Abstract
Disorders of iron metabolism has been implicated in cardiovascular disease. However, the association of serum iron stores and coronary artery disease (CAD) remains inconsistent. Here, we investigated the associations of serum iron metabolism with the incidence of CAD, the severity of coronary artery stenosis, metabolic biomarkers, and the risk of major adverse cardiovascular event (MACE). A total of 643 CAD patients and 643 healthy controls were enrolled to assess the associations of serum iron status with the presence of CAD, the severity of CAD, and the risk of MACE. Serum iron metabolism and other metabolic markers were measured in all subjects. All statistical analyses were analyzed using SPSS22.0 software and STATA statistical package. Serum level of iron metabolism markers, including serum iron, unsaturated transferrin iron binding capacity (UIBC), Total iron binding capacity (TIBC) levels, in CAD groups was significantly lower than the control group (P < 0.001). UIBC and TIBC were negatively correlated with ferritin in both sexes. Each unit increase of serum iron and TIBC were found to have a protective role for CAD in women (iron: OR 0.794, 95% CI (0.647–0.973), TIBC: OR 0.891, 95% CI (0.795–0.999), P < 0.05). However, high ferritin level was significant associated the CAD incident in both sexes (OR 1.029, 95% CI (1.002–1.058) in men, OR 1.013, 95% CI (1.0–1.025) in women, P < 0.05). Serum iron metabolism markers exhibited no significant association with the severity of CAD. Increased serum level of iron and TIBC levels were found to have a protective role for CAD in women, but not in men. Elevated serum ferritin is independently and positively associated with CAD in men and women.
Similar content being viewed by others
Introduction
Iron plays an important role in several fundamental biological processes such as erythropoiesis and cell metabolism. Iron status is a modifiable feature associated with cardiovascular disease. The role of body iron indices has been reviewed in the pathogenesis of coronary artery disease (CAD)1,2,3,4,5. Iron metabolism disorders, either deficiency or overload, were associated with increased cardiovascular morbidity and mortality6,7. Iron overload was positively correlated with the risk factors of cardiovascular disease, such as the risk of metabolic syndrome8, insulin resistance (IR)9,10 and new-onset type 2 diabetes mellitus11. Excessive iron accumulation accelerates the formation of atherosclerosis through several putative mechanisms. Firstly, iron catalyzes Fenton reaction to produce reactive oxygen species (ROS). ROS promote LDL peroxidation and induce endothelial dysfunction by reducing the bioavailability of nitric oxide4,12,13,14,15,16,17. ROS increased the expression of LOX-1 receptor on endothelial cells, resulting in mitochondrial DNA damage and autophagy activation18. Secondly, accumulated iron in adipocytes leads to adipocyte IR by increasing lipolysis and by decreasing insulin-stimulated glucose transport7. Adipocyte IR accelerates atherosclerosis19.
There were lots of studies supported that iron overload was positively correlated with CAD incident9,20,21,22,23,24. Sullivan proposed that reduced iron stores can protect against ischemic heart disease25. Salonen et al. observed that a higher level of iron is a risk factor for myocardial infarction in Finnish men26. Previous researchers have tried to reduce blood iron content in various ways to decrease the CAD incident. It has been reported that reducing iron stores through phlebotomy could decelerate the progression of atherosclerotic plaque13,27. Iron chelation in patients with CAD has been shown to be associated with improved endothelial function28.
However, some studies found no evidence that reducing iron storage can prevent cardiovascular disease, and the results of others were even contrary to the hypothesis27,29,30,31,32. Iron deficiency was associated with an increased risk for CAD and had detrimental effects in patients with CAD6,32. Weather reduced iron can protect CAD incident or not, and to what level iron should be lowered to reduce CAD risk need further study. So we conducted a case–control study enrolled a total of 643 CAD patients and 643 healthy controls to analyze the associations of serum iron levels with the presence of CAD, the severity of coronary artery stenosis, and major adverse cardiovascular event (MACE) after revasculation. MACE included ischemic stroke, myocardial infarction, and hospitalization for heart failure.
Subjects and methods
Study population
The control group consisted of 643 (male/female 381/262) healthy persons without cardiovascular disease via physical examination and electrocardiogram. The controls were frequency-matched to the cases on age and sex. The CAD group consisted of 643 patients, 349 men and 294 women. All CAD patients underwent physical examination and review of their medical history. Patients were excluded if they (1) had heart failure, acute myocardial infarction, coronary bypass surgery or angioplasty, coronary spasm, or myocardial bridge; (2) had cardiac diseases such as cardiomyopathy, valvular or congenital heart disease, arrhythmia; (3) patients who had malignant tumors, acute or chronic infection, iron deficiency anemia, digestive system disease, fever, connective tissue disease, autoimmune disease; (4) patients whose Blood pressure ≥ 180/110 mmHg after taking standard antihypertensive drugs, severe hepatic or renal dysfunction; (5) A history of major surgical trauma, pregnancy, mental illness, or any other cause of active blood loss within 2 weeks. The Zhengzhou University ethics Committee approved this study (The approval number 2020-KY-172). Informed consent was obtained from all participants. All the investigations were performed in accordance with the principles of the Declaration of Helsinki.
Methods
All subjects underwent anthropometrical evaluation with measurements of weight, height, and body mass index (BMI). Smoking was defined as at least 1 cigarette per day for more than 1 year. Drinker was defined as alcohol intake of at least 90 or 45 g of liquor per day for more than 1 year for men or women, respectively.
Laboratory determinations
All blood samples were obtained after overnight fasting. Fasting plasma glucose (FPG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides (TG), high sensitive C-reactive protein (hs-CRP), iron metabolism biomarkers, liver function and renal function markers were measured on a Cobas 8000 Analyzer (Roche Diagnostics, Germany) using Roche Reagent following the manufacturer’s manual.
FPG was measured by the hexokinase method; TG, TC, LDL-C and HDL-C, Alanine aminotransferase (ALT) and aspartate aminotransferase (AST), creatinine, urea nitrogen (UREA), uric acid (UA) concentrations were evaluated by enzymatic methods. Hemoglobin A1c (HbA1c) was assayed by high-performance liquid chromatography on a Primer-Premier Hemoglobin Testing System (reagents were supplied by Primus). hs-CRP, homocysteine (Hcy), Apolipoprotein A (ApoA), Apolipoprotein B (ApoB) and serum ferritin levels were measured using an immune-turbid metric assay. The serum iron and unsaturated transferrin iron binding capacity (UIBC) was measured using a colorimetric test. Total iron binding capacity (TIBC) equaled the serum iron levels plus serum UIBC levels. Iron deficiency was defined as: ferritin < 100 ng/mL or 100–300 ng/mL with transferrin saturation (Tfs) < 20%33. Finally, estimated glomerular filtration rate (eGFR) was computed by using the chronic kidney disease epidemiology (CKD-EPI) collaboration equation34. Selective cardioangiography (CAG) was performed in all patients using the standard Judkins technique. The localization of coronary artery disease and the rate of lumen stenosis were determined by CAG. Structured interviews with a standardized questionnaire, including the demographics, Diabetes management, vascular risk factors (such as smoking and drinking status), and medical history, were performed by trained investigations.
Follow-up and study outcomes
The primary outcome was the first occurrence of major adverse cardiovascular events (MACE), including ischemic stroke, myocardial infarction, and hospitalization for heart failure. Patients were followed up starting from the index date (at the health screening examination date) until two years after revasculation.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (normally distributed data). Categorical variables are expressed as the frequency and its percentage. Continuous variables were analyzed using Student's t-test in normally distributed data, and Mann–Whitney test in non-normally distributed data. Chi-squared test was utilized for categorical variables. The association between continuous variables was assessed by Pearson correlation. The association of iron metabolism biomarkers with CAD was analyzed by logistic regression models with three progressive degrees of adjustment. Model 1 was a crude model without any confounders; model 2 was adjusted for age and cardiovascular risk factors including smoking habit, alcohol drinking habit, body mass index (BMI), hypertension, and dyslipidemia; model 3 was additionally adjusted for laboratory tests including HbA1C, ALT, AST, TC, TG, hs-CRP, eGFR. To avoid collinearity, we did not include iron and transferrin saturation into the multiple liner regression models because these two variables lied into the same causal pathway between ferritin and CAD. All the statistical analyses were executed using Statistical Package for Social Science (SPSS, version 22.0) and STATA statistical package (version 13, Texas, USA).
Results
Clinical characteristics
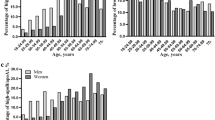
Table 1 showed the general characteristics of the study groups according to the sexual groups. There was no significant difference in age and diastolic blood pressure (DBP) between CAD group and controls. Overall, CAD patients were more likely to be smokers, alcohol drinkers, obese, hypertensive, dyslipidemia and hyperglycemia. Comparing to the controls, individuals in the male CAD group showed significantly lower plasma concentrations of Total proteins (TP), Albumin (ALB), ApoA, Tfs and serum iron levels. While blood pressure, body mass index (BMI) and the plasma concentrations of ferritin, HbA1c, fasting blood glucose (FBG), hs-CRP, liver function and renal function markers were significantly higher in the CAD group compared to the control (P < 0.05) (Table 1, Fig. 1). Additionally, significant differences were found in the diabetes management styles between male and female patients with CAD (Supplement Table S1).
Correlation between ferritin and other metabolic biomarkers in CAD patients
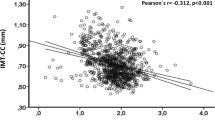
We analyzed the correlation between ferritin and other metabolic biomarkers in CAD by Pearson correlation. We totally tested the correlation between serum ferritin and 16 biomarkers, i.e. blood lipids (LDL-C, HDL-C, TC, TG, ApoA, ApoB and Lp(a)), blood glucose (HbA1c), blood pressure (systolic blood pressure, SBP and diastolic blood pressure, DBP), eGFR, proinflammatory measures (hs-CRP), adiposity measure (BMI) and other iron metabolism markers. TC, TG, LDL-C and Tfs were positively correlated with ferritin in men. HDL-C, ApoA, and eGFR were negatively correlated with ferritin in women, while BMI was positively associated with ferritin in women (Table 2). UIBC and TIBC were negatively correlated with ferritin in both sexes. Interestingly, HbA1C level was negatively correlated with ferritin in men, while inversely associated with ferritin in women.
Logistic regression analysis of iron metabolic markers and the risk of CAD and MACE risk
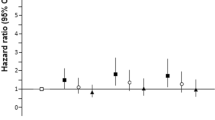
To illustrate the continuous relationships between parameters of iron metabolism and the risk of CAD, we assessed the concentration-risk relationship between serum ferritin levels and CAD risk by multivariate random-effects meta-regression based on the restricted cubic spline model with four knots (Fig. 2). Visual inspection revealed J-shaped relationships between ferritin and the ORs for the risk of CAD.
From an unadjusted multivariable logistic regression analysis, each unit increase in ferritin was associated with a 1.003-fold (95% confidence interval (CI), 1.002–1.004) and 1.01-fold (95% CI, 1.000–1.01) increased Odds Ratio (OR) of CAD in male and female groups respectively (Table 3). Each unit increase of serum iron level and Tfs were found to have decreased OR for CAD in both sexes (iron OR 0.83, 95% CI 0.803–0.858 in men, OR 0.894, 95% CI 0.860–0.929 in women, Tfs OR 0.896, 95% CI 0.869–0.911 in men, OR 0.943, 95% CI 0.921–0.967 in women, P < 0.05, Table 3). Increased serum level of TIBC had significantly lower risks for CAD in women (OR 0.943, 95% CI (0.921–0.967), P < 0.001, Table 3).
When further adjusted for age, BMI, SBP, DBP, HR, smoking habit, alcohol drinking habit, hypertension, diabetes status, dyslipidemia, medicine and family history of CAD (model 2), the effect modification by serum iron, ferritin levels and Tfs on the CAD risk remained similar significant in both sexes. Additional adjustment for laboratory tests including HbA1C, ALT, AST, TC, TG, hs-CRP, eGFR (model 3) there was no significant direct correlation for serum iron and Tfs on the CAD risk in men (P > 0.05). Increased serum level of iron (OR 0.794, 95% CI (0.647–0.973), and TIBC (OR 0.891, 95% CI (0.795–0.999) were found to have a protective role for CAD in women (P < 0.05, Table 3). The OR for ferritin was significant in the both sexes (OR 1.029, 95% CI (1.002–1.058) in men, OR 1.013, 95% CI (1.00–1.025) in women, P < 0.05, Table 3). Regrettably, there was no statistical significance between the correlation of iron metabolism markers and MACE after calibrating for confounders (model 3, P > 0.05, Table 4).
Association of iron metabolism markers with the severity of CAD
The severity of CAD was quantified by the modified Gensini scores based on the number and the extent of lesions in coronary arteries35. The detail calculation of modified Gensini scores was described in our previous study36. Gensini score of coronary artery equals the sum of all segment scores37. Each segment score equals segment weighting factor multiplied by a severity score. Segment weighting factor assigned to specific coronary segment are 5 for left main coronary artery, 2.5 for proximal left anterior descending coronary artery (LAD) and proximal left circumflex branch, 1.5 for mid-segment of LAD, 0.5 for second diagonal branch and posterolateral branch, and 1 for other branches. Severity score allocated to the definite percentage luminal diameter reduction are 1 for 0–25%, 2 for 26–50%, 4 for 51–75%, 8 for 76–90%, 16 for 91–99%, and 32 for 100% stenosis. Serum iron metabolism markers exhibited no significant association with the severity of CAD (Supplement Table S2).
Discussion
Although iron status was implicated in cardiovascular disease, the relationship between iron states and CAD has long been a controversial topic in the literature. This study was conducted to assess the association between serum iron metabolism markers and CAD. For physiological reasons, the reference intervals for iron metabolism markers in women are different from men; we analyzed the association of iron status and CAD stratified by gender. In a cohort of 643 CAD patients and 643 controls, iron imbalance, as characterized by either high serum ferritin or low iron levels, was associated with an increased risk of CAD.
Overall, our findings suggested that increased serum level of iron and TIBC levels showed a protective role for CAD in women, but not in men. Iron is an essential mineral, which participates in different functions of the organism under physiological conditions. Numerous biological processes, such as oxygen transport and lipid metabolism, protein production, cellular respiration, and DNA synthesis, require the presence of iron38. Iron depletion may thus lead to the impaired function of different tissues including central nervous system, muscle tissue, the myocardium, the immune system and the thyroid gland. Maintaining iron metabolism is important for cardiovascular health as its high energy consumption and high mitochondrial activity39. Intravenous iron administration in acute myocardial infarction (MI) exerted beneficial effects in MI patients40.
However, we did not find iron deficiency was associated with increased risk for CAD, which was inconsistent with some previous report. The patients’ status and comorbidities might explain the inconsistence. In our study, we enrolled low percentage (less than 30%) of iron deficiency in the patient group, and we exclude the CAD patients with clinical anemia. However, there was a high prevalence of iron deficiency in acute coronary syndrome and its association with poor outcome41. Moreover, the definition of iron deficiency to be applied in heart disease remains controversial. The most frequently used definition of iron deficiency in patients with cardiovascular disease is ferritin < 100 μg/L or ferritin 100–299 μg/L and transferrin saturation (TSAT) < 20%41.
Iron is a trace element that exists in serum at low concentration of mg/dL. Iron values exhibit diurnal variation depending on dietary iron intake or patient condition42. Various previous studies have evaluated serum ferritin instead of serum iron level. Ferritin is an iron-binding molecule that stores iron in a biologically available form, which is essential to iron homeostasis43. Moreover, serum ferritin is a well-known acute-phase reactant. In our study, the serum iron level was lower in CAD group, but the ferritin level in CAD group was higher than the control group. We speculated that the increase level of ferritin was mainly due to the underlying inflammation. A batch of studies have shown a similar positive correlation between ferritin and the risk of CAD1,20,22,23,29,30,44,45,46. Xu et al. found that elevated serum ferritin was independently significantly associated with carotid atherosclerosis in women (Xu et al. 2017). We also found serum ferritin was independently significantly associated with CAD in both sexes after adjusting for inflammation marker such as hs-CRP. On the other hand, some other studies demonstrated that the higher risk of CAD is not related to the serum ferritin levels30,47. These studies implied that ferritin might be an inflammatory marker for atherosclerosis. Most of these studiers lack complete iron metabolism markers, it was hard to tell whether high ferritin levels reflect chronic inflammation caused by hyperglycemia or indicate iron overload, which can also lead to inflammation.
There are limited data concerning serum iron and iron saturation in CAD patients and the results are also inconsistent. The discrepancies among the studies may be partly attributable to the differences in race, dietary habits, sample size and confounding factors. Within the reference range, increased serum levels of iron and TIBC were found to have a protective role for CAD in women, but not in men. Increased serum ferritin was independently associated with CAD incident in men and women. Our study indicated that reduced serum iron level could not protect CAD incident, but increased the risk of CAD in the elder female.
The main limitation of the study is its cross-sectional design; therefore, a causal relationship could not be established. In addition, the measurements of hepcidin, a well-known regulator of body iron fluxes, were not available. However, we evaluated transferrin saturation which is an important determinant of hepcidin release48. Moreover, the daily iron intake needs to be estimated in the future study. Finally, there is a pragmatic need to identify circulating iron biomarkers reliably characterizing iron status within tissues.
Abbreviations
- UIBC:
-
Unsaturated iron-binding capacity
- TIBC:
-
Total iron-binding capacity
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- CAG:
-
Cardioangiography
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BMI:
-
Body mass index
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- hs-CRP:
-
High sensitivity C reactive protein
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- γ-GT:
-
γ-Glutamyl transferase
- eGFR:
-
Estimated glomerular filtration rate
- BP:
-
Blood pressure
- Tfs:
-
Transferrin saturation
- IR:
-
Insulin resistance
- TC:
-
Total cholesterol
- UA:
-
Uric acid
- HbA1c:
-
Hemoglobin A1c
- Hcy:
-
Homocysteine
- ApoA:
-
Apolipoprotein A
- ApoB:
-
Apolipoprotein B
- TP:
-
Total proteins
- ALB:
-
Albumin
- MACE:
-
Major adverse cardiovascular event
References
Danesh, J. & Appleby, P. Coronary heart disease and iron status: Meta-analyses of prospective studies. Circulation 99, 852–854. https://doi.org/10.1161/01.cir.99.7.852 (1999).
Lapice, E., Masulli, M. & Vaccaro, O. Iron deficiency and cardiovascular disease: An updated review of the evidence. Curr. Atheroscler. Rep. 15, 358. https://doi.org/10.1007/s11883-013-0358-0 (2013).
Munoz-Bravo, C., Gutierrez-Bedmar, M., Gomez-Aracena, J., Garcia-Rodriguez, A. & Navajas, J. F. Iron: Protector or risk factor for cardiovascular disease? Still controversial. Nutrients 5, 2384–2404. https://doi.org/10.3390/nu5072384 (2013).
You, S. A. & Wang, Q. Ferritin in atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 357, 1–16. https://doi.org/10.1016/j.cccn.2005.02.001 (2005).
Ma, H. et al. Serum ferritin levels are associated with carotid atherosclerosis in Chinese postmenopausal women: The Shanghai Changfeng Study. Br. J. Nutr. 114, 1064–1071. https://doi.org/10.1017/S0007114515001944 (2015).
von Haehling, S., Jankowska, E. A., van Veldhuisen, D. J., Ponikowski, P. & Anker, S. D. Iron deficiency and cardiovascular disease. Nat. Rev. Cardiol. 12, 659–669. https://doi.org/10.1038/nrcardio.2015.109 (2015).
Wlazlo, N. et al. Iron metabolism is associated with adipocyte insulin resistance and plasma adiponectin: The Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Diabetes Care 36, 309–315. https://doi.org/10.2337/dc12-0505 (2013).
Rodriguez-Mortera, R., Caccavello, R., Hermo, R., Garay-Sevilla, M. E. & Gugliucci, A. Higher hepcidin levels in adolescents with obesity are associated with metabolic syndrome dyslipidemia and visceral fat. Antioxidants 10, 751. https://doi.org/10.3390/antiox10050751 (2021).
Altamura, S. et al. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol. Metab. 51, 101235. https://doi.org/10.1016/j.molmet.2021.101235 (2021).
Vaquero, M. P., Martinez-Maqueda, D., Gallego-Narbon, A., Zapatera, B. & Perez-Jimenez, J. Relationship between iron status markers and insulin resistance: An exploratory study in subjects with excess body weight. PeerJ 8, e9528. https://doi.org/10.7717/peerj.9528 (2020).
Diaz-Lopez, A. et al. Association between iron status and incident type 2 diabetes: A population-based cohort study. Nutrients https://doi.org/10.3390/nu12113249 (2020).
Habib, A. & Finn, A. V. The role of iron metabolism as a mediator of macrophage inflammation and lipid handling in atherosclerosis. Front. Pharmacol. 5, 195. https://doi.org/10.3389/fphar.2014.00195 (2014).
Qayyum, R. & Schulman, P. Iron and atherosclerosis. Clin. Cardiol. 28, 119–122 (2005).
Minqin, R. et al. The iron chelator desferrioxamine inhibits atherosclerotic lesion development and decreases lesion iron concentrations in the cholesterol-fed rabbit. Free Radic. Biol. Med. 38, 1206–1211. https://doi.org/10.1016/j.freeradbiomed.2005.01.008 (2005).
Sengoelge, G., Sunder-Plassmann, G. & Horl, W. H. Potential risk for infection and atherosclerosis due to iron therapy. J. Renal Nutr. 15, 105–110. https://doi.org/10.1053/j.jrn.2004.09.018 (2005).
Kruszewski, M. The role of labile iron pool in cardiovascular diseases. Acta Biochim. Pol. 51, 471–480 (2004).
Yuan, X. M. & Li, W. The iron hypothesis of atherosclerosis and its clinical impact. Ann. Med. 35, 578–591. https://doi.org/10.1080/07853890310016342 (2003).
Ding, Z. et al. LOX-1, oxidant stress, mtDNA damage, autophagy, and immune response in atherosclerosis. Can. J. Physiol. Pharmacol. 92, 524–530. https://doi.org/10.1139/cjpp-2013-0420 (2014).
Cannizzo, B. et al. Insulin resistance promotes early atherosclerosis via increased proinflammatory proteins and oxidative stress in fructose-fed ApoE-KO mice. Exp. Diabetes Res. 2012, 941304. https://doi.org/10.1155/2012/941304 (2012).
Haidari, M., Javadi, E., Sanati, A., Hajilooi, M. & Ghanbili, J. Association of increased ferritin with premature coronary stenosis in men. Clin. Chem. 47, 1666–1672 (2001).
Kang, P., Liu, T., Tian, C., Zhou, Y. & Jia, C. Association of total iron binding capacity with coronary artery disease. Clin. Chim. Acta Int. J. Clin. Chem. 413, 1424–1429. https://doi.org/10.1016/j.cca.2012.05.01 (2012).
Sung, K. C. et al. Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arterioscler. Thromb. Vasc. Biol. 32, 2525–2530. https://doi.org/10.1161/ATVBAHA.112.253088 (2012).
Zhou, Y., Liu, T., Kang, P. & Jia, C. Association of better iron status biomarkers and coronary artery disease risk. Intern. Med. J. 44, 846–850. https://doi.org/10.1111/imj.12508 (2014).
Silvestre, O. M. et al. Ferritin levels and risk of heart failure-the Atherosclerosis Risk in Communities Study. Eur. J. Heart Fail. 19, 340–347. https://doi.org/10.1002/ejhf.701 (2017).
Sullivan, J. L. Iron and the sex difference in heart disease risk. Lancet 1, 1293–1294. https://doi.org/10.1016/s0140-6736(81)92463-6 (1981).
Salonen, J. T. et al. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 86, 803–811. https://doi.org/10.1161/01.cir.86.3.803 (1992).
Zheng, H., Cable, R., Spencer, B., Votto, N. & Katz, S. D. Iron stores and vascular function in voluntary blood donors. Arterioscler. Thromb. Vasc. Biol. 25, 1577–1583. https://doi.org/10.1161/01.ATV.0000174126.28201.61 (2005).
Duffy, S. J. et al. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation 103, 2799–2804. https://doi.org/10.1161/01.cir.103.23.2799 (2001).
Reunanen, A., Takkunen, H., Knekt, P., Seppanen, R. & Aromaa, A. Body iron stores, dietary iron intake and coronary heart disease mortality. J. Intern. Med. 238, 223–230. https://doi.org/10.1111/j.1365-2796.1995.tb00926.x (1995).
Sun, Q. et al. Excessive body iron stores are not associated with risk of coronary heart disease in women. J. Nutr. 138, 2436–2441. https://doi.org/10.3945/jn.108.097766 (2008).
Ekblom, K. et al. Iron stores and HFE genotypes are not related to increased risk of first-time myocardial infarction: A prospective nested case-referent study. Int. J. Cardiol. 150, 169–172. https://doi.org/10.1016/j.ijcard.2010.04.001 (2011).
Yu, P. H. et al. Low serum iron is associated with anemia in CKD stage 1–4 patients with normal transferrin saturations. Sci. Rep. 11, 8343. https://doi.org/10.1038/s41598-021-87401-w (2021).
Jankowska, E. A. et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 31, 1872–1880. https://doi.org/10.1093/eurheartj/ehq158 (2010).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Rasouli, M., Kiasari, A. M. & Arab, S. Indicators of dehydration and haemoconcentration are associated with the prevalence and severity of coronary artery disease. Clin. Exp. Pharmacol. Physiol. 35, 889–894. https://doi.org/10.1111/j.1440-1681.2008.04932.x (2008).
Guo, S. et al. Serum complement C1q activity is associated with obstructive coronary artery disease. Front. Cardiovasc. Med. 8, 618173. https://doi.org/10.3389/fcvm.2021.618173 (2021).
Montorsi, P. et al. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: The COBRA trial. Eur. Heart J. 27, 2632–2639. https://doi.org/10.1093/eurheartj/ehl142 (2006).
Ravingerova, T. et al. The molecular mechanisms of iron metabolism and its role in cardiac dysfunction and cardioprotection. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21217889 (2020).
Weidmann, H. et al. Iron metabolism contributes to prognosis in coronary artery disease: Prognostic value of the soluble transferrin receptor within the atherogene study. J. Am. Heart Assoc. 9, e015480. https://doi.org/10.1161/JAHA.119.015480 (2020).
Florian, A. et al. Positive effect of intravenous iron-oxide administration on left ventricular remodelling in patients with acute ST-elevation myocardial infarction: A cardiovascular magnetic resonance (CMR) study. Int. J. Cardiol. 173, 184–189. https://doi.org/10.1016/j.ijcard.2014.02.016 (2014).
Reinhold, J., Papadopoulou, C., Baral, R. & Vassiliou, V. S. Iron deficiency for prognosis in acute coronary syndrome: A systematic review and meta-analysis. Int. J. Cardiol. 328, 46–54. https://doi.org/10.1016/j.ijcard.2020.12.021 (2021).
Henjum, S., Groufh-Jacobsen, S., Stea, T. H., Tonheim, L. E. & Almendingen, K. Iron status of vegans, vegetarians and pescatarians in Norway. Biomolecules. https://doi.org/10.3390/biom11030454 (2021).
Knovich, M. A., Storey, J. A., Coffman, L. G., Torti, S. V. & Torti, F. M. Ferritin for the clinician. Blood Rev. 23, 95–104. https://doi.org/10.1016/j.blre.2008.08.001 (2009).
Rajapurkar, M. M. et al. Association of catalytic iron with cardiovascular disease. Am. J. Cardiol. 109, 438–442. https://doi.org/10.1016/j.amjcard.2011.09.032 (2012).
Steen, D. L. et al. Prognostic evaluation of catalytic iron in patients with acute coronary syndromes. Clin. Cardiol. 36, 139–145. https://doi.org/10.1002/clc.22089 (2013).
Holay, M. P., Choudhary, A. A. & Suryawanshi, S. D. Serum ferritin-a novel risk factor in acute myocardial infarction. Indian Heart J. 64, 173–177. https://doi.org/10.1016/S0019-4832(12)60056-X (2012).
Reyes, C. et al. Association between serum ferritin and acute coronary heart disease: A population-based cohort study. Atherosclerosis 293, 69–74. https://doi.org/10.1016/j.atherosclerosis.2019.12.011 (2020).
Alexander, J. & Kowdley, K. V. HFE-associated hereditary hemochromatosis. Genet. Med. 11, 307–313. https://doi.org/10.1097/GIM.0b013e31819d30f2 (2009).
Acknowledgements
Thanks to Liming Liu (Department of Cardiology, The First Affiliated Hospital of Zhengzhou University) for the Guidance in Coronary artery disease.
Funding
The First Affiliated Hospital of Zhengzhou University financially supported this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.G. performed the experiments, analyzed the data, prepared figures and tables, and reviewed drafts of the paper. X.M. performed the data collection and analysis, X.L. and H.O. performed the laboratory tests and clinical data collection. All authors have approved the submitted version and agreed to be accountable for all aspects of work ensuring integrity and accuracy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, S., Mao, X., Li, X. et al. Association between iron status and incident coronary artery disease: a population based-cohort study. Sci Rep 12, 17490 (2022). https://doi.org/10.1038/s41598-022-22275-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22275-0
- Springer Nature Limited