Abstract
Intrahepatic cholestasis of pregnancy (ICP) is a common liver disease during pregnancy, that has serious complications. This study aimed to compare the blood inflammation and biochemical markers of pregnant women with ICP in Southwest China and analyse their diagnostic value for ICP. A controlled cross-sectional study was conducted, and routine blood and biochemical indicators of 304 diagnosed ICP patients and 363 healthy pregnant women undergoing routine prenatal examination were assessed. The blood inflammatory indicators and biochemical indicators were compared between the ICP groups and normal groups. In this study, the levels of the ALT, AST, GGT, TBIL and DBIL biochemical indicators and the levels of WBC, neutrophils, NLR and PLR inflammatory indicators in the ICP group were significantly higher than those in healthy pregnant women (p < 0.001). The PA and lymphocytes of the ICP group were significantly lower than those of the normal group (p < 0.001). ROC curves showed that ALT and the NLR had higher predictive value for ICP. The GGT, TBA and NLR of pregnant women with ICP in the preterm group were significantly higher than those in the term group, and the combined NLR and TBA had a certain predictive value for preterm birth.
Similar content being viewed by others
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is an idiopathic disease of pregnancy that occurs in the middle and third trimesters of pregnancy. It is characterized by mild to severe skin pruritus and abnormal liver function. Postpartum, symptoms are rapidly relieved, and liver function returns to normal1. The incidence of ICP worldwide is between 0.2 and 25%. In China, the incidence of ICP is between 2.3 and 6.0%, and the recurrence rate in a second pregnancy is 40% and 60%2,3. At present, the exact cause of ICP is not clear, but heredity, hormone, nutrition and environmental factors may be related to it4. In clinical practice, the underdiagnosis, misdiagnosis and untimely treatment of ICP without obvious causes still occur from time to time. Long-term high bile acid levels will cause obvious vasospasm on the surface of placental villi, reduce blood flow through the intervillous area of the placenta, and eventually lead to fetal insufficiency and complications of the mother and fetus5,6,7. The pathogenesis of ICP is often accompanied by liver injury and changes in liver enzyme levels to varying degrees, and abnormal levels of γ-glutamyl transferase (GGT) and bile acid can support the diagnosis of ICP8. Therefore, the early diagnosis, screening and treatment of ICP is very important. For pregnant women with no obvious cause of skin pruritus during pregnancy, it is very important to use new detection indicators for diagnosis.
The exact pathogenesis of ICP is complex, and inflammation may play an important role9. The increase in the pathological concentration of total bile acid (TBA) in the pathogenesis of ICP can induce hepatocyte cells to produce inflammatory response and induce a variety of inflammatory response to promote the infiltration of neutrophils, lymphocytes and macrophages in the blood9,10. It has been reported that the levels of inflammatory factors, such as Iinterleukin-6 (IL-6), interleukin-17 (IL-17) and tumor necrosis factor α (TNF-α) are elevated in parturient women with ICP are significantly higher than those in normal pregnant women. These elevated inflammatory markers disrupt the immune balance between the mother and fetus, thus reflecting the severity of the condition11,12,13. The white blood cell (WBC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) can be used as indicators of inflammation and are widely used in the prediction of gestational diabetes mellitus, atherosclerosis and tumors14,15. However, the diagnostic value of the above indicators for ICP is unknown. In this study, we explored the predictive value of routine blood and biochemical indicators for ICP.
Results
Study population
The baseline characteristics of the 667 included pregnant women are shown in Table1. Notably, the serum levels of ALT (alanine aminotransferase), AST (aspartate aminotransferase), GGT (γ-glutamyl transferase), TBIL (total bilirubin), DBIL (direct bilirubin) and TBA (total bile acid) and the WBC (white blood cell) count, neutrophil count, NLR (neutrophil-to-lymphocyte ratio) and PLR (platelet-to-lymphocyte ratio) in pregnant women with ICP were significantly higher than those in healthy pregnant women. However, the levels of PA (prealbumin) and lymphocytes in pregnant women with ICP were significantly lower than those in healthy pregnant women (p < 0.001, see Table 1).
We further compared the biochemical and blood inflammatory indicators among the mild ICP group, the severe ICP group and the normal group. We found that except for platelets, the biochemical and blood inflammatory indicators in the mild and severe groups were all significantly different from those in the normal group (p < 0.001, shown in Table 2). The TBA level in the severe ICP group was higher than that in the mild ICP group. However, there were no significant differences in other biochemical or inflammatory indicators between the two groups (shown in Table 2).
Correlation analysis between TBA and blood inflammatory indicators
We found that the TBA level in pregnant women with ICP was positively correlated with the WBC (r = 0.113, p = 0.003), neutrophil count (r = 0.130, p = 0.001), NLR (r = 0.267, p < 0.001) and PLR (r = 0.248, p < 0.001) and negatively correlated with the lymphocyte count (r = -0.204, p < 0.001).
Predictive value of biochemical and blood inflammation indicators for ICP
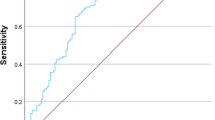
We used ROC curves to analyse the predictive value of the biochemical indicators and found that ALT, AST, GGT, TBIL and DBIL levels were significantly different between the ICP and normal groups. Among the biochemical indicators, ALT (AUC = 0.855, 95% confidence interval (CI): 0.825, 0.880) had the highest predictive value for ICP (p < 0.001, see Table 3, Fig. 1). The optimal cut-off value of ALT for diagnosing ICP was 28.0 U/L, with high sensitivity (86.14%) and specificity (84.57%) (see Table 3, Fig. 1).
Similarly, we analyzed the predictive value of blood inflammatory indicators for ICP using ROC curves, and found that the WBC count, neutrophil count, lymphocyte count, NLR and PLR were significantly different between the ICP group and normal groups (see Table4). Predictive analysis of ROC curves based on inflammatory indicators showed that the NLR (AUC = 0.753) had certain predictive value for ICP with a specificity of 94.21% and sensitivity of 44.41%. This predictive value was higher than that of the WBC count, neutrophil count, lymphocyte count and PLR (see Table 4, Fig. 2).
Multivariate analysis of independent factors predicting ICP with inflammatory indicators
Following adjustment for age and gestational weeks, the multivariate logistic regression model showed that the WBC count, neutrophil count, lymphocyte count, NLR and PLR were independent risk factors for ICP (p < 0.05, shown in Table 5).
Predictive value of biochemical and blood inflammation indicators for preterm delivery in the ICP group
There were significant differences in the levels of GGT, TBA, neutrophils, lymphocytes and NLR between the ICP preterm subgroup (GW < 37 weeks) and the ICP full-term subgroup (GW ≥ 37 weeks) (p < 0.05, shown in Table 6). The results of ROC analysis of the above differential inflammatory indicators and biochemical indicators showed that the NLR blood inflammatory indicator and the TBA biochemical indicator had some predictive value for preterm delivery. The area under the curve of the NLR was 0.635 (95% confidence interval (CI): 0.571, 0.700), and the area under the curve of TBA levels was 0.579 (95% confidence interval (CI): 0.514, 0.664). Multivariate logistic regression analysis was used to assess the diagnostic efficacy of the NLR and TBA levels for the combined diagnosis of ICP. The combined diagnosis of NLR and TBA levels increased the area under the curve (AUC = 0.669) and the sensitivity (92.63%) of diagnosis (shown in Table 7, Fig. 3).
Discussion
ICP is a hepatic disease associated with pregnancy and is characterized by pruritus and concomitant liver dysfunction and typically increased serum levels of total bile acid16. In recent years, the implementation of the two-child policy in China has led to an increase in the proportion of pregnant women with advanced maternal age, with a concomitant increase in the incidence of ICP. The incidence of ICP has significant geographical characteristics, with a prevalence of 1.0–4.0% in the Yangtze River region and a high incidence in Sichuan, China17,18. The etiology of ICP is multifactorial, estrogen-bile acid axis and immunological factors like inflammatory factors may underlie the effect of cholestasis effect on hepatocytes19. Because of the great influence of ICP on the fetus, it can lead to the occurrence of adverse pregnancy outcomes, such as preterm delivery and fetal distress1. Therefore, research on its mechanism has become increasingly popular, and early screening and diagnosis of the disease during pregnancy is increasingly important. Most of the current studies support that inflammatory mediators are involved in the development of ICP and that stagnant bile acid directly activates the inflammatory signaling pathway in the liver, promoting the development of inflammatory reactions and the accumulation of proinflammatory mediators that further lead to liver injury10,20,21. Therefore, ICP is often accompanied by varying degrees of abnormalities in inflammatory factors and liver enzyme levels22.
At present, the laboratory diagnosis of ICP is mainly performed by using serum TBA. However, the diagnostic cut-off values of TBA also vary greatly according to the measurement method used, fasting status, study population and gestational age at the time of diagnosis, so screening for appropriate clinical indicators can be crucial as an adjunct to ICP diagnosis22,23,24. Previous studies have demonstrated that bile acids have some cytotoxic effects, causing cell apoptosis and necrosis and leading to increased levels of ALT and TBIL. Additionally, bilirubin can also interact with bile and aggravate hepatocyte damage, leading to abnormal elevation of biochemical indicators25. In clinical practice, changes in biochemical indicators such as liver enzymes are important markers of hepatocyte injury and are also widely used26,27. To determine the diagnostic value of biochemical indicators for ICP, we evaluated the diagnostic efficacy of liver function indicators for ICP. In the ICP group, the levels of ALT, AST, GGT, TBIL and DBIL were significantly higher than those in the healthy group, and ROC curve analysis showed that ALT had higher a predictive value for ICP with a sensitivity of 86.14% and a specificity of 84.57%. This finding is consistent with previous studies28. The above results confirm that blood biochemical indicators have a certain predictive value for ICP. For pregnant women who lack a high level of TBA, the combination of biochemical indicators and clinical symptoms may be helpful for the diagnosis of ICP.
It has been reported that the levels of inflammatory factors can be used to assess the degree of abnormal liver function in patients with ICP. It may participate in the process of liver injury and disrupt the immune balance between the mother and fetus, thus indicating the severity of the condition19,21,29. However, the specific role of inflammation in the pathogenesis of ICP is not known. Previous studies have reported that cytokines IL-8, IL-10 and IL-12 play a certain role in the pathogenesis of ICP, but the above indicators cannot be widely used in clinical diagnosis in developing countries due to technical and application problems21,30,31. To this end, we evaluated the diagnostic value of routine blood examination in routine clinical obstetrical examination for ICP. In our study, we found that the numbers of WBC and neutrophils, the NLR and the PLR were significantly higher in the ICP group than in the normal group, while the lymphocyte levels was significantly lower in the ICP group than in the normal group (shown in Table 1). By correlation analysis, we found that TBA was positively correlated with the WBC count, neutrophil count, NLR and PLR and negatively correlated with lymphocyte count. Furthermore, our study also confirmed that the WBC count, neutrophil count, lymphocyte count, NLR and PLR are independent risk factors for ICP, and ROC curve analysis confirmed that the NLR had a higher diagnostic value for ICP (shown in Tables 4 and 5). The above changes are consistent with the reports in the literature21. The above results suggest that the elevation of TBA is consistent with the elevation of routine blood inflammatory indicators and that the occurrence of ICP may be directly related to inflammation.
Although ICP has little risk for mothers, it is related to adverse perinatal outcomes, leading to preterm birth, stillbirth32. Preterm birth is a common clinical complication of ICP. Cohort studies have confirmed that an increase in the maternal serum TBA concentration will increase the incidence of spontaneous preterm birth33. Long-term low-grade chronic inflammation increases the risk of preterm birth34. Therefore, we analyzed the blood inflammation and biochemical indicators of preterm and term births in pregnant women with ICP. We found that GGT, TBA, neutrophils and NLR of preterm delivery in pregnant women with ICP were higher than full-term delivery groups. We used ROC curves to predict the diagnostic value of the abovementioned indicators for preterm birth in pregnant women with ICP. The NLR had certain predictive value for preterm birth, but the sensitivity and specificity were not high enough. However, the combined detection of the NLR and TBA level increases the predictive efficacy and sensitivity for preterm birth in ICP. The mechanisms of preterm delivery in ICP are still unclear. The accumulation of the inflammatory response induced by ICP, which leads to the progression of ICP, and the accumulation of TBA may be involved in the onset of preterm delivery35,36.
ICP is the most common pregnancy-specific liver disease37. The current clinical grading criteria for ICP are mainly based on the level of serum TBA38. In this study, we considered that the critical value of TBA for diagnosing ICP was 10 µmol/L; the TBA level of 10–40 µmol/L was defined as mild ICP, and the TBA level > 40 µmol/L was defined as severe ICP, as suggested in the literature30. As serum TBA could not be used to distinguish the ICP patients with low pruritus from normal pregnant women, and there is no consensus on the diagnostic threshold of liver enzymes, which may limit the early diagnosis of ICP21,30,32. Since the pathogenesis of ICP is closely related to inflammation, in our study, we used routine blood tests, which are commonly used in clinical practice, for the ancillary diagnosis of ICP and its complications leading to preterm delivery. Our study confirmed that biochemical indicators and blood inflammatory indicators are related to ICP and its complications in preterm delivery. Some indicators can be used alone or in combination for the prediction and auxiliary diagnosis of ICP. For patients with clinical ICP symptoms and a lack of typical symptoms, multi-indicator combined detection should be used to reduce the occurrence of complications.
Materials and methods
Study subjects
This study was a cross-sectional analysis of 304 pregnant women with diagnosed ICP and 363 normal pregnant women in Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, from January 2020 to June 2021. The inclusion criteria of the ICP group were as follows39: (1) pregnant women with pruritus without rash and elevated TBA level ≥ 10 µmol/L and/or aminotransferase levels detectable in their blood sample; (2) patients were classified as having mild or severe cholestasis based on TBA concentrations of 10–40 or ≥ 40 µmol/L, respectively (3) patients without diabetes, hepatobiliary diseases, immunological diseases or other pregnancy complications; (4) patients whose gestational age was determined based on the first day of the last menstrual period and/or first trimester ultrasonographic measurements; (5) patients with a singleton pregnancy confirmed by ultrasound; (6) patients with voluntary participation; and (7) patients with preterm delivery, defined as delivery before 37 completed weeks of gestation.
Data collection
All venous blood samples were collected from peripheral vein blood in a vacutainer tube after the participants had fasted for 12 h. The hemogram samples were collected in ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes, and blood serum samples were collected into standard gel separator tubes for biochemical tests. All samples were detected within 1 h after collection. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), prealbumin (PA) and total bile acid (TBA) levels were measured by standard laboratory techniques using a Hitachi automatic analyzer (Hitachi 7600 automatic analyzer, Tokyo, Japan). The CBC included the white blood cell (WBC) count, platelet (PLT) count, neutrophil count, and lymphocyte count. The NLR was calculated as the neutrophil count divided by the lymphocyte count, and the PLR was calculated as the platelet count divided by the lymphocyte count. All CBC analyses were performed with a Sysmex Hemato analyzer (Sysmex XN-1000 Hemato analyzer, Kobe, Japan).
Statistical analysis
All data were analyzed by SPSS 22.0 (IBM, Chicago, USA). The normally distributed measurement data are expressed as the mean ± standard deviation, and the measurement data that did not conform to a normal distribution are expressed as the median (interquartile range (IQR)). The Mann‒Whitney U test was used to perform comparisons between groups. Receiver operating characteristic (ROC) analysis was used to identify the best cut-off value, and the sensitivity and specificity of each indicator for ICP diagnosis were screened. Spearman correlation coefficients were computed to examine the association between TBA and blood inflammatory indicator levels. A multivariate logistic regression model was used to analyse the risk factors for ICP and the diagnostic value of the combined indices. A p < 0.05 was considered statistically significant.
Ethical approval
This study was approved by the ethics committee of the Women and Children Affiliated Hospital of the Medical College of the University of Electronic Science and Technology, and clinically informed written consent was obtained from each patient. All methods were carried out in accordance with the Declaration of Helsinki.
Limitations
Our study provides evidence that blood inflammatory indicators and biochemical indicators have a direct correlation with ICP. At present, the method of diagnosing ICP based on serum inflammatory markers and biochemical markers still needs to be studied, and larger and further prospective studies to evaluate the use of the markers and in-depth mechanistic studies of ICP are needed.
Data availability
The authors confirm that all the data-based findings are fully available without restriction. All relevant data are included in the paper and references. The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Change history
19 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-26533-z
References
Lee, R. H., Mara, G., Metz, T. D. & Pettker, C. M. Society for maternal-fetal medicine consult series #53: Intrahepatic cholestasis of pregnancy: Replaces consult #13, April 2011. Am. J. Obstet. Gynecol. 224, B2–B9. https://doi.org/10.1016/j.ajog.2020.11.002 (2021).
Qi, H. B., Shao, Y., Wu, W. X. & Zhang, J. H. Grading of intrahepatic cholestasis of pregnancy. Zhonghua Fu Chan Ke Za Zhi 39, 14–17 (2004).
Joshi, D., James, A., Quaglia, A., Westbrook, R. H. & Heneghan, M. A. Liver disease in pregnancy. Lancet 375, 594–605. https://doi.org/10.1016/S0140-6736(09)61495-1 (2010).
Dixon, P. H. & Williamson, C. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin. Res. Hepatol. Gastroenterol. 40, 141–153. https://doi.org/10.1016/j.clinre.2015.12.008 (2016).
Tayyar, A. T. et al. Role of ischemia-modified albumin in the evaluation of oxidative stress in intrahepatic cholestasis of pregnancy. J. Matern. Fetal Neonatal. Med. 32, 3836–3840. https://doi.org/10.1080/14767058.2018.1474871 (2019).
Sharifzadehgan, S., Hermann, M., Nedellec, S., De Luca, D. & Benachi, A. Intrahepatic cholestasis of pregnancy: Shorter duration of labor?. Eur. J. Obstet. Gynecol. Reprod. Biol. 225, 258–259. https://doi.org/10.1016/j.ejogrb.2018.03.045 (2018).
Feng, C. et al. Impacts of different methods of conception on the perinatal outcome of intrahepatic cholestasis of pregnancy in twin pregnancies. Sci. Rep. 8, 3985. https://doi.org/10.1038/s41598-018-22387-6 (2018).
Williamson, C. & Geenes, V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 124, 120–133. https://doi.org/10.1097/AOG.0000000000000346 (2014).
Kosters, A. & Karpen, S. J. The role of inflammation in cholestasis: Clinical and basic aspects. Semin. Liver Dis. 30, 186–194. https://doi.org/10.1055/s-0030-1253227 (2010).
Allen, K., Jaeschke, H. & Copple, B. L. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 178, 175–186. https://doi.org/10.1016/j.ajpath.2010.11.026 (2011).
Gabay, C. K. I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454. https://doi.org/10.1056/NEJM199902113400607 (1999).
Kirbas, A. et al. The role of interleukin-17 in intrahepatic cholestasis of pregnancy. J. Matern. Fetal Neonatal. Med. 29, 977–981. https://doi.org/10.3109/14767058.2015.1028354 (2016).
Biberoglu, E. et al. Role of inflammation in intrahepatic cholestasis of pregnancy. J. Obstet. Gynaecol. Res. 42, 252–257. https://doi.org/10.1111/jog.12902 (2016).
Liu, J. F. et al. Association between neutrophil-to-lymphocyte ratio and differentiated thyroid cancer: A meta-analysis. Sci. Rep. 6, 38551. https://doi.org/10.1038/srep38551 (2016).
Shi, L. et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 8, 18792–18801. https://doi.org/10.18632/oncotarget.13320 (2017).
Sitaula, D., Timalsina, S., Sharma, B., Pokharel, B. & Thapa, R. Prevalence and pregnancy outcomes of intrahepatic cholestasis of pregnancy. J. Nepal. Health Res. Counc. 19, 321–326. https://doi.org/10.33314/jnhrc.v19i2.3455 (2021).
Wei, W. & Hu, Y. Y. Expression of hypoxia-regulated genes and glycometabolic genes in placenta from patients with intrahepatic cholestasis of pregnancy. Placenta 35, 732–736. https://doi.org/10.1016/j.placenta.2014.06.372 (2014).
Shan, D. et al. Intrahepatic cholestasis of pregnancy in women with twin pregnancy. Twin Res. Hum. Genet. 19, 697–707. https://doi.org/10.1017/thg.2016.74 (2016).
Xiao, J. et al. Molecular pathogenesis of intrahepatic cholestasis of pregnancy. Can. J. Gastroenterol. Hepatol. 2021, 6679322. https://doi.org/10.1155/2021/6679322 (2021).
Woolbright, B. L. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 18, 4985. https://doi.org/10.3748/wjg.v18.i36.4985 (2012).
Kirbas, A. et al. Neutrophil-to-lymphocyte ratio as a diagnostic marker of intrahepatic cholestasis of pregnancy. Eur. J. Obstetr. Gynecol. Reprod. Biol. 180, 12–15. https://doi.org/10.1016/j.ejogrb.2014.05.042 (2014).
Palmer, K. R., Xiaohua, L. & Mol, B. W. Management of intrahepatic cholestasis in pregnancy. The Lancet 393, 853–854. https://doi.org/10.1016/s0140-6736(18)32323-7 (2019).
Pathak, B., Sheibani, L. & Lee, R. H. Cholestasis of pregnancy. Obstet. Gynecol. Clin. North Am. 37, 269–282. https://doi.org/10.1016/j.ogc.2010.02.011 (2010).
Huang, X. et al. ROC curve analysis of the sensitivity and specificity of biochemical detection of intrahepatic cholestasis during pregnancy. Z. Geburtshilfe Neonatol. 225, 327–332. https://doi.org/10.1055/a-1299-2298 (2021).
Ekiz, A. et al. Alanine aminotransferase as a predictor of adverse perinatal outcomes in women with intrahepatic cholestasis of pregnancy. Pak. J. Med. Sci. 32, 418–422. https://doi.org/10.12669/pjms.322.9057 (2016).
Zhu, P. Z. S. et al. Correlation of lipid peroxidation and atp enzyme on erythrocyte membrane with fetal. Eur. Rev. Med. Pharmacol. Sci. 23, 2318–2324. https://doi.org/10.26355/eurrev_201903_17371 (2019).
Celik, S. & Caliskan, C. The impact of assisted reproductive technology in twin pregnancies complicated by intrahepatic cholestasis of pregnancy: A retrospective cohort study. Z. Geburtshilfe Neonatol. 225, 34–38. https://doi.org/10.1055/a-1129-7358 (2021).
Hagenbeck, C. et al. Intrahepatic cholestasis of pregnancy. Gynakologe 54, 1–16. https://doi.org/10.1007/s00129-021-04787-4 (2021).
Larson, S. P., Kovilam, O. & Agrawal, D. K. Immunological basis in the pathogenesis of intrahepatic cholestasis of pregnancy. Expert Rev. Clin. Immunol. 12, 39–48. https://doi.org/10.1586/1744666X.2016.1101344 (2016).
Mays, J. K. The active management of intrahepatic cholestasis of pregnancy. Curr. Opin. Obstet. Gynecol. 22, 100–103. https://doi.org/10.1097/GCO.0b013e328337238d (2010).
Celik, S., Guve, H., Caliskan, C. & Celik, S. The role of melatonin, IL-8 and IL-10 in intrahepatic cholestasis of pregnancy. Z. Geburtshilfe Neonatol. 225, 238–243. https://doi.org/10.1055/a-1233-9084 (2021).
Ovadia, C. et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: Results of aggregate and individual patient data meta-analyses. The Lancet 393, 899–909. https://doi.org/10.1016/s0140-6736(18)31877-4 (2019).
Glantz, A., Marschall, H. U. & Mattsson, L. A. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology 40, 467–474. https://doi.org/10.1002/hep.20336 (2004).
Morisaki, N. et al. Maternal blood count parameters of chronic inflammation by gestational age and their associations with risk of preterm delivery in the Japan Environment and Children’s Study. Sci. Rep. 11, 15522. https://doi.org/10.1038/s41598-021-93101-2 (2021).
Wang, L., Lu, Z., Zhou, X., Ding, Y. & Guan, L. Effects of intrahepatic cholestasis of pregnancy on hepatic function, changes of inflammatory cytokines and fetal outcomes. Exp. Ther. Med. 17, 2979–2984. https://doi.org/10.3892/etm.2019.7312 (2019).
Türkmen, G. G. et al. Effect of intrahepatic cholestasis of pregnancy on maternal serum screening tests. J. Neonatal. Perinatal. Med. 9(4), 411–415. https://doi.org/10.3233/NPM-161618 (2016).
Westbrook, R. H., Geoffrey, D. & Williamson, C. Pregnancy and liver disease. J. Hepatol. 64(4), 933–945. https://doi.org/10.1016/j.jhep.2015.11.030 (2016).
Bicocca, M. J., Sperling, J. D. & Chauhan, S. P. Intrahepatic cholestasis of pregnancy: Review of six national and regional guidelines. Eur. J. Obstet. Gynecol. Reprod. Biol. 231, 180–187. https://doi.org/10.1016/j.ejogrb.2018.10.041 (2018).
Liu, L. et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. The Lancet 385, 430–440. https://doi.org/10.1016/s0140-6736(14)61698-6 (2015).
Funding
The clinical case screening and data collection were supported by the Chengdu Science and Technology Bureau: Technology Innovation Research and Development Project [2021-YF05-00648-SN] and Sichuan Medical Association Wound Disease Special Scientific Research Project [2021TG33].
Author information
Authors and Affiliations
Contributions
L.W., M.J.L. contributed to the study design and prepared the manuscripts; Y.Z.W., D.C.C. and H.B.Y. analyzed the data; C.G.L. revised the manuscripts; S.Y.W. and W.S. completed all tests. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Mangjun Luo was incorrectly marked as a corresponding author. The correct corresponding authors for this Article are Li Wang and Chenggui Liu. In addition, Li Wang and Mengjun Luo were omitted as equally contributing authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, M., Wang, L., Yao, H. et al. Diagnostic and prognostic value of blood inflammation and biochemical indicators for intrahepatic cholestasis of pregnancy in Chinese pregnant women. Sci Rep 12, 20833 (2022). https://doi.org/10.1038/s41598-022-22199-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22199-9
- Springer Nature Limited