.08 [0.70, 1.27]).
Adjusted odds ratios for a high hyperactivity-inattention symptom score (≥ 7) in 5.5 year old children of the ELFE (n = 10,898; black points) and EPIPAGE 2 cohorts (n = 2646; grey points). aAdjusted for maternal age at birth, maternal education, household income (ELFE), household socioeconomic category (EPIPAGE 2), parity, sex, psychological problems during pregnancy (ELFE), anxiety symptoms (EPIPAGE 2), singleton pregnancy (EPIPAGE 2), maternal physical activity during pregnancy (ELFE), maternal healthy diet score during pregnancy (ELFE), maternal alcohol intake during pregnancy (ELFE), maternal smoking during pregnancy, child age at evaluation, gestational age, breastfeeding, cause of prematurity (EPIPAGE 2) childcare at 2 years, screentime at 2 years (ELFE), HOME score (EPIPAGE 2), difficulties falling asleep at 2 years (ELFE) and frequent night waking at 2 years (EPIPAGE 2). bMissing covariates are imputed and weighted using IPW.
Gestational weight gain adjustment and interactions
Additional adjustment for gestational weight gain did not significantly change the odds ratios between maternal obesity or overweight and a high HIS score in ELFE. A significant interaction between gestational age and maternal BMI was observed in ELFE (p = 0.02), but not EPIPAGE 2 (p = 0.77). In ELFE, we observed a stronger association between maternal pre-pregnancy obesity and high HIS scores with decreasing gestational age (Fig. 3). Children born preterm (32–37 weeks) had 2.3× increased odds of a high HIS score if born to an obese mother (aOR 2.29 [0.83, 6.33]), while children born between 37 and 39.9 weeks had 1.4× greater odds of a high HIS score (aOR 1.36 [1.04, 1.77]) and children born at full-term (≥ 40 weeks) did not display a significantly increased odds of a high HIS score if born to an obese mother (aOR 1.18 [0.90, 1.56]). None of the other interactions tested were significant (p > 0.20).
Adjusted Odds ratios for maternal pre-pregnancy obesity and high hyperactivity-inattention symptom scores (≥ 7) by gestational age group in children 5 years old from the ELFE (n = 10,898) and EPIPAGE 2 (n = 2646) cohort studies .aAdjusted for maternal age at birth, maternal education, household income (ELFE), household socioeconomic category (EPIPAGE 2), parity, sex, psychological problems during pregnancy (ELFE), anxiety symptoms (EPIPAGE 2), singleton pregnancy (EPIPAGE 2), maternal physical activity during pregnancy (ELFE), maternal healthy diet score during pregnancy (ELFE), maternal alcohol intake during pregnancy (ELFE), maternal smoking during pregnancy, child age at evaluation, gestational age, breastfeeding, cause of prematurity (EPIPAGE 2) childcare at 2 years, screentime at 2 years (ELFE), HOME score (EPIPAGE 2), difficulties falling asleep at 2 years (ELFE) and frequent night waking at 2 years (EPIPAGE 2). bMissing covariates are imputed and weighted using IPW.
Similar adjustment factors
Using the same adjustment factors in both ELFE and EPIPAGE 2 did not change our estimates from the fully adjusted models (Supplementary Table S6).
Discussion
Maternal pre-pregnancy obesity was associated with higher HIS scores in offspring at 5.5 years. The magnitude of the association appears to vary by gestational age, with a more pronounced effect in children born preterm. This is the first study to examine this association in a large cohort of preterm infants and to compare the magnitude of the association among gestational age groups. The trends remained after adjustment for paternal BMI, maternal lifestyle variables and factors characterizing the postnatal environment which most previous studies could not account for.
Our results are in accordance with previous studies, which have reported a relationship between HIS and maternal obesity or both obesity and overweight9. We also previously reported a positive association between maternal obesity and child HIS trajectories from 3 to 8 years in the French EDEN cohort study10. The magnitude of the association in both ELFE and EPIPAGE 2 is somewhat smaller than that of EDEN, where we observed almost two-fold increased odds of a high HIS score trajectory with maternal obesity. Overall, meta-analyses suggest that high maternal pre-pregnancy BMI is associated with child ADHD/HIS9. However, several studies have not observed an association between high maternal BMI and child ADHD or HIS26,27,28, and in others the association was not robust among assessment types or evaluators11,29,30. To our knowledge, this is the first study to investigate whether the association between maternal obesity and HIS extends to very, moderate and late preterm infants, and to compare the magnitude across a range of gestational ages, including both infants born at term and preterm. In a study of extremely preterm infants (born < 28 weeks), van der Burg et al. found more than 2 × increased odds of parent-reported, but not teacher-reported, HIS in children at 10 years old born to mothers who were obese before pregnancy compared to mothers with a normal pre-pregnancy BMI11. This study was severely limited in the potential confounding factors it took into account and it is likely the small sample size also played a role (n < 200). Unfortunately, we did not have enough infants born before 28 weeks to be able to stratify our analyses and our results cannot be directly compared to this study.
In studies considering siblings born at term, positive associations were observed when considering the study population as a whole, but the relationship was attenuated to null in family-level analyses31,32, suggesting unmeasured familial confounding may be responsible for the overall association observed in many other studies. In this case, paternal BMI is a valuable negative control, as an association between paternal BMI and child HIS could suggest bias from familial lifestyle, genetics, or shared exposures rather than a direct intrauterine effect of maternal BMI. Indeed, several social factors have been associated with ADHD in offspring such as financial difficulties, social housing tenure and single parent status, which may act through mediators such as parent involvement and adversity risk33. However, in contrary to sibling studies, almost all studies adjusting for paternal BMI did not find any association between offspring HIS and paternal BMI27,34,35,36, including our own results in the EPIPAGE 2 or EDEN cohorts10. Only Mikkelson et al. observed an association between paternal obesity and child HIS, and observed the greatest behavioural difficulties in children when both parents were overweight or obese, suggesting some influence of the shared familial environment or genetics on the development of HIS37. The fact that we observed an association with paternal BMI in ELFE indicates that we cannot attribute the effects on increased HIS solely to an intrauterine mechanism in this study population. A very recent study conducted a Mendelian randomization analysis to test the hypothesis of a causal association between maternal BMI during pregnancy and offspring HIS38. The authors observed that the positive association between maternal BMI and offspring HIS remained, though slightly attenuated, after adjusting for both BMI and ADHD polygenic risk scores. This is consistent with the hypothesis that prenatal exposure to high maternal BMI may affect the offspring’s HIS risk through both shared genetic factors and intrauterine mechanisms.
There are several theories of possible biological mechanisms in the relationship between high maternal pre-pregnancy BMI and offspring hyperactivity. Obesity is a state of chronic inflammation and obese women have higher levels of pro-inflammatory factors compared to women with normal weight4,39. Proinflammatory cytokines have been linked to oxidative stress and inflammation in the placenta, altered cytokine expression, and changes in fetal brain gene expression4. Some cytokine levels, at birth (cord blood) or cross-sectionally at 5 years old, have also been associated with behavioural problems in children40,41,42. In a cross-sectional study of 5-year-old French children, Barbosa et al. found that low levels of the cytokine C–C motif chemokine ligand (CCL)2, responsible for monocyte recruitment to sites of inflammation43, were associated with higher HIS scores41. However, cord blood cytokines were not associated with HIS scores in children at 5 years40. On the other hand, in a follow-up study of infants born extremely preterm (< 28 weeks) previously mentioned, van der Burg et al. found that offspring of overweight or obese mothers were more likely to have increased concentrations of proinflammatory cytokines shortly after birth, but only among infants delivered for maternal or fetal indications and not among infants spontaneously born preterm44. As pregnancy disorders related to early spontaneous births are more likely to have inflammatory features than disorders leading to preterm delivery for maternal or fetal indications, they concluded that the role of maternal obesity may be obscured by the inflammation associated with spontaneous indications.
Behavioural development could also be altered by changes to the fetal steroid or hormonal environment due to increased levels of leptin and leptin resistance in obese women4. Higher maternal leptin levels have been correlated to higher leptin levels in the placenta, umbilical cord and fetus4. Leptin is believed to have a role in fetal brain development, specifically behavioural regulation45. In a Japanese cohort, Minatoya et al. observed a positive relationship between maternal pre-pregnancy obesity and high total behavioural problems46. In a later study, they unexpectedly found a significant association between increased cord blood leptin and decreased HIS SDQ scores47. Future studies are needed to examine the relationship between leptin levels and hyperactivity, as leptin has been shown to vary greatly by ethnicity and could explain the surprising results observed in this Japanese cohort48.
However, the effects of maternal pre-pregnancy obesity may not be linked to single metabolites, but rather the effect on the entire maternal metabolome. Girchenko et al. found 43 metabolites were significantly associated with early pregnancy BMI49. This profile was significantly associated with higher hazards of any mental and behavioural disorder in children up to 12 years old, as well as higher risk of increasing comorbid disorders49. Mediation analyses suggested that up to 60% of the effects of maternal BMI on offspring mental health could be attributed to the metabolomic profile49. Further mediation analyses are needed to confirm this potential indirect pathway.
There are several limitations in our study. As both cohorts are observational, there is a possibility that unmeasured confounding remains. We were also not able to adjust for precisely the same factors in both cohorts, which may account for some of the differences in the magnitudes of our estimates. In ELFE, pre-pregnancy weight relied on maternal self-report at delivery and could have caused some measurement error. However, maternal self-reported weight has been found to correlate highly with clinically assessed pre-pregnancy weight50. In addition, pre-pregnancy weight is more likely to be under-reported and thus would have attenuated our estimates rather than inflate them51. Symptoms of hyperactivity-inattention relied on parental report rather than clinical diagnoses. These scores may reflect biases that vary by evaluator, ethnicity, gender, or socioeconomic factors, but adjustment for maternal country of birth yielded similar results and the interaction between country of birth and SDQ scores was not significant. The SDQ has also been found to be a valid and reliable tool and we used a French-specific threshold for a probable clinical diagnosis score20,21. Finally, it’s possible that we observed a smaller risk of HIS symptoms in preterm infants than is true due to the competing risk of neonatal death. High pre-pregnancy BMI is associated with greater risk of perinatal death25 and descriptive analyses support this phenomenon in EPIPAGE 2. By conditioning on both live birth and survival to 5 years, the association between maternal obesity and offspring HIS may be distorted and diminished in preterm infants52.
This is the first large study of the effect of maternal pre-pregnancy BMI on the development of HIS symptoms in preterm and term infants. Other study strengths include the longitudinal follow-up of two large, nationwide cohort studies. We were able to improve efficiency and reduce bias due to missing data and loss to follow-up using multiple imputation and inverse probability weighting. This is also one of the few studies with available data on important confounders such as maternal diet, physical activity, alcohol consumption, child sleeping patterns and home stimulation. We also had data on paternal BMI available to allow us to use it as a negative control.
Conclusion
Maternal pre-pregnancy obesity was associated with increased odds of a high hyperactivity-inattention symptom score in both preterm and term children around 5 years of age. The magnitude of the association appears to increase with decreasing gestational age. Paternal BMI was also associated with child HIS scores in ELFE, suggesting that not all of the observed effect can be attributable solely to intrauterine programming due to maternal obesity. Given the growing proportion of obesity in pregnancy, future studies should focus on elucidating possible biological mechanisms involved, such as inflammation and leptin signaling. Efforts should be strengthened to ensure healthy weights in women of child bearing age, and health-care providers should be made aware of the possible increased risk of hyperactivity in the offspring of obese women in order to provide more timely management of the disorder.
Data availability
The datasets generated during and analysed during the current study are not publicly available due to privacy laws set by the Commission nationale de l’informatique et des libertés (CNIL). Anonymized data may be made available upon reasonable request to any public or private research team and with permission of the ELFE and EPIPAGE 2 scientific committees. Data requests concerning ELFE can be made through the website: https://pandora-elfe.inserm.fr/public/index.php. Data requests concerning EPIPAGE 2 can be made using through the email: accesdonnees.epipage@inserm.fr by using information provided on the data access website: https://epipage2.inserm.fr/index.php/en/related-research/265-data-access-and-questionnaires.
References
Chen, C., Xu, X. & Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 13, e0202183 (2018).
Leddy, M. A., Power, M. L. & Schulkin, J. The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 1, 170–178 (2008).
Godfrey, K. M. et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 5, 53–64 (2017).
Edlow, A. G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 37, 95–110 (2017).
Sayal, K., Prasad, V., Daley, D., Ford, T. & Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 5, 175–186 (2018).
Harpin, V. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch. Dis. Child 90, i2–i7 (2005).
Harpin, V., Mazzone, L., Raynaud, J. P., Kahle, J. & Hodgkins, P. Long-term outcomes of ADHD: A systematic review of self-esteem and social function. J. Atten. Disord. 20, 295–305 (2016).
Shook, L. L., Kislal, S. & Edlow, A. G. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 40, 1126–1137 (2020).
Jenabi, E., Bashirian, S., Khazaei, S. & Basiri, Z. The maternal prepregnancy body mass index and the risk of attention deficit hyperactivity disorder among children and adolescents: A systematic review and meta-analysis. Korean J. Pediatr. 62, 374–379 (2019).
Dow, C., Galera, C., Charles, M.-A. & Heude, B. Maternal pre-pregnancy BMI and offspring hyperactivity–inattention trajectories from 3 to 8 years in the EDEN birth cohort study. Eur. Child. Adolesc. Psychiatry https://doi.org/10.1007/s00787-022-02047-x (2022).
van der Burg, J. W. et al. Maternal obesity and attention-related symptoms in the preterm offspring. Early Hum. Dev. 115, 9–15 (2017).
Franz, A. P. et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: A meta-analysis. Pediatrics 141, e20171645 (2018).
Pierrat, V. et al. Neurodevelopmental outcomes at age 5 among children born preterm: EPIPAGE-2 cohort study. The BMJ 2021, 373 (2021).
Johnson, S. & Marlow, N. Early and long-term outcome of infants born extremely preterm. Arch. Dis. Child 102, 97–102 (2017).
Oldenburg, K. S., O’Shea, T. M. & Fry, R. C. Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Semin. Fetal. Neonatal Med. 25, 101115 (2020).
Sharma, A., Jen, R., Butler, A. & Lavoie, P. The developing human preterm neonatal immune system: A case for more research in this area. Clin. Immunol. 145, 61–68 (2012).
Cnattingius, S. et al. Maternal obesity and risk of preterm delivery. JAMA 309, 2362 (2013).
Charles, M. A. et al. Cohort Profile: The French national cohort of children (ELFE): Birth to 5 years. Int. J. Epidemiol. 49, 368–369 (2021).
Lorthe, E. et al. Cohort Profile: The Etude Epidémiologique sur les Petits Ages Gestationnels-2 (EPIPAGE-2) preterm birth cohort. Int. J. Epidemiol. 50, 1428–1429m (2021).
Goodman, R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 40, 1337–1345 (2001).
Shojaei, T., Wazana, A., Pitrou, I. & Kovess, V. The strengths and difficulties questionnaire: Validation study in French school-aged children and cross-cultural comparisons. Soc. Psychiatry Psychiatr. Epidemiol. 44, 740–747 (2009).
Kadawathagedara, M. et al. Adéquation des consommations alimentaires des femmes enceintes de l’étude ELFE aux recommandations du Programme national nutrition santé. Cahiers de Nutrition et de Diététique 52, 78–88 (2017).
Bradley, R. H., Caldwell, B. M. & Corwyn, R. F. The Child Care HOME Inventories: Assessing the quality of family child care homes. Early Child Res. Q. 18, 294–309 (2003).
Nguyen, C. D., Carlin, J. B. & Lee, K. J. Model checking in multiple imputation: An overview and case study. Emerg. Themes Epidemiol. 14, 1–12 (2017).
Marchi, J., Berg, M., Dencker, A., Olander, E. K. & Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 16, 621–638 (2015).
Brion, M.-J. et al. Maternal pre-pregnancy overweight and child cognition and behavior: Exploring intrauterine effects in two pregnancy cohorts. Pediatrics 127, e202–e211 (2011).
Casas, M. et al. Maternal pre-pregnancy obesity and neuropsychological development in pre-school children: A prospective cohort study. Pediatr. Res. 82, 596–606 (2017).
Li, M. et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 137, e20152206 (2016).
Jo, H. et al. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 135, e1198–e1209 (2015).
Mina, T. H. et al. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr. Res. 82, 47–54 (2017).
Chen, Q. et al. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: A population-based cohort study using a sibling-comparison design. Int. J. Epidemiol. 43, 83–90 (2014).
Musser, E. D. et al. Maternal prepregnancy body mass index and offspring attention-deficit/hyperactivity disorder: A quasi-experimental sibling-comparison, population-based design. J. Child Psychol. Psychiatry 58, 240–247 (2017).
Russell, A., Ford, T. & Russell, G. Socioeconomic associations with ADHD: Findings from a mediation analysis. PLoS ONE 10, e0128248 (2015).
Daraki, V. et al. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: The Rhea mother–child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 26, 703–714 (2017).
Brion, M. J. et al. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 127, e202 (2011).
Robinson, S. L. et al. Parental weight status and offspring behavioral problems and psychiatric symptoms. J. Pediatr. 220, 227-236.e1 (2020).
Mikkelsen, S. H. et al. Parental body mass index and behavioral problems in their offspring: A Danish national birth cohort study. Am. J. Epidemiol. 186, 593–602 (2017).
Karhunen, V. et al. The link between attention deficit hyperactivity disorder (ADHD) symptoms and obesity-related traits : Genetic and prenatal explanations. Transl. Psychiatry https://doi.org/10.1038/s41398-021-01584-4 (2021).
Rivera, H. M., Christiansen, K. J. & Sullivan, E. L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 9, 1–16 (2015).
Barbosa, S. et al. Immune activity at birth and later psychopathology in childhood. Brain Behav. Immun. Health 8, 100141 (2020).
Barbosa, S. et al. Serum cytokines associated with behavior: A cross-sectional study in 5-year-old children. Brain Behav. Immun. 87, 377–387 (2020).
Anand, D., Colpo, G. D., Zeni, G., Zeni, C. P. & Teixeira, A. L. Attention-deficit/hyperactivity disorder and inflammation: What does current knowledge tell us? A Systematic Review. Front Psychiatry 8, 228 (2017).
Bose, S. & Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 36, 1039–1050 (2013).
van der Burg, J. W. et al. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 79, 3–12 (2016).
Valleau, J. C. & Sullivan, E. L. The impact of leptin on perinatal development and psychopathology. J. Chem. Neuroanat. 61–62, 221–232 (2014).
Minatoya, M. et al. Associated factors of behavioural problems in children at preschool age: The Hokkaido study on environment and children’s health. Child Care Health Dev. 43, 385–392 (2017).
Minatoya, M. et al. Association between fetal adipokines and child behavioral problems at preschool age: The hokkaido study on environment and children’s health. Int. J. Environ. Res. Public Health 15, 120 (2018).
Mente, A. et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 33, 1629–1634 (2010).
Girchenko, P. et al. Maternal early-pregnancy body mass index-associated metabolomic component and mental and behavioral disorders in children. Mol. Psychiatry https://doi.org/10.1038/s41380-022-01723-3 (2022).
Lederman, S. A. & Paxton, A. Maternal reporting of prepregnancy weight and birth outcome: Consistency and completeness compared with the clinical record. Matern. Child Health J. 2, 123–126 (1998).
Headen, I., Cohen, A. K., Mujahid, M. & Abrams, B. The accuracy of self-reported pregnancy-related weight: A systematic review. Obes. Rev. 18, 350–369 (2017).
Kramer, M. S., Zhang, X. & Platt, R. W. Analyzing risks of adverse pregnancy outcomes. Am. J. Epidemiol. 179, 361–367 (2014).
Acknowledgements
The authors would like to thank the participants of the ELFE and EPIPAGE 2 studies and their families for their continued participation in these studies.
Funding
CD’s salary was funded by a Grant from the Fondation pour la Recherche Médicale (the French Foundation for Medical Research): SPF201909009122. EPIPAGE 2: The French National Institute of Public Health Research (IRESP TGIR 2009–01 programme)/Institute of Public Health and its partners [the French Health Ministry, the National Institute of Health and Medical Research (INSERM), the National Institute of Cancer, the National Solidarity Fund for Autonomy (CNSA)], the National Research Agency through the French programme of investments for the future (Grants No. ANR-11-EQPX-0038 and ANR-10-COHO-0001)) and the PremUp Foundation. Additional funding was obtained from the Fondation pour la Recherche Médicale (SPF 20160936356) and Fondation de France [00050329 and R18202KK (Grand Prix)]. The Elfe survey is a joint project between the French Institute for Demographic Studies (INED) and the National Institute of Health and Medical Research (INSERM), in partnership with the French blood transfusion service (Etablissement français du sang, EFS), Santé publique France, the National Institute for Statistics and Economic Studies (INSEE), the Direction générale de la santé (DGS, part of the Ministry of Health and Social Affairs), the Direction générale de la prévention des risques (DGPR, Ministry for the Environment), the Direction de la recherche, des études, de l’évaluation et des statistiques (DREES, Ministry of Health and Social Affairs), the Département des études, de la prospective et des statistiques (DEPS, Ministry of Culture), and the Caisse nationale des allocations familiales (CNAF), with the support of the Ministry of Higher Education and Research and the Institut national de la jeunesse et de l’éducation populaire (INJEP). Via the RECONAI platform, it receives a government Grant managed by the National Research Agency under the "Investissements d'avenir" programme (ANR-11-EQPX-0038 and ANR-19-COHO-0001).
Author information
Authors and Affiliations
Contributions
C.D., E.L., C.G., M.A.C., and B.H. contributed to study design and conceptualization. Data curation was performed by C.D., M.T., and L.M.M. Formal analyses were undertaken by C.D. C.D., P.Y.A., M.A.C. and B.H. participated in funding acquisition. C.D., E.L., M.A.C. and B.H. were responsible for investigation and methodology. C.D. was responsible for writing the original draft and all authors contributed to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dow, C., Lorthe, E., Marchand-Martin, L. et al. Maternal pre-pregnancy obesity and offspring hyperactivity-inattention symptoms at 5 years in preterm and term children: a multi-cohort analysis. Sci Rep 12, 18190 (2022). https://doi.org/10.1038/s41598-022-22750-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22750-8
- Springer Nature Limited