Abstract
There is growing evidence that pesticides may be among the causes of worldwide bee declines, which has resulted in repeated calls for their increased scrutiny in regulatory assessments. One recurring concern is that the current frameworks may be biased towards assessing risks to the honey bee. This paradigm requires extrapolating toxicity information across bee species. Most research effort has therefore focused on quantifying differences in sensitivity across species. However, our understanding of how responses to pesticides may vary within a species is still very poor. Here we take the first steps towards filling this knowledge gap by comparing acute, lethal hazards in sexes and castes of the eusocial bee Bombus terrestris and in sexes of the solitary bee Osmia bicornis after oral and contact exposure to the pesticides sulfoxaflor, Amistar (azoxystrobin) and glyphosate. We show that sensitivity towards pesticides varies significantly both within and across species. Bee weight was a meaningful predictor of pesticide susceptibility. However, weight could not fully explain the observed differences, which suggests the existence of unexplored mechanisms regulating pesticide sensitivity across bee sexes and castes. Our data show that intra-specific responses are an overlooked yet important aspect of the risk assessment of pesticides in bees.
Similar content being viewed by others
Introduction
There is growing consensus that anthropogenic activities are causing the worldwide decline of wild bees1,2,3. The mechanisms regulating this phenomenon are likely multifactorial and interactive4,5. However, there is growing evidence that, among these stressors, the role of pesticides may be pivotal4,5,6. As bees are key pollinators7, their loss may affect food system stability8,9 and biodiversity10, with regionally unsustainable consequences6. Increasing awareness of the role of pesticides in this paradigm has sparked growing interest in how their risks are characterised before they enter the market11 and how pesticides are used following regional authorisation12.
One recurring concern is that environmental risk assessment, because of its reductionist nature, is centred towards testing standard model species13. In the case of risk assessment of bee pollinators, honey bees are widely used as a standard model species. In other words, pesticide hazards characterised using this model species are extrapolated to all other bee taxa, which are not routinely tested11. The uncertainty resulting from such inference is counterbalanced by using assessment factors14,15, which are meant to correct for the inter-specific variation of responses.
Many studies have investigated whether such assessment factors may be sufficiently protective16,17,18,19 or not20,21 of particularly sensitive species. Likewise, efforts have been made to characterise how differences in bee ecology and life history traits across species may influence bee vulnerability to pesticide exposure, encouraging further investigation of pesticide risks across species other than the honey bee22,23,24,25. In contrast, data investigating intra-specific acute sensitivity to pesticides, i.e., between sexes and, in the case of eusocial species, castes are surprisingly scarce. By inference, it might be assumed that intraspecific variation in sensitivity to pesticides may be largely explained by size differences across castes and sexes, as is often the case for interspecific sensitivity differences17. However, neonicotinoid exposure has been shown to trigger differential expression of detoxification26,27, immune28, and stress response29 genes across bee castes and sexes, which suggests factors other than body weight may similarly regulate intraspecific sensitivity.
Here we characterise the acute, lethal hazard of three pesticides upon oral and contact exposure in the eusocial buff-tailed bumble bee (Bombus terrestris L.) and the solitary red mason bee (Osmia bicornis L. Syn. Osmia rufa L.) following ring-tested study designs. B. terrestris and O. bicornis were used as candidate species because—unlike honey bees—their sexes and castes have a similar likelihood of exposure in the field23,24,25. Moreover, these species have been proposed as suitable models by recent regulatory risk assessment schemes14, and recent standardised acute testing protocols30,31,32.
We used the sulfoximine insecticide sulfoxaflor, the methoxy-acrylate fungicide Amistar (azoxystrobin 250 g/l, Suspension Concentrate, see supplementary methods, S1) and the glycine herbicide glyphosate (as active substance, RoundUp ProActive or RoundUp FL, see supplementary methods, S1) as model substances. Our choice was justified by their widespread use, regulatory status and systemic uptake in plants. Because of these characteristics, the likelihood of bees being exposed in the field was considered similarly plausible across model substances. Additionally, we are not aware of published evidence of the acute toxicity of these substances across castes and sexes of B. terrestris and O. bicornis.
Using these model substances, we performed a comparative analysis of acute lethal hazards across sexes and castes of B. terrestris and O. bicornis by analysing sensitivity ratios (SR) between median lethal doses (LD50)33,34.
As we are not aware of published studies that have systematically quantified pesticide toxicity across sexes and castes of bee species upon different exposure routes, here we address this knowledge gap by testing the following hypotheses: (i) that the bee species B. terrestris and O. bicornis show different levels of acute pesticide sensitivity; (ii) that the sensitivity across castes and sexes within these two species varies; and (iii) that body size per se does not explain intraspecific variation in sensitivity.
Results
The positive control dimethoate and the negative control in all dose response analyses were in line with standardised study protocols30,31 at timepoints selected for the comparative analysis. Specifically, the positive control caused mortality above 50%, while the negative control caused below 10% mortality for bumble bees and 15% for O. bicornis35,36,37.
O. bicornis and B. terrestris show differential sensitivity to sulfoxaflor and Amistar
Osmia bicornis females were more sensitive to sulfoxaflor than bumble bee workers, both before and after doses were normalised by body weight (Tables 1, 2; Fig. 1).
The acute toxicity of Amistar (oral) and sulfoxaflor (oral and contact) across sexes and castes of B. terrestris and O. bicornis. Dots represent the median lethal doses, while horizontal error bars represent the 95% confidence intervals. The y-axis lists the bee castes and sexes, while the x-axis reports the doses (i.e., non-normalised = µg/bee or normalised = ng/mg body weight) on a logarithmic scale.
Similarly, O. bicornis males were more sensitive to sulfoxaflor than B. terrestris males both before and after doses were normalised by body weight (Tables 1, 2; Fig. 1).
Conversely, upon oral exposure to Amistar, O. bicornis was less sensitive than B. terrestris before and after normalisation by body weight (Tables 1, 2; Fig. 1).
Responses across castes and sexes differ for reasons other than body weight in B. terrestris
Bumble bee queens were less sensitive than workers and males upon contact and oral exposure to sulfoxaflor and oral exposure to Amistar, before doses were normalised by bee weight (Table 2; Fig. 2). For sulfoxaflor, such differences were smaller but remained significantly different after doses were normalised by body weight.
The intra-specific sensitivity across sexes and castes of B. terrestris and O. bicornis. Dots represent the Sensitivity Ratios (SR), while error bars represent the 95% confidence interval. The y-axis reports the relevant comparisons, while the x-axis reports the SR (dimensionless). The vertical dashed line (SR = 1) represents the case where the two compared LD50s are equal, below which the sensitivity is higher. SRs are considered significant when their confidence bounds do not cross 1.
For Amistar, after body weight normalisation and with oral exposure, the median lethal dose of queens differed from males but not workers (Table 2; Fig. 2). This suggests that body weight was a key, but not the only factor explaining the differences in sensitivity across castes for this substance.
Body weight does not explain pesticide sensitivity across sexes in B. terrestris
Bumble bee males were more sensitive than workers to sulfoxaflor and Amistar before doses were normalised by body weight (Table 2; Fig. 2). Such differences were still significant after doses were normalised by fresh weight. This suggests that bumble bee males were less resilient than workers to pesticide exposure for reasons other than differences in body weight.
O. bicornis males and females are equally sensitive to sulfoxaflor and Amistar
Osmia bicornis females and males were equally sensitive to sulfoxaflor upon oral and contact exposure, both before and after pesticide intake was normalised by body weight (Table 2; Fig. 2), despite females being on average 0.97 times heavier than males (Supplementary material S2 Table S1).
Similarly, upon oral exposure to Amistar, Osmia bicornis females and males showed equal sensitivity before and after dose normalisation (Table 2; Fig. 2).
Azoxystrobin and glyphosate (as active substance or formulation) do not cause mortality in limit tests across castes and sexes of O. bicornis and B. terrestris
Azoxystrobin and glyphosate were either tested as active substance or formulation using a single, high (i.e., ‘limit’) dosing regime. None of the test items caused significant mortality levels (Table 3). Additional details on the methodology and results of the limit tests are reported in the supplementary material (S6).
Discussion
We hypothesised that B. terrestris and O. bicornis show different levels of acute pesticide sensitivity (i). Here we show that O. bicornis was less sensitive than bumble bees to Amistar, but more sensitive to sulfoxaflor, both before and after dose normalisation by weight. Before dose normalisation, O. bicornis females were 10.1 times more sensitive orally to sulfoxaflor than bumble bee workers. This result confirms previous evidence38, which reported similar toxicity levels (i.e., LD50: 0.083 and 0.009 µg/bee for B. terrestris and O. bicornis respectively) and a ninefold difference in sensitivity between these two species 48 h after oral exposure to sulfoxaflor.
When comparing the toxicity of sulfoxaflor to O. bicornis in our study with the LD50 reported in the EU assessment39 for A. mellifera, O. bicornis was found to be 11.2 times orally and 7.4 times topically more sensitive than honey bee workers.
Across dose response experiments we showed that castes and sexes of B. terrestris, but not O. bicornis, responded differently to pesticide exposure, partially confirming our hypothesis (ii). However, the confidence interval around the sensitivity ratios for O. bicornis upon oral exposure was relatively wide, compared to B. terrestris. This aspect, which may be partly explained by a less precise LD50 estimate, may add uncertainty to the conclusions drawn on the comparative analysis for this species.
Bumble bee queens were more resilient than workers and males to both pesticides and exposure routes. However, once doses were normalised by bee weight, the lower sensitivity of queens relative to workers was much less evident for sulfoxaflor and non-significant for Amistar. Bumble bee males always showed higher sensitivity than workers and queens, both before and after doses were normalised by body weight.
Our results confirmed body weight as a meaningful predictor of acute sensitivity across bumble bee workers and queens. However, consistent with our initial hypothesis (iii), body weight alone did not predict the observed differences in sensitivity across bumble bee castes. A possible explanation for this pattern is that, as queens are larger than workers and males40 and are likely to have a thicker cuticle, the diffusion of pesticides through their exoskeleton to the target site may be affected by their robust morphology. Consistent with this hypothesis, toxicokinetic (i.e., the efficiency of cuticular penetration) was shown to regulate neonicotinoid sensitivity in honey bees41. Nonetheless, as differences in sensitivity were observed across both oral and contact exposure, we do not expect cuticular penetration to be a meaningful predictor of pesticide toxicity across all exposure routes.
Conversely, we found no evidence that the sensitivity to our model substances varies significantly between sexes of O. bicornis, both before and after dose normalisation, despite their differences in body weight (Supplementary material S2).
This evidence confirms that species may differ in patterns of inter-sex sensitivity and builds on previous studies showing that males of A. mellifera29 and B. terrestris26, but not O. bicornis42, were more sensitive than females to neonicotinoid exposure. From a mechanistic standpoint, our results highlight the presence of species- and substance-specific patterns of pesticide sensitivity across sexes and castes, which are not explained by body size alone.
Pesticide metabolization may also explain the differences in pesticide sensitivity observed in our experiments. Recent studies characterising the role of cytochrome P450 enzymes in neonicotinoid detoxification attributed species- and substance-specific differences in neonicotinoid sensitivity to their relative presence and site interaction in bee43,44,45,46,47. The molecular mechanism behind neonicotinoid detoxification in O. bicornis was determined to be underpinned by metabolic activity regulated by cytochrome P450 enzymes within the CYP9BU subfamily45, similar, but not identical, to the P450 of the CYP9Q subfamily responsible for this mechanism in bumble bees44,45,46. Thus, the differences in sensitivity between species may be influenced by differential detoxification mechanisms. We are not aware of published studies comparing the expression of cytochrome P450 enzymes across bee sexes and castes. However, we suggest that differences in their relative expression, if any, may predict responses of sexes and castes to pesticide exposure. Consistent with this assumption, bumble bee queens have significantly higher fat body reserves than other castes48. As fat bodies express P450 enzymes in bumble bee queens49, we hypothesise that the higher fat reserves in queens may parially explain why they were the most resilient caste to pesticide exposure in our study.
As mortality caused by Amistar has previously been connected to gut damage caused by etoxylated alcohols50. As we found significant differences across and within species in the sensitivity to Amistar, we show that differential physiological responses may regulate pesticide sensitivity not only to active ingredients but also to co-formulants.
Consistent with previous evidence35, we found sulfoxaflor to be more toxic via oral than contact exposure. With oral and contact LD50s of 0.126 and 6.323 µg/bee respectively, we found B. terrestris workers to be similarly or less sensitive than honey bee workers to sulfoxaflor39. Additionally, B. terrestris was 7 times less sensitive than Bombus impatiens (LD50 = 0.177 µg/bee)51 upon oral exposure to sulfoxaflor. This difference may be explained by the lower body weight of Bombus impatiens relative to our tested species. The oral toxicity of sulfoxaflor in B. terrestris workers fell between the values reported for the same species in a recent regulatory report39 for two sulfoxaflor-based formulations (LD50 = 0.027 and 0.15 µg/bee for GF-2032 and GF-2626 respectively). Moreover, the contact toxicity estimated in our study was comparable to that reported in the same regulatory document for GF-2032 (LD50 = 7.55 µg/bee) but not GF-2626 (LD50 = 23.3 µg/bee). While we cannot explain such differences, we hypothesise that the presence of co-formulants in these products may have influenced the toxicity of sulfoxaflor50.
Bombus terrestris queens and O. bicornis females may be directly exposed to pesticides during their life cycles due to their life history traits. B. terrestris queens may experience prolonged contact or inhalation exposure while overwintering or nesting in soil25. Moreover, reproductive of both species may be orally or topically exposed while foraging for pollen and nectar in early spring23,25. This is particularly concerning, since exposure of reproductives may have downstream effects on wild bee population dynamics52. Our data provide evidence that the consequently high risk of bumble bee queens to pesticide exposure might be partially buffered by their lower sensitivity upon acute exposure. However, this may not be the case for O. bicornis, where females and males showed similar sensitivity to our model substances. Additionally, we found bumble bee males to be significantly more sensitive than workers and queens. As pesticides may impact the mating success and fertility of bumble bee males53, their exposure might have negative impacts on population dynamics.
None of the study groups showed an acute mortality response to topical azoxystrobin exposure (Supplementary materials S2 and S6). This corroborates previous findings50, in which Amistar’s oral toxicity was found to be due to a co-formulant in the commercial preparation, at higher doses than in the limit tests of our study (supplementary material S6). We also confirm glyphosate (as a.s. or formulation) to have low toxicity for B. terrestris and, for the first time, for O. bicornis, as no increase in mortality was observed in any caste or sex at doses ranging from 100 to 200 µg/bee (Supplementary materials S2 and S6), exceeding realistic exposure estimates in the field54.
Additionally, to our knowledge this is the first attempt to adapt regulatory-ready designs to the testing of bee castes other than workers, which we expect may significantly improve the standardisation of future toxicological studies with queen and male bees.
In conclusion, this is the first systematic characterisation of lethal acute hazards across castes and sexes of two bee species. As such, we believe our data provide an important baseline for further exploration of the mechanisms behind intra-specific differences in sensitivity to pesticides.
Materials and methods
Model substances
We used the sulfoximine insecticide sulfoxaflor, the methoxy-acrylate fungicide Amistar (azoxystrobin 250 g/l, Suspension Concentrate, see supplementary methods, S1) and the glycine herbicide glyphosate (as active substance, RoundUp ProActive or RoundUp FL, see supplementary methods, S1) as model substances. Our choice was justified by their widespread use, regulatory status and systemic uptake in plants. Because of these characteristics, the likelihood of bees being exposed in the field was considered similarly plausible across model substances. Additionally, we are not aware of published evidence of the acute toxicity of these substances across castes and sexes of B. terrestris and O. bicornis.
Sulfoxaflor is a relatively novel insecticide55,56,57, developed to replace or complement the use of older chemical classes against which insect pest populations had developed resistance57. However, because of its risks to bees58, its uses have been recently restricted in the EU to indoor growing conditions. As a nicotinic acetylcholine receptor (nAChR) competitive modulator, sulfoxaflor targets the same neural receptor as the bee-harming neonicotinoid insecticides55,56,57. Despite evidence that sulfoxaflor may target the nAChR in a distinct way compared to recently banned neonicotinoids55,56,57, these substances were shown to be similarly lethal in acute exposure laboratory settings for individuals of Apis mellifera, B. terrestris and O. bicornis38. Additionally, sulfoxaflor was shown to reduce reproduction59,60,61 (but not learning62,63) in bumble bees under field-realistic laboratory settings. When applied pre-flowering in a semi-field study design, sulfoxaflor impacted colony growth, colony size and foraging in bumble bees64 but not honey bees65. Azoxystrobin is a broad-spectrum, systemic fungicide, which has been widely used in agriculture since its first marketing authorisation in 199666. Azoxystrobin shows low acute toxicity to honey bees67. Azoxystrobin residues were found in nectar and pollen from treated crops68,69 and subsequently in the bodies of wild bees70. In a semi-field experimental setting, foraging, but not colony growth, was significantly impaired in B. terrestris exposed to Amistar (azoxystrobin 250 g/L SC)64, while no lethal or sublethal effects could be observed in honey bees65 or in O. bicornis71. However, a recent study showed that, when formulated as Amistar this pesticide induced acute mortality in bumble bees at high doses, which was attributed to the dietary toxicity of the co-formulant C16-18 alcohol ethoxylates50.
Glyphosate is a broad-spectrum systemic herbicide and the most widely used pesticide in the world72. Products containing glyphosate may be applied to flowering weeds73 and contaminate their pollen and nectar54, thus driving bee contact and oral exposure. Glyphosate showed low lethal hazards in regulatory-ready laboratory74 and semi-field designs when dosed as pure active substance or as MON 52276 (SL formulation containing 360 g glyphosate/L)75. A recent study found ready-to-use consumer products containing glyphosate to be lethally hazardous to bumble bees73. However, this toxicity was attributed to co-formulants, rather than the active substance itself.
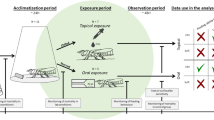
We characterised the acute oral and contact toxicity to B. terrestris and O. bicornis of sulfoxaflor, azoxystrobin and glyphosate as either pure active substances or formulation (see supplementary material S2 Table S1). Each test was repeated across castes and sexes of these two species. For bumble bees we used workers, males and gynes (i.e., unmated queens), hereby referred to as queens, whereas for O. bicornis we used males and females. Bumble bee experiments were designed following OECD protocols30,31, while O. bicornis was tested following published76 and ring-tested protocols32, as an OECD protocol for this latter species is not yet available.
We used a dose response design whenever the test item was found to drive significant mortality in the tested species. In all other cases, a limit test design using a single, high pesticide dose was used. Details on the methods and results of the limit tests are reported in the supplementary materials (S2 and S4).
Pesticide treatments
All dose response tests were performed with pure sulfoxaflor, while azoxystrobin was tested as a plant protection product (Amistar 250 g a.s./l, SC, Syngenta, UK) in all oral tests, as its solubility in water was insufficient (6.7 mg a.s./L, see EFSA, 2010) to achieve the desired concentrations. Amistar contains co-formulants with hazard classification (54 C16-18 alcohols, ethoxylated < 20% w/w; naphthalenesulfonic acid, dimethyl-, polymer with formaldehyde and methylnaphthalenesulfonic acid, sodium salt < 10% w/w and 1,2-benzisothiazol-3(2H)-one < 0.05% w/w) and other unknown compounds. Therefore, to reduce potential bias due to differences in composition, we used the same production batch of Amistar across all dose response experiments. Pesticide treatments were freshly prepared on each day of exposure by means of serial dilutions of concentrated stock solutions. At this stage, stock and treatment solutions were stored at − 20 °C for subsequent chemical analysis. A subset of these solutions was analysed for absolute quantification of active substance (Supplementary material S3). Each dose response included a minimum of 5 serial dilutions of the test item spaced by a factor ≤ 2, in addition to one untreated control, an acetone control (in cases where the test substance was dissolved in such solvent) and a positive control (dimethoate).
Further details on the test solutions and dosing regimen are given in the supplementary materials (S1 and S2).
B. terrestris: oral and contact toxicity tests
Oral exposure experiments were carried out in the UK using B. terrestris ssp. audax, while contact exposure experiments were conducted in Estonia using B. terrestris ssp. terrestris. Our design did not specifically investigate how responses to pesticide exposure may vary across these two subspecies.
Test organisms and conditions
For oral exposure, B. terrestris ssp. audax colonies were purchased from a local supplier (Agralan, UK) as queen-right standard hives (i.e., > 80 workers). Upon arrival, bees were screened for the most prevalent gut parasites (Apicystis bombi, Crithidia spp., and Nosema spp.) through microscopic examination of faecal samples (n = 5 per colony box) using a Nikon eclipse (50i) compound microscope at 400X magnification. No infections were detected. Males were either collected from the same commercial colonies described above or, when this was not possible, by direct purchase (Agralan, UK). All queens were collected from queen-right colonies provided pro bono by a commercial supplier (Koppert, Slovakia), which were screened using the same methods described above.
For the contact exposure experiments, B. terrestris ssp. terrestris were purchased as queen‐right standard hives (i.e., > 80 workers; A.M. Ozoli, Latvia), while males and queens of the same subspecies were obtained from queen-less boxes (A.M. Ozoli, Latvia). These bees were not microscopically examined for parasite infections. However, their health status was visually checked upon arrival.
For both exposure routes, bees were kept and tested in complete darkness in a rearing room at 26˚C and the humidity at ∼ 60%. Bee handling was undertaken under red light. Before and after exposure, orally exposed bees were fed ad libitum inverted syrup (45% w/w, Thornes, UK), while topically exposed bees were given ad libitum access to sucrose syrup (50% w/v). Prior to exposure, bees were given a provision of fresh-frozen, honey bee-collected pollen pellets (Agralan, UK and A. M. Ozoli, Latvia for oral and contact tests respectively). We could not analyse all pollen batches used across bumble bee acute oral exposure studies. However, screening of this pollen source found only low levels of miticides used in honeybee hives (results not shown). The pollen used in contact exposure experiments was obtained by a local supplier who distributes pollen from certified organic beekeeping. This pollen source was not analysed. However, considering it was sourced from organically managed apiaries, it is considered unlikely that this pollen source was contaminated with relevant concentrations of our model agrochemicals.
Experimental design
For both exposure routes and 1 day prior to their chemical exposure, bees of unknown age were weighed to the nearest milligram, before being individually housed in plastic cages (Nicot, Nicotplast, FR) for acclimatisation. At this stage, exceptionally large or small bees30,31 were visually excluded. On the following day, bees were allocated to treatments by colony of origin and body weight. When this was not possible (i.e., directly purchased males), the experimental design only controlled for body weight effects. Depending on the experiment, 3 to 9 colonies per test were used.
For oral exposure, following 4 h starvation, bees were given a 40 µL provision of pesticide-spiked or untreated sucrose syrup through a 2 ml syringe (Becton Dickinson, USA) with the tip removed. Four hours after exposure, syringes were visually inspected to ensure complete consumption of the treatment solution. At this stage, bees that did not consume the entire pesticide provision were removed. Across oral dose–response experiments, the average initial sample size per treatment group (i.e., test item and negative control) was 33 for workers, 34 for males and 43 for queens. Upon exclusion of unexposed bees, the average final sample size was 29 for workers (range: 20–35), 24 for males (range: 15–33) and 20 for queens (range: 7–36) (Supplementary material S2, Table S1). The lower limit of the range of sample sizes reported above corresponded to most concentrated treatment group in the Amistar experiments. For this compound we observed a dose-dependent feeding inhibition, which we attributed to the high dosing and viscosity of the treatment solution.
For contact exposure, following cold anesthetization, a droplet of treatment solution that was either spiked or not with the test solution, was applied to the dorsal side of the thorax (i.e., mesonotum) of each bee. The treatment volume was adjusted by bee size, with workers and males being exposed via a 2 µL droplet while queens were exposed to 4 µL. Upon topical exposure, bees were given ad libitum access to sucrose syrup through a 2 ml syringe with the tip removed (Terumo, Belgium).
Mortality was recorded at 24, 48, 72 and 96 h post-exposure. In dose response contact experiments, we tested 46 workers, 40 males and 30 queens per treatment group except for the untreated and solvent controls in the queen experiment, in which 15 individuals were tested per control group (Supplementary material S2, Table S1). Across experiments we tested 3685 bumblebees (workers: 1397; males: 1283 and queens: 1005; Supplementary material, S2).
Osmia bicornis: oral and contact toxicity tests
All O. bicornis experiments were carried out in Germany.
Diapausing O. bicornis males and females were purchased from a commercial rearing facility (Pollinature GhmB, Konstanz, DE). Upon arrival, cocoons were visually sorted by sex and stored in darkness at 4 °C in plastic bags.
To induce emergence from diapause, cocoons were placed in an incubator (Memmert, DE) at 21 ± 1 °C and 40% relative humidity. Cocoons were checked twice daily and, upon emergence, bees were transferred back to 4 °C to keep them dormant for maximum 4 days until test initiation.
Emerged males or females were allocated by day of hatching to rearing plastic boxes (27 * 14 * 16 cm) in groups of 10–20. Two feeders (Eppendorf 2 mL tubes with a 2 mm hole at the bottom) with a visual cue in the form of a petal (Brassica rapa or Diplotaxis tenuifolia) were provided in each cage. After ∼ 4 h of group housing in daylight, the now meconium-free bees were weighed to the nearest milligram and transferred to individual Nicot cages. Unusually small or large individuals were removed upon visual inspection and randomization was performed so that the treatments had and equal distribution of age classes (i.e., days after emergence) and body weights (mg). Bees were then left to starve at 21 °C overnight, after which each bee was presented with 20 µL of sucrose syrup containing pesticide or control treatment. The dose was presented in a cut-off tip of a 0.5 mL Eppendorf tube with a petal identical to the ones used in the hoarding cage in order to stimulate feeding behaviour. Using this method, we visually confirmed consumption, and found 74.5% of males and 88.3% of females (Supplementary material S2 Table S1; mean ratio across treatment groups) consumed the solution within 3 h. Bees that had not consumed the entire droplet within 3 h were considered non-feeders and were excluded. Consequently, across oral dose–response experiments, the average initial sample size per treatment group (i.e., test item and negative control) was 32 for females and 36 for males, while the final sample size was 27 for females [range 33–15], 245 for males [32–13]; Supplementary material S2, Table S1). Similar to bumble bees, the lower limit of the range of sample sizes reported above correspond to Amistar treatments. For this compound we observed feeding inhibition which we attributed to the high dosing and viscosity of the treatment solution.
For contact exposure, the above experimental procedure was repeated up to the allocation to treatments, after which bees were immediately cold-anaesthetized and topically exposed to the treatments. 1µL of solution was pipetted onto the dorsal part of the thorax between the wing-bases and bees were immediately returned to their individual Nicot holding cages. Across dose response contact experiments, a total of 30 individuals were included per treatment group dosed with the test item, and 20 individuals were included per control group (untreated control and solvent control; Supplementary material S2, Table S1). During the test phase, bees were kept in individual Nicot cages of the same design as described above. Bees were kept in an incubator at 21 °C, 16:8 h light:dark cycle for the remainder of the test. 50% w/v sugar solution was provided ad libitum in honey bee queen feeding cups (Nicot, Nicotplast, FR), covered by a metal mesh at the bottom of the cage. Mortality was recorded at 24, 48, 72 and 96 h post-exposure.
Across experiments we tested 1668 O. bicornis (females: 819 and males: 849; supplementary material, S2).
Data curation and statistical analysis
We defined the last observation timepoint at which increases in mortality were lower than 10% as a steady-state mortality level. We used this timepoint as baseline for hazard characterisation and comparative analysis across bee sexes, castes and species. This approach was preferred over the arbitrary selection of a fixed time point (e.g., 48 h) across experiment, as it enabled a more realistic characterisation of acute hazards. This choice was compliant with standard OECD methods30,31, which recommend extending the test duration beyond 48 h and up to 96 h whenever the mortality rate in the treated groups increases by ≥ 10% within a 24 h timeframe, whilst control mortality remains at acceptable (low) levels. The rationale behind the OECD recommendation is that the onset of lethal effects upon acute exposure may be delayed in time, in which case, selecting 48 h as timepoint for LD50 derivation may underestimate hazard.
However, when O. bicornis females were orally exposed to sulfoxaflor, the latest timepoint at which the control mortality was below 15% was 48 h. Therefore, for this latter test the LD50 was calculated at 48 h. For consistency, the same timepoint was selected for O. bicornis males exposed to sulfoxaflor38. Similarly, for Amistar, 48 h was selected as a valid timepoint for both sexes, as the control mortality of O. bicornis males exceeded 15% at 72 h.
To minimise statistical bias in comparative analyses, we used the same statistical model for dose response analysis across all experiments. After selection of mortality timepoints, dose responses were fitted using a log-normal model, based on which we estimated the median lethal doses (LD50s) and its asymptotic-based delta confidence interval. Analyses were first carried out by expressing doses as a function of the pesticide intake per bee (i.e., µg/bee) and then by normalising pesticide intake by fresh bee weight (i.e., ng/mg body weight). This enabled us to determine if, and to what extent, differences in bodyweight predicted the responses to pesticide exposure across bee castes, sexes and species. Doses were expressed as measured concentration whenever the chemical analysis of the test solutions deviated from the nominal concentration by more than 20%77 (Supplementary material S3). Mortality rates were corrected using Schneider-Orelli’s formula whenever control mortality at the steady-state timepoint exceeded 5%. Whenever acetone was used, we tested a solvent (acetone) control and a water control in parallel37, which were compared using a Fisher’s exact test. No difference in mortality between the two groups was observed.
Pesticide hazards across sexes and castes were compared with LD50 values (Table 1, Supplementary material S5 Fig. S3), which were used to calculate sensitivity ratios (SR) and relative confidence intervals using the comped function in drc33,34. Statistical analyses and data visualisation were performed in R78 using the packages dplyr79, drc71 and ggplot280.
For the limit tests, mortality rates were compared between controls and exposure groups using a Fisher’s exact test. Whenever appropriate, p-values were adjusted using the Benjamini–Hochberg correction.
Data availability
The datasets generated during and analysed during the current study are available in the Zenodo repository, https://doi.org/10.5281/zenodo.7065152.
Change history
06 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-25623-2
References
Goulson, D., Lye, G. C. & Darvill, B. The decline and conservation of bumblebees. Annu. Rev. Entomol. 53, 191–208 (2009).
Nieto, A. et al. European red list of bees. Publ. Off. Eur. Union 98 (2014) https://doi.org/10.2779/77003.
IPBES. Summary for policymakers of the assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. (2016).
Vanbergen, A. J. et al. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 11, 251–259 (2013).
Siviter, H. et al. Agrochemicals interact synergistically to increase bee mortality. Nature 596, 389–392 (2021).
Dicks, L. V. et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 5, 1453–1461 (2021).
Potts, S. G. et al. Safeguarding pollinators and their values to human well-being. Nature 540, 220–229 (2016).
Gallai, N., Salles, J. M., Settele, J. & Vaissière, B. E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821 (2009).
Garibaldi, L. A., Aizen, M. A., Klein, A. M., Cunningham, S. A. & Harder, L. D. Global growth and stability of agricultural yield decrease with pollinator dependence. Proc. Natl. Acad. Sci. 108, 5909–5914 (2011).
Biesmeijer, J. C. et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 (2006).
Topping, C. J. et al. Holistic environmental risk assessment for bees. Science 371, 897–897 (2021).
Mesnage, R. et al. Improving pesticide-use data for the EU. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-021-01574-1 (2021).
Van Leeuwen, C. J. & Vermeire, T. G. Risk Assessment of Chemicals: An Introduction (Springer Netherlands, 2007). https://doi.org/10.1007/978-1-4020-6102-8.
EFSA. Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 11, 3295 (2013).
USEPA. Guidance for Assessing Pesticide Risks to Bees. (2014).
Arena, M. & Sgolastra, F. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23, 324–334 (2014).
Uhl, P. et al. Interspecific sensitivity of bees towards dimethoate and implications for environmental risk assessment. Sci. Rep. 6, 1–7 (2016).
Heard, M. S. et al. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species?. Sci. Total Environ. 578, 357–365 (2017).
Ansell, G. R., Frewin, A. J., Gradish, A. E. & Scott-Dupree, C. D. Contact toxicity of three insecticides for use in tier I pesticide risk assessments with Megachile rotundata (Hymenoptera: Megachilidae). PeerJ 9, e10744 (2021).
Scott-Dupree, C. D., Conroy, L. & Harris, C. R. Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J. Econ. Entomol. 102, 177–182 (2009).
Roessink, I., Van Der Steen, J., Kasina, M., Gikungu, M. & Nocelli, R. Is the European honeybee (Apis mellifera mellifera) a good representative for other pollinator species?. Julius-Kühn-Archiv https://doi.org/10.5073/jka.2012.437.047 (2012).
Franklin, E. L. & Raine, N. E. Moving beyond honeybee-centric pesticide risk assessments to protect all pollinators. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-019-0987-y (2019).
Boyle, N. K. et al. Workshop on pesticide exposure assessment paradigm for non-apis bees: Foundation and summaries. Environ. Entomol. 48, 4–11 (2019).
EFSA PPR. Scientific opinion on the science behind the development of a risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 10, 2668 (2012).
Gradish, A. E. et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): Implications for risk assessments. Environ. Entomol. 48, 12–21 (2019).
Mobley, M. W. & Gegear, R. J. One size does not fit all: Caste and sex differences in the response of bumblebees (Bombus impatiens) to chronic oral neonicotinoid exposure. PLoS ONE 13, 1–16 (2018).
Colgan, T. J. et al. Caste- and pesticide-specific effects of neonicotinoid pesticide exposure on gene expression in bumblebees. Mol. Ecol. 28, 1964–1974 (2019).
Brandt, A. et al. Immunosuppression response to the neonicotinoid insecticide thiacloprid in females and males of the red mason bee Osmia bicornis L. Sci. Rep. 10, 1–10 (2020).
McAfee, A., Metz, B. N., Milone, J. P., Foster, L. J. & Tarpy, D. R. Drone honey bees are disproportionately sensitive to abiotic stressors despite expressing high levels of stress response proteins. Commun. Biol. 5, 1–13 (2022).
OECD. Guideline for the testing of chemicals 246. Bumblebee, acute contact toxicity test. (2017).
OECD. Guideline for the testing of chemicals 247. Bumblebee, acute oral toxicity test. (2017).
Roessink, I. et al. A method for a solitary bee (Osmia sp.) first tier acute contact and oral laboratory test: An update. In Hazards Pestic. to Bees—13th Int. Symp. ICP-PR Bee Prot. Gr. 158 (2017) https://doi.org/10.5073/jka.2018.462.045.
Wheeler, M. W., Park, R. M. & Bailer, A. J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 25, 1441–1444 (2006).
Ritz, C., Baty, F., Streibig, J. C. & Gerhard, D. Dose-Response Analysis Using R (2015)https://doi.org/10.1371/journal.pone.0146021.
Uhl, P., Awanbor, O., Schulz, R. S. & Brühl, C. A. Is Osmia bicornis an adequate regulatory surrogate? Comparing its acute contact sensitivity to Apis mellifera. PLoS ONE 14, e0201081 (2019).
EPFO. Side-effects on honeybees. EPPO Bull. 40, 313–319 (2010).
Medrzycki, P. et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 52, 1–60 (2013).
Azpiazu, C. et al. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci. Rep. 11, 1–9 (2021).
EFSA. Peer review of the pesticide risk assessment for the active substance sulfoxaflor in light of confirmatory data submitted. EFSA J. 18 (2020).
Goulson, D. Bumblebees: Behaviour, Ecology, and Conservation (Oxford University Press, 2009).
Zaworra, M., Koehler, H., Schneider, J., Lagojda, A. & Nauen, R. Pharmacokinetics of three neonicotinoid insecticides upon contact exposure in the western honey bee, Apis mellifera. Chem. Res. Toxicol. 32, 35–37 (2019).
Robinson, A. et al. Comparing bee species responses to chemical mixtures: Common response patterns?. PLoS ONE 12, 1–21 (2017).
Haas, J. & Nauen, R. Pesticide risk assessment at the molecular level using honey bee cytochrome P450 enzymes: A complementary approach. Environ. Int. 147, 106372 (2021).
Manjon, C. et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 28, 1137-1143.e5 (2018).
Beadle, K. et al. Genomic insights into neonicotinoid sensitivity in the solitary bee Osmia bicornis. PLoS Genet. 15, 1–19 (2019).
Troczka, B. J. et al. Identification and functional characterisation of a novel N-cyanoamidine neonicotinoid metabolising cytochrome P450, CYP9Q6, from the buff-tailed bumblebee Bombus terrestris. Insect Biochem. Mol. Biol. 111, 103171 (2019).
Hayward, A. et al. The leafcutter bee, Megachile rotundata, is more sensitive to N-cyanoamidine neonicotinoid and butenolide insecticides than other managed bees. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-019-1011-2 (2019).
Alford, D. V. Studies on the fat-body of adult bumble bees. J. Apic. Res. 8, 37–48 (1968).
Costa, C. P. et al. Transcriptome analysis reveals nutrition- and age-related patterns of gene expression in the fat body of pre-overwintering bumble bee queens. Mol. Ecol. 29, 720–737 (2020).
Straw, E. A. & Brown, M. J. F. Co-formulant in a commercial fungicide product causes lethal and sub-lethal effects in bumble bees. Sci. Rep. 11, 1–10 (2021).
Mundy-Heisz, K. A., Prosser, R. S. & Raine, N. E. Acute oral toxicity and risks of four classes of systemic insecticide to the Common Eastern Bumblebee (Bombus impatiens). Chemosphere 295, 133771 (2022).
Baron, G. L., Jansen, V. A. A., Brown, M. J. F. & Raine, N. E. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nat. Ecol. Evol. 1, 1308–1316 (2017).
Straub, L. et al. Thiamethoxam as an inadvertent anti-aphrodisiac in male bees. Toxicol. Rep. 9, 36–45 (2022).
Thompson, H. M. et al. Evaluating exposure and potential effects on honeybee brood (Apis mellifera) development using glyphosate as an example. Integr. Environ. Assess. Manag. 10, 463–470 (2014).
Zhu, Y. et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 59, 2950–2957 (2011).
Sparks, T. C. et al. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pest. Biochem. Physiol. 107, 1–7 (2013).
Watson, G. B., Siebert, M. W., Wang, N. X., Loso, M. R. & Sparks, T. C. Sulfoxaflor—A sulfoximine insecticide: Review and analysis of mode of action, resistance and cross-resistance. Pest. Biochem. Physiol. 178, 104924 (2021).
European Commission. Commission implementing regulation (EU) 2022/686 of 28 April 2022 amending implementing regulations (EU) 2015/1295 and (EU) No 540/2011 as regards the conditions of approval of the active substance sulfoxaflor. 18–22 (Official journal of the European Union, 2022).
Siviter, H., Brown, M. J. F. & Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 561, 109–112 (2018).
Linguadoca, A., Rizzi, C., Villa, S. & Brown, M. J. F. Sulfoxaflor and nutritional deficiency synergistically reduce survival and fecundity in bumblebees. Sci. Total Environ. 795, 148680 (2021).
Siviter, H., Horner, J., Brown, M. J. F. & Leadbeater, E. Sulfoxaflor exposure reduces egg laying in bumblebees Bombus terrestris. J. Appl. Ecol. https://doi.org/10.1111/1365-2664.13519 (2019).
Vaughan, O. P., Straw, E. A., Linguadoca, A. & Brown, M. J. F. No effect of dual exposure to sulfoxaflor and a trypanosome parasite on bumblebee olfactory learning. Sci. Rep. 12, 1–10 (2022).
Siviter, H. et al. No evidence for negative impacts of acute sulfoxaflor exposure on bee olfactory conditioning or working memory. PeerJ 7, e7208 (2019).
Tamburini, G. et al. Fungicide and insecticide exposure adversely impacts bumblebees and pollination services under semi-field conditions. Environ. Int. 157, 106813 (2021).
Tamburini, G. et al. Sulfoxaflor insecticide and azoxystrobin fungicide have no major impact on honeybees in a realistic-exposure semi-field experiment. Sci. Total Environ. 778, 146084 (2021).
Bartlett, D. W. et al. The strobilurin fungicides. Pest. Manag. Sci. 58, 649–662 (2002).
EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance azoxystrobin. EFSA J. 8, 1542 (2010).
Mullin, C. A. et al. High levels of miticides and agrochemicals in north American apiaries: implications for honey bee health. PLoS ONE 5, e9754 (2010).
Sanchez-Bayo, F. & Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 9, e94482 (2014).
Hladik, M. L., Vandever, M. & Smalling, K. L. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 542, 469–477 (2016).
Schwarz, J. M. et al. No evidence for impaired solitary bee fitness following pre-flowering sulfoxaflor application alone or in combination with a common fungicide in a semi-field experiment. Environ. Int. 164, 107252 (2022).
Benbrook, C. M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 28, 3 (2016).
Straw, E. A., Carpentier, E. N. & Brown, M. J. F. Roundup causes high levels of mortality following contact exposure in bumble bees. J. Appl. Ecol. 58, 1167–1176 (2021).
Straw, E. A. & Brown, M. J. F. No evidence of effects or interaction between the widely used herbicide, glyphosate, and a common parasite in bumble bees. PeerJ https://doi.org/10.7717/peerj.12486 (2021).
EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 13, (2016).
Ladurner, E., Bosch, J., Kemp, W. P. & Maini, S. Evaluation of a standard artificial flower design to feed individual bees known amounts of pesticides. Apidologie 36, 379–287 (2005).
OECD. Guidelines for the Testing of Chemicals 237. Honey Bee (Apis mellifera) Larval Toxicity Test, Single Exposure. Vol. 23 (2013).
World Health Organ. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Wickham, H., François, R., Henry, L. & Müller, K. dplyr: A grammar of data manipulation. R package version 0.8.3. (2019).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, New York, 2016).
Acknowledgements
Thanks to Anna Friedel, Lucie Marie Baltz, Tamara Mecklenburg and Lewis Armstrong for technical support, to the ICPPR non-Apis working group, Piotr Medrzycki and Monica Colli for experimental advice. This project received funding from the European Horizon 2020 research and innovation programme under grant agreement no.773921 and the European Union's European Regional Development Fund (Estonian University of Life Sciences ASTRA project “Value-chain based bioeconomy”).
Author information
Authors and Affiliations
Contributions
S.H.; M.J., A.L.: conceptualization, methodology, investigation, data curation, writing (original draft). A.L.: data curation, visualization, formal analysis. E.A.S.: methodology, investigation, writing (review and editing). P.S.: resources. R.K.: supervision, writing (review and editing). C.C. G.S. R.C.: methodology, investigation, resources. M.M. M.J.F.B. R.J.P.: conceptualization, supervision, funding acquisition, writing (review & editing).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. The views expressed in this article are the authors only and do not reflect the views of the European Food Safety Authority.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Competing interests section in the original version of this Article was incorrect. It now reads: “The authors declare no competing interests. The views expressed in this article are the authors only and do not reflect the views of the European Food Safety Authority.”
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Linguadoca, A., Jürison, M., Hellström, S. et al. Intra-specific variation in sensitivity of Bombus terrestris and Osmia bicornis to three pesticides. Sci Rep 12, 17311 (2022). https://doi.org/10.1038/s41598-022-22239-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22239-4
- Springer Nature Limited
This article is cited by
-
A new exposure protocol adapted for wild bees reveals species-specific impacts of the sulfoximine insecticide sulfoxaflor
Ecotoxicology (2024)
-
Neither sulfoxaflor, Crithidia bombi, nor their combination impact bumble bee colony development or field bean pollination
Scientific Reports (2023)
-
Risk assessment requires several bee species to address species-specific sensitivity to insecticides at field-realistic concentrations
Scientific Reports (2023)