Abstract
In adult Lepidoptera the labial palps are best known for their role in CO2 detection, but they can also bear sensilla chaetica which function is unknown. The number and distribution of sensilla chaetica in labial palps was studied using a bright field microscope. To determine if these sensilla have a gustatory function, we performed single sensillum electrophysiology recordings from palp and antennal sensilla of adult moths of Cydia pomonella (L.), Grapholita molesta (Busck) and Lobesia botrana (Denis and Shieffermüller). Each sensillum was stimulated with 3 doses of one of four test stimulus (sucrose, fructose, KCl and NaCl). Overall, responses (spikes/s−1) increased with dose, and were higher in the palps than in the antennae, and higher to sugars than to salts. With sugars the response increased with concentration in the palp but not in the antenna. With salts there was a drop in response at the intermediate concentration. The number and position of sensilla chaetica on labial palps was variable among individuals. Sensilla were located in the most exposed areas of the palp. Differences in sensilla distribution were detected between species. Such differences among species and between palps and antenna suggest that taste sensilla on the palps have an unforeseen role in adaptation.
Similar content being viewed by others
Introduction
Reception of gustatory stimuli (also referred to as gustation, taste or contact chemoreception) plays a vital role in many aspects of insect fitness, such as in food selection and oviposition choices1,2. Tastants are detected by gustatory receptor neurons (GRNs), which are typically present in groups of 2 to 4 inside gustatory sensilla2. Gustatory sensilla have been found in almost any part of the adult body, especially those in direct contact with taste stimuli, such as the tarsi, mouthparts, food channel, ovipositor and antennae2. There are different morphological types of gustatory sensilla2,3. Sensilla chaetica are long, hair-like structures that can be differentiated from similar sensilla (e.g. sensilla trichoidea) by having thicker walls3,4. The presence of a terminal pore in sensilla chaetica is usually associated with gustatory function and a flexible basal socked with tactile function3. Sensilla chaetica typically have a contact chemoreception function, with two to four GRNs and one mechanosensory neuron which terminates in a tubular body at the base of the sensilla4,5.
Gustation in the mouthparts of adult insects is generally found in the galeae, in the maxillary palps of the maxilla, and in the labial palps of the labium. The labial palps of insects with standard chewing mouthparts (e.g., Coleoptera, Orthopteroids) are usually small and bear gustatory sensilla6,7,8. In insects with mouthpart modified for sucking and licking (e.g., Diptera, Hemiptera), the labium and associated labial palps retain their taste function9,10,11,12. In Lepidoptera, the galeae have joined in a long and coiled proboscis, and in most lineages the rest of the mouthparts structures are largely reduced, except for the labial palps, which are relatively large and cover most of the front part of the insect head at each side of the proboscis13. The labial palps of Lepidoptera present a cavity at the tip of the apical segment, the labial-palp pit organ (LPO), which contains sensilla that respond to changes in CO2 concentration14. A ventral glomerulus in the antennal lobe receives the input of the CO2 sensory cells. CO2 detection may help Lepidoptera in host-plant selection14. In some moth species, the labial palps bear chaetica-like sensilla15,16, which often have a gustatory function in other insect appendages3,4,5. The presence of putative gustatory receptors of the sugar clade in the labial palps of Helicoverpa armigera (Hübner)17 further indicates the presence of gustation in the labial palps, but as far as we know, there is no physiological evidence that the labial palps of Lepidoptera have a gustatory function. The antennae of Lepidoptera are well known for their olfactory function performed by numerous sensilla trichoidea, but they also bear some gustatory sensilla chaetica in each flagellum2. In noctuid moths the gustatory function of antennal sensilla chaetica has been demonstrated18,19,20. The gustatory function of sensilla chaetica on the antennae of tortricid moths21,22,23 remains to be shown.
The objective of this study is to determine if the sensilla present of the surface of the labial palp of moths respond to gustatory stimuli. To this end we performed electrophysiological recordings from adults of three tortricid moths, Cydia pomonella (L.), Lobesia botrana (Denis and Shiffermüller) and Grapholita molesta (Busck), which are key pest species of fruit trees and vines worldwide24,25. Two sugars (fructose and sucrose) and two salts (KCl and NaCl) were tested at 3 concentrations. In addition, we tested the response of sensilla chaetica in the antennae. In order to determine the biological relevance of sugars in these species we also studied their effect on the adult longevity.
Results
Labial palp sensilla
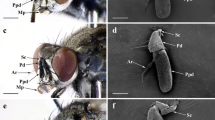
The labial palps were composed of three segments densely covered with scales, with sensilla protruding from the scales in the apical segment (Fig. 1). These sensilla have a typical chaetica morphology, they are hair-like structures relatively straighter and more rigid than sensilla trichoidea, and with a flexible socket at the base3,4,5. The second segment was the longest (G. molesta 356.5 µm, L. botrana 464 µm and C. pomonella 636 µm on average), it was curved upwards and projected forward on each side of the head (Fig. 1). The apical segment was oval, between 120 and 237 µm long and 60 to 143 µm wide depending on the species and sex (Table 1). The apical segment was longest in C. pomonella, followed by L. botrana and G. molesta and widest in C. pomonella than in the other two species. It was wider and longer in females than in males (Supplementary Table S1). The number of sensilla in the apical segment of the labial palp ranged between 5 and 15 (Table 1), and it was positively correlated with the area of the segment (Person correlation coefficient = 0.73, t58 = 8.15, p < 0.001). In general females had 1.3 more sensilla than males and the number of sensilla was 1.3 times higher in C. pomonella than in G. molesta (Supplementary Table S2).
Labial palps of a female G. molesta. (a) Lateral view, (b) ventral view, scales have been partially removed from the right palp, (c) close up of "b" showing sensilla chaetica clearly protruding among the scales from the third segment, (d) third segment digested with KOH and stained with gencian violet to show the labial pit organ (elongated dark sac inside the palp) and the sensilla chaetica on the surface. White arrows in panels “(a)” and “(b)” indicate the apical segment of labial palp.
The position of sensilla chaetica in the apical segment of the labial palp was highly variable between individuals and no consistent location could be determined for any sensillum. However, some general patterns could be observed. Sensilla tended to be located on the most exposed areas (of the resting palp). In addition, many sensilla occurred around the LPO opening (39% in L. botrana, 30% in C. pomonella and 23% in G. molesta), (Fig. 1, Supplementary Fig. S1). In all species, more than 80% of sensilla were located on the distal half of the segment and more than half of the sensilla (62% in L. botrana, 66% in G. molesta and 75% in C. pomonella) were lateral. The most substantial difference between species was found between dorsal and ventral regions. In G. molesta 90% of the sensilla were ventral, while in the other two species the number of sensilla in the dorsal and ventral regions was similar (Supplementary Fig. S1, Supplementary Table S3). The results of the ANOVA of the distribution of the sensilla among the areas defined by anatomical symmetry axes showed differences among species (Deviance = 106.28, df = 14, p [Chi] < 0.001), but not between sexes (Deviance = 11.53, df = 7, p [Chi] = 0.117) and no interaction between species and sexes was detected (Deviance = 10.23, df = 14, p [Chi] = 0.745). Within each species, the number of sensilla varied between areas. The ventro-lateral-distal area of the palp had the highest number of sensilla in all species, however substantial differences could be observed between species in sensilla distribution among areas (Tukey: p < 0.05, Supplementary Fig. S1, Supplementary Table S3).
Effect of diet on survival
Diet had a significant impact on adult survival in all three species (Log-rank test: p < 0.0001), the longest survival time was detected in C. pomonella with access to sugared water (35 days) and the lowest in G. molesta without water (1 day). In the absence of water, median longevity decreased by 75% in G. molesta, 50% in L. botrana and 40% in C. pomonella. Addition of sugar to the diet increased median longevity in G. molesta and C. pomonella by 6 and 4.5 days respectively (Log-rank test: p < 0.0001, Supplementary Fig. S2).
Electrophysiology
For each combination of factors (stimulus, species, sex and appendage) between 16 and 34 sensilla from between 4 and 6 individuals were sampled, with a total of 3690 recordings from 1057 sensilla sampled from antenna and palp together (Supplementary Table S4). The highest number of spikes/s (68) was observed on the labial palp of a female C. pomonella in response to KCl, but in general the number of spikes was relatively low (2.84 on average) (Fig. 3). The average spike amplitude was relatively variable between recordings of the same sensillum and between sensilla (Fig. 2). Thus, although several spike amplitude classes could be detected in some recordings, we did not sort spikes. Instead, for the purpose of this study, we analyzed the sum of all the spikes in a given recording, irrespective of whether they belong to one or more neuron types. Different number of spikes between electrolyte and sugars indicate that the electrolyte alone did not stimulate sensilla more than the test stimuli (Dunnett test: p < 0.01, Supplementary Table S5).
Representative single-sensillum recording traces (left) of sensilla chaetica and superimposed spikes (right) detected in the recording (6 ms section). Each group (a–d) shows the response of the same sensillum to increasing concentrations of test stimulus. (a) Labial palp of C. pomonella female, (b) antenna of C. pomonella male, (c) labial palp of C. pomonella female, and (d) antenna of L. botrana male. NaCl 1mM=electrolite.
The model that best explained responses to stimuli contain all main factors with a 4th-order interaction (Likelihood-ratio test: p < 0.01, Supplementary Table S6). According to the ANOVA (Table 2), appendage (i.e., antenna or palp) explained 23.95% of the deviance, the interaction between stimulus and concentration explained 13.07% of the deviance, concentration 9.98%, stimulus 7.77%, and species 6.8%. The remaining 25 factors of the model together explained 33.56% of the deviance, each contributing 4.79% or less to the total deviance. On average, response was 2.76 times higher in palpi than in antennae, and the number of spikes increased with stimulus concentration (Tukey: p < 0.05, Table 3). There were 1.2 times more spikes/s to sugars (fructose and sucrose combined) than to salts (NaCl and KCl combined), and sucrose was the strongest of the four stimuli tested (4.30 ± 0.26 spikes/s) (Tukey: p < 0.05, Table 3, Fig. 3). C. pomonella produced 1.8 times more spikes/s than G. molesta (Tukey: p < 0.05, Table 3, Fig. 3). Sex had a significant, but very low, impact on the number of spikes (0.76 more in females than in males, Tukey: p < 0.05, Table 3), and so the two sexes were combined for graphic display (Fig. 3).
Response of gustatory receptor neurons of sensilla chaetica located on the apical segment of the labial palps and antenna of three tortricid moth species upon a 2 s stimulation with three doses of NaCl, KCl, fructose or sucrose. N = 16–34 sensilla of 4–6 individuals of each sex. Males and females have been combined in this plot. Box plots show median (horizontal line), first and third quartile (box), 1.5*inter-quartile range (error bars), and data points (•).
The effect of dose was different for salts and sugars. In salts the highest response occurred at 1 M, then at 10 mM and the lowest at 100 mM (Tukey: p < 0.05, Supplementary Table S6, Fig. 3). With sugars, no differences were found among concentrations when pooling antennal and palp responses (Tukey: p < 0.05, supplementary Table S6). However, in antenna the response to sugars decreased as the dose increased, whereas in the palp responses increased with concentration (Tukey: p < 0.05, Supplementary Table S6, Figs. 2 and 3).
From highest to lowest, the mean responses to sugars and salts were as follows: sugars in palps (5.33 ± 0.27 spikes/s), salts in palps (3.60 ± 0.27 spikes/s), and sugars and salts in antennae (1.40 ± 0.11 and 1.81 ± 0.14 respectively) (Tukey: p < 0.05, Supplementary Table S6, Fig. 3). Responses to sugars in C. pomonella and L. botrana were in average 1.9 times higher than in G. molesta, but no differences were detected between species with salts. Females produced more spikes than males in L. botrana and G. molesta (Tukey: p < 0.05, Supplementary Table S6).
Discussion
We present evidence that the labial palps of moths have a gustatory function. Furthermore, we found significant differences between appendages, stimuli, species and sexes. As far as we know, this may be the first direct evidence that the labial palps of moths, or perhaps any Lepidoptera, have a gustatory function, while it is a common feature in other insect orders. The presence of a flexible socket at the base of the sensilla chaetica suggests mechanosensory function3,4, however observations should be conducted to confirm this role. We do not know the extent of gustation in the labial palps of Lepidoptera because reports of uniporous sensilla chaetica (i.e., with a putative gustatory function) on the external part of labial palps are scarce. Chaetica-like sensilla with a terminal pore have been reported in a species of the Cossidae family16. Sensilla chaetica are described in other Cossidae and in members of the Tortricidae and Pyralidae families, but it is not indicated if they bear a pore at the tip, and thus they may not be gustative15,26,27. In a Neopseustidae specie, aporous sensilla chaetica have been described28. Several studies on members of the Gelechiidae, Pyralidae, Sphingidae, Noctuidae, Plutellidae, Erebidae and Nymphalidae families fail to report sensilla chaetica or any other type of sensilla with a putative gustatory function in the labial palps29,30,31,32,33,34,35,36,37,38.
Obtaining electrophysiological recordings from sensilla chaetica of the labial palps and antenna was challenging because we experienced a persistent difficulty to make stable electric contacts with these sensilla, whereas it was relatively easy on sensilla styloconica of the proboscis (data not shown). Conditions improved when the humidity of the rearing chamber was increased, but consistent contacts were only obtained when the sensilla tip was wiped repeatedly with an empty glass capillary before making contact. Some authors have encountered similar problems in other insects and suggest that debris accumulates on the pore at the tip of the sensilla. Städler et al.39 report that low relative humidity reduced the quality of the recordings of Rhagoletis cerasi L. tarsi, and that flies that were allowed to walk on the plants were easier to record (implying that the sensilla were "unplugged" by the contact with the substrate). Canney and Gardner40 report that cleanness of the pore of sensilla chaetica improved the quality of electrophysiological recordings in Ostrinia nubilalis (Hübner).
Sugars and salts play a role on the fitness and behavior of the three tortricid moths. Our longevity test confirms that adults of the three species do not live long without water and that sugars prolong longevity41,42,43. When given access to flowers under laboratory conditions the longevity of C. pomonella increased with respect to a no-food control, but a dilution of sugar in water was even better than the flowers44. Peach extrafloral nectar, a natural solution rich in sugars45, enhanced G. molesta adult longevity42. Despite the importance of sugar on adult fitness, only L. botrana has been observed feeding in the wild. It visits the flowers of tansy (Tanacetum vulgare L.) (a non-host plant located around the vineyards) at dusk to feed on the pollen and nectar46. Sugars stimulate oviposition in C. pomonella and L. botrana47,48, and gustatory sensilla in the ovipositor of L. botrana detect fructose and sucrose48. KCl is also perceived by sensilla in the ovipositor of L. botrana, and it affects oviposition48. Sugars and salts could impact larval fitness too49,50,51. Therefore, the presence of taste receptors on antennae and palps could contribute to the detection of salts and sugars, but they may also be specialized on the detection of other stimuli not tested yet.
Gustatory sensilla of insects typically have 4 different GRNs, generally associated to different taste modalities2,3. GRNs in the antennae of adult Lepidoptera respond to sugars, water, salts, amino acids and bitter compounds18,19,20. The number of spikes that we observed in tortricids was relatively lower than what has been found in antennal sensilla of noctuid moths18,19,20. We also observed that the response to sugars in antennal sensilla decreased as concentration increased, contrary to what has been observed in Chloridera (formely Heliothis) virescens (Fabricus) and Spodoptera littoralis (Boisduval)19,20. Many noctuids are flower visitors, but this behavior is rare in tortricids52, and so different sugar requirements between members of the two families may explain differences in sugar sensitivity. Water and salt intake regulation is crucial for animals to maintain osmotic homeostasis1,53, and in many insects some cells respond to low salt concentrations (water GRN) and some to high salt concentrations (salt GRN), which allows for fine detection of salts19,20,53. In our tests, the relatively high number sensilla responding to the electrolyte control, and the unusual shape of the dose–response curve to salts (with a drop at the intermediate concentration) suggests that palp and antennal sensilla chaetica of tortricids have a water and a salt GRN, which may help in salt detection.
There are few reports on the putative behaviors where Lepidoptera may use the gustatory sensilla detected on the labial palps. Grant54 observed the response of the tortricid moth Choristoneura fumiferana (Clemens) to a rubber septum loaded with female sex pheromone. The tips of the labial palps contacted with the septum ("the palps themselves pulled away from the head in lever-like fashion") right before attempting copulation (Fig. 1 in54). Also during courtship, G. molesta males and females perform a dance where there is mutual contact with the antennae and probably the palps too55. Thus, gustatory receptors on palp and antennae may be used to detect conspecific signals, such as cuticular hydrocarbons, which often present sexual dimorphism in insects56,57. Gustatory function of the labial palps may also be related to oviposition choice behavior because in the moth Cactoblastis cactorum (Berg) females actively touch the plant with the labial palps while searching for oviposition sites58.
The discovery of gustation in the labial palps of moths may shed new light on the innervation of the labial palp nerves in the moth brain. Unilateral palp backfills typically reveal bilateral projections ascending to the antennal lobe where they stain a basal glomerulus, equivalent in both males and females, the "labial pit organ glomerulus" (LPOG)33,59,60. This glomerulus receives the input from the CO2 sensory neurons housed in the labial pit organ. However, palp backfills show additional arborizations in the suboesophageal ganglion (SOG)33,59. To clarify this point, Pramod et al.60 performed selective mass staining from both, the inside of the labial pit organ and the outer surface of the distal labial palp segment of H. armigera and compared their arborizations. LPO sensory neurons projected exclusively to the LPOG, whereas the non-LPO sensory neurons targeted the gnathal ganglion and the ventral nerve cord. It is very likely that the axons that do not innervate the LPOG correspond to mechano/gustatory neurons located on gustatory sensilla because the mechano/gustatory input from other cephalic appendages ends in the SOG2,3. Interestingly, an SEM picture of the labial palp of H. armigera does not show sensilla chaetica33. Sensilla chaetica are rather conspicuous and resilient61 so it is unlikely that they are accidentally knocked off when scales were removed to observe the palps. If they did not fall during scale removal, then other type of sensilla may contain the palp neurons that innervate the SOG. Thus, further studies are required to determine which other Lepidoptera species have gustatory function in the labial palps.
Material and methods
Insects
Larvae were reared on a semi-artificial diet modified from that of Ivaldi-Sender62 at 25 °C under a 16:8 light:dark photoperiod. Pupae were sexed and kept in separate environmental chambers with unrestricted access to a 10% sucrose in water dilution. Before electrophysiological recordings, adults were anaesthetized with CO2 to restrain them inside a modified pipette tip, with their head, antennae and palps fixed with melted dental wax (ref: R3712-00, Leone s.p.a., Firenze, Italy). All insects tested were between 1 and 3 days old.
Labial palp sensilla
To investigate the location of sensilla chaetica in the labial palps, 10 palpi from different individuals were observed for each species and sex in a bright field microscope. We removed the scales, cleared the cuticle and stained the preparations according to George and Nagy63. Detached heads were boiled in 10% KOH until the scales fell off and then washed in distilled water and cleared in 2.5% bleach until they became transparent. After a second wash in distilled water, they were immersed in 0.5% crystal violet until the sensilla were clearly visible. Samples were placed on a drop of glycerol on a microscope slide under a cover slip.
The maximum length and width of the apical segment of the labial palp was measured with an eyepiece micrometer. To assert sensilla position, the apical segment was divided in 8 areas (Supplementary Fig. S1) resulting from the intersection of the 3 axes of symmetry (sagittal, transversal and frontal), and the number of sensilla in each area was recorded.
Electrophysiology
Gustatory stimuli [NaCl (CAS: 7647-14-5, ref: S7653-1 KG), KCl (CAS:7647-14-5, ref: S7653-1 KG), sucrose (CAS: 57-50-1, ref: S9378-1 KG) and fructose (CAS: 50-48-7, ref: F0127-1 KG), Sigma-Aldrich, Madrid, Spain] were diluted in deionized water. Sucrose and fructose dilutions contained 1 mM NaCl as electrolyte. Three concentrations of each stimulus (10, 100, 1000 mM) were prepared in 1 ml aliquots and kept at − 20 °C. During the experiments, one aliquot of each concentration was defrosted and kept at 4 °C for up to one week.
For each individual, two to six sensilla chaetica from either the antenna or the apical segment of the labial palp were tested. The antenna was sampled up to the apical segment. On the antenna, sensilla chaetica were differentiated from sensilla trichoidea by their morphology (straighter and thicker), their orientation (perpendicular to the surface) and their optical characteristics (brighter under the stereo microscope). On the outside of the labial palp only sensilla chaetica were present. Only one stimulus was used for a given individual, starting from lowest to highest concentration. The contact with each sensillum was limited to 2 s, allowing ≥3 min between stimulations to avoid adaptation. Sugars stimuli were preceded by stimulation with the electrolyte (1 mM NaCl) as a control.
Electrophysiological recordings from sensilla chaetica were obtained using the tip recording technique64. Glass capillary recording electrodes (Hirschmann Laborgeräte GmbH & Co, Germany) were pulled to obtain a 3.5 μm wide tip (PP-830, Narishige, Japan), which was filled with the test solution and connected to a 0.5 mm-wide platinum wire, fitted to a preamplifier probe (Taste Probe, Syntech, Germany). The recording electrode was placed over the sensillum tip using a micromanipulator (NMN-25, Narishige, Japan) under a stereomicroscope (Leica M125, objective 2x, oculars 25x, zoom range 0.8–10, Leica microsystems, Spain). The reference electrode was a sharpened tungsten wire (0.125-mm diameter, 99.98% purity, Advent Research Materials Ltd, UK), inserted into the eye with the help of a micromanipulator (UM-3C, Narishige, Japan). The recording electrode was filled with the stimulus solution just before stimulation, it was discarded after 2 minutes and replaced by a new one if needed.
The signal from the recording electrode was pre-amplified (10x) and filtered (10 Hz high-pass) using a Taste Probe amplifier (65; Syntech, Germany) and further amplified (50x) and filtered (3000 Hz low-pass filter) (AC/DC differential amplifier, A-M systems Inc., WA, USA). The signal was digitalized and analyzed (Micro 1041-3 and Spike2, respectively, Cambridge Electronic Design Limited, UK), and spike detection was performed using dbWave software66.
Effect of water and sucrose on adult longevity
We determined the effect of water and sugar on the survival of 20–24 adults in order to assess the importance of these stimuli on adult fitness. To this end, groups of 4 to 9 newly emerged adults of both sexes picked randomly from the colony were placed in ventilated 100 mL plastic bottles after CO2 anesthesia. Four bottles were prepared for each treatment: control (dry cotton swab), water: (a cotton swab soaked in distilled water) and sugar (cotton swab soaked in 10% domestic sucrose diluted in distilled water). Cotton was replaced regularly so that it was never dry. Bottles were inspected daily (with sporadic 2 to 3 day gaps for the longest-lived species, C. pomonella) until all individuals were dead, dead individuals were scored and removed.
Data analysis
Statistical analyses were run in R 4.1.2 software67. Generalized lineal models (GLM) were used for all the analyses with specific error families and link functions where needed. Pairwise comparisons used the Tukeys´s test of the package “emmeans”, unless specified otherwise. To analyze length and width of the apical segment of the labial palp a GLM with Gaussian error distribution was used. The total number of sensilla in the apical segment of the labial palps and the distribution of sensilla, within each species, among the delimited areas (Supplementary Fig. S1) was analyzed with GLM with Poisson error distribution and a logit link. For spike counts analysis only the response to stimuli, and not to the 1 mM NaCl electrolyte, was analyzed. A quasi-Poisson error distribution was used because overdispersion was detected. A different model was run to compare the number of spikes between each sugar concentration and the electrolyte control using Dunnett test.
Model selection started from the simplest model containing no main effects, then main factors and interactions were added sequentially. For comparison between models, the likelihood ratio test (LRT) and the Akaike information criterion (AIC) were used, and models with lower AIC values and significantly different LRT were selected. The model with the best fit was used to conduct pairwise comparison between relevant groups of significant factors using estimated marginal means.
To check the relationship between number of sensilla and labial palp size, a Pearson correlation test was conducted between total number of sensilla and the surface of the labial palp. The area of the 3rd segment of the labial palp was estimated as the area of a cylinder with diameter equal to the maximum width of the segment and length equal to the length of the segment. The comparison of sensilla distribution among the areas of a labial palp was conducted with a “vector GLM” using the package “VGAM”, an ANOVA was used to calculate the significance level of species and sexes.
The effect of diet on survival curves was calculated using the Kaplan–Meier method68, and a log-rank test was used to detect overall differences among curves. Log-rank test and Benjamini–Hochberg correction was used for multiple pairwise comparisons among curves.
Data availability
Raw data and R-script for statistical analysis are available online at https://doi.org/10.34810/data222.
References
Cobb, M., Scott, K. & Pankratz, M. Gustation in Drosophila melanogaster. In Insect Taste (eds Newland, P. et al.) 1–38 (Taylor & Francis, 2009).
Marion-Poll, F. The gustatory system in insects. In The Senses: A Comprehensive Reference (ed. Fritzsch, B.) 148–168 (Elsevier, 2020).
Mitchell, B. K., Itagaki, H. & Rivet, M. P. Peripheral and central structures involved in insect gustation. Microsc. Res. Tech. 47, 401–415 (1999).
Hallberg, E. Fine-structural characteristics of the antennal sensilla of Agrotis segetum (Insecta: Lepidoptera). Cell Tissue Res. 218, 209–218 (1981).
Koh, Y. H., Park, K. C. & Boo, K. S. Antennal sensilla in adult Helicoverpa assulta (Lepidoptera, Noctuidae): Morphology, distribution, and ultrastructure. Ann. Entomol. Soc. Am. 88, 519–530 (1995).
Chapman, R. F. & Ascoli-Christensen, A. Sensory coding in the grasshopper (Orthoptera: Acrididae) gustatory system. Ann. Entomol. Soc. Am. 92, 873–879 (1999).
Ohmura, W., Ozaki, M. & Yamaoka, R. Behavioral and electrophysiological investigation on taste response of the termite Zootermopsis nevadensis to wood extractives. J. Wood Sci. 52, 261–264 (2006).
Seada, M. A. & Hamza, A. M. Differential morphology of the sensory sensilla of antennae, palpi, foretarsi and ovipositor of adult Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Ann. Agric. Sci. 63, 1–8 (2018).
Powell, G., Tosh, C. R. & Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 51, 309–330 (2006).
Montell, C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 19, 345–353 (2009).
Barrozo, R. B., Reisenman, C. E., Guerenstein, P., Lazzari, C. R. & Lorenzo, M. G. An inside look at the sensory biology of triatomines. J. Insect Physiol. 97, 3–19 (2017).
Bestea, L. et al. Peripheral taste detection in honey bees: What do taste receptors respond to?. Eur. J. Neurosci. 54, 4417–4444 (2021).
Krenn, H. W. Feeding mechanisms of adult Lepidoptera: Structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 55, 307–327 (2010).
Guerenstein, P. G. & Hildebrand, J. G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 53, 161–178 (2008).
Song, Y. Q., Sun, H. Z. & Wu, J. X. Ultrastructural characteristics of the proboscis and the labial palp pit organ in the oriental fruit moth, Grapholita molesta. Bull. Insectol. 69, 59–66 (2016).
Hu, P. et al. Sensilla on six olfactory organs of male Eogystia hippophaecolus (Lepidoptera: Cossidae). Microsc. Res. Tech. 81, 1059–1070 (2018).
Guo, M., Chen, Q., Liu, Y., Wang, G. & Han, Z. Chemoreception of mouthparts: Sensilla morphology and discovery of chemosensory genes in proboscis and labial palps of adult Helicoverpa armigera (Lepidoptera: Noctuidae). Front. Physiol. 9, 970 (2018).
Calatayud, P. A. et al. Sexual dimorphism of antennal, tarsal and ovipositor chemosensilla in the African stemborer, Busseola fusca (Fuller) (Lepidoptera: Noctuidae). Ann. Soc. Entomol. Fr. 42, 403–412 (2006).
Jørgensen, K., Almaas, T. J., Marion-Poll, F. & Mustaparta, H. Electrophysiological characterization of responses from gustatory receptor neurons of sensilla chaetica in the moth Heliothis virescens. Chem. Senses 32, 863–879 (2007).
Popescu, A. et al. Function and central projections of gustatory receptor neurons on the antenna of the noctuid moth Spodoptera littoralis. J. Comp. Physiol. A 199, 403–416 (2013).
Roh, H. S., Park, K. C., Oh, H. W. & Park, C. G. Morphology and distribution of antennal sensilla of two tortricid moths, Cydia pomonella and C. succedana (Lepidoptera). Microsc. Res. Tech. 79, 1069–1081 (2016).
Ammagarahalli, B. & Gemeno, C. Response profile of pheromone receptor neurons in male Grapholita molesta (Lepidoptera: Tortricidae). J. Insect Physiol. 71, 128–136 (2014).
Godoy, R. et al. Antennal morphology and localization of a pheromone-binding protein of Lobesia botrana (Denis & Schiffermüller)(Lepidoptera: Tortricidae). Neotrop. Entomol. 48, 422–432 (2019).
Ioriatti, C. et al. Chemical ecology and management of Lobesia botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 104, 1125–1137 (2011).
Knight, A. L., Judd, G. J., Gilligan, T., Fuentes-Contreras, E. & Walker, W. B. Integrated management of tortricid pests of tree fruit. In Integrated Management of Diseases and Insect Pests of Tree Fruit (eds Xu, X. & Fountain, M.) 377–424 (Burleigh Dodds Science Publishing, 2019).
Chen, J. & Hua, B. Sexual dimorphism of adult labial palps of the peach fruit moth Carposina sasakii Matsumura (Lepidoptera: Carposinidae) with notes on their sensilla. Acta Zool. 97, 42–48 (2016).
Vargas-Fernández, I., Castro-Gerardino, D. J. & Llorente-Bousquets, J. E. Labial palps of the butterfly Prestonia clarki (Lepidoptera: Pieridae). Ultrastructure of sensilla and sensory organs. Rev. Biol. Trop. 66, 1324–1346 (2018).
Faucheux, M. J. Mouthparts and associated sensilla of a South American moth, Synempora andesae (Lepidoptera: Neopseustidae). Rev. Soc. Entomol. Argent. 67, 21–33 (2008).
Bogner, F., Boppré, M., Ernst, K. D. & Boeckh, J. CO2 sensitive receptors on labial palps of Rhodogastria moths (Lepidoptera: Arctiidae): Physiology, fine structure and central projection. J. Comp. Physiol. A 158, 741–749 (1986).
Kent, K. S., Harrow, I. D., Quartararo, P. & Hildebrand, J. G. An accessory olfactory pathway in Lepidoptera: The labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res. 245, 237–245 (1986).
Faucheux, M. J. Morphology and distribution of sensilia on the cephalic appendages, tarsi and ovipositor of the European sunflower moth, Homoeosoma nebulella Den. & Schiff. (Lepidoptera: Pyralidae). Int. J. Insect Morphol. Embryol. 20, 291–307 (1991).
Krenn, H. W. & Penz, C. M. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): A search for anatomical adaptations to pollen-feeding behavior. Int. J. Insect Morphol. Embryol. 27, 301–309 (1998).
Zhao, X. C. et al. Fine structure and primary sensory projections of sensilla located in the labial-palp pit organ of Helicoverpa armigera (Insecta). Cell Tissue Res. 353, 399–408 (2013).
Dong, J. et al. Morphology, type and distribution of the labial-palp pit organ and its sensilla in the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). Acta Entomol. Sin. 57, 681–687 (2014).
Barcaba, T. & Krenn, H. W. The mouthparts of adult Indian meal moths, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). Entomol. Austriaca 22, 91–105 (2015).
Li, Y. P., Xiao, D. U., Liu, F. F., Yin, L. I. & Liu, T. X. Ultrastructure of the sensilla on antennae and mouthparts of larval and adult Plutella xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 17, 1409–1420 (2018).
Chen, Q. et al. Fine structure and olfactory reception of the labial palps of Spodoptera frugiperda. Front. Physiol. 12, 880 (2021).
Abd El-Ghany, N. M. & Faucheux, M. J. The mouthparts and sensilla of the adult tomato leafminer moth, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae). Arthropod Struct. & Dev. 67, 101144 (2022).
Städler, E., Schöni, R. & Kozolwski, M. W. Relative air humidity influences the function of the tarsal chemoreceptor cells of the cherry fruit fly (Rhagoletis cerasi). Physiol. Entomol. 12, 339–346 (1987).
Canney, P. J. & Gardener, D. R. Effects of artificial and natural diets on success in tip recording and on galeal chemosensillum morphology of European corn borer larvae. Physiol. Entomol. 14, 13–19 (1989).
Savopoulou-Soultani, M., Milonas, P. G. & Stavridis, D. G. Role of availability of food to the adult Lobesia botrana (Lepidoptera: Tortricidae) in its reproductive performance. J. Econ. Entomol. 91, 1341–1348 (1998).
Atanassov, A. & Shearer, P. W. Peach extrafloral nectar impacts life span and reproduction of adult Grapholita molesta (Busck) (Lepidoptera: Tortricidae). J. Agric. Urban Entomol. 22, 41–47 (2005).
Wenninger, E. J. & Landolt, P. J. Apple and sugar feeding in adult codling moths, Cydia pomonella: Effects on longevity, fecundity, and egg fertility. J. Insect Sci. 11, 161 (2011).
Mátray, S. & Herz, A. Do floral resources affect fitness of adult Cydia pomonella (Linnaeus 1758) (Lepidoptera: Tortricidae)?. Bull. Entomol. Res. 111, 726–732 (2021).
Caldwell, D. L. & Gerhardtt, K. O. Chemical analysis of peach extrafloral nectary exudate. Phytochemistry 25, 411–413 (1986).
Gabel, B. Tansy flowers attract European grapevine moth females, Lobesia botrana Den. & Schiff. (Lep., Tortricidae). J. Appl. Entomol. 113, 153–158 (1992).
Lombarkia, N. & Derridj, S. Incidence of apple fruit and leaf surface metabolites on Cydia pomonella oviposition. Entomol. Exp. Appl. 104, 79–87 (2002).
Maher, N., Thiery, D. & Städler, E. Oviposition by Lobesia botrana is stimulated by sugars detected by contact chemoreceptors. Physiol. Entomol. 31, 14–22 (2006).
Savopoulou-Soultani, M., Stavridis, D. G., Vassiliou, A., Stafilidis, J. E. & Iraklidis, I. Response of Lobesia botrana (Lepidoptera: Tortricidae) to levels of sugar and protein in artificial diets. J. Econ. Entomol. 87, 84–90 (1994).
Pszczolkowski, M. A. & Brown, J. J. Effect of sugars and non-nutritive sugar substitutes on consumption of apple leaves by codling moth neonates. Phytoparasitica 31, 283–291 (2003).
Su, S. et al. Life-history traits and flight capacity of Grapholita molesta (Lepidoptera: Tortricidae) using artificial diets with varying sugar content. J. Econ. Entomol. 114, 112–121 (2021).
Van Zandt, P. A. et al. Which moths might be pollinators? Approaches in the search for the flower-visiting needles in the Lepidopteran haystack. Ecol Entomol. 45, 13–25 (2020).
Kaushik, S., Kumar, R. & Kain, P. Salt an essential nutrient: Advances in understanding salt taste detection using Drosophila as a model system. J. Exp. Neurosci. 12, 1–12 (2018).
Grant, G. G. Copulatory behavior of spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae): Experimental analysis of the role of the sex pheromone and associated stimuli. Ann. Entomol. Soc. Am. 80, 78–88 (1987).
Baker, T. C. & Cardé, R. T. Courtship behavior of the oriental fruit moth (Grapholitha molesta): Experimental analysis and consideration of the role of sexual selection in the evolution of courtship pheromones in the Lepidoptera. Ann. Entomol. Soc. Am. 72, 173–188 (1979).
Ferveur, J. F. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35, 279 (2005).
Gemeno, C. et al. Cuticular hydrocarbons discriminate cryptic Macrolophus species (Hemiptera: Miridae). Bull. Entomol. Res. 102, 624–631 (2012).
Myers, J. H., Monro, J. & Murray, N. Egg clumping, host plant selection and population regulation in Cactoblastis cactorum (Lepidoptera). Oecologia 51, 7–13 (1981).
Varela, N. et al. Three-dimensional antennal lobe atlas of the oriental fruit moth, Cydia molesta (Busck) (Lepidoptera: Tortricidae): Comparison of male and female glomerular organization. Cell Tissue Res. 337, 513–526 (2009).
Pramod, K. C. et al. Revisiting the labial pit organ pathway in the noctuid moth, Helicoverpa armigera. Front. Physiol. 11, 202 (2020).
Ryan, M. F. The chemoreceptive organs: Structural aspects. In Insect Chemoreception: Fundamental and Applied (ed. Ryan, M. F.) 113–139 (Kluwer Academic Publishers, 2002).
Ivaldi-Sender, C. Techniques simples pour un elevage permanent de la Tordeuse orientale, Grapholita molesta (Lepidoptera, Tortricidae) sur milieu artificiel. Ann. Zool. Ecol. Anim. 6, 337–343 (1974).
George, J. A. & Nagy, B. A. Morphology, distribution, and ultrastructural differences of sensilla trichodea and basiconica on the antennae of the oriental fruit moth, Grapholitha molesta (Busck) (Lepidoptera: Tortricidae). Int. J. Insect Morphol. Embryol. 13, 157–170 (1984).
Hodgson, E. S., Lettvin, J. Y. & Roeder, K. D. Physiology of a primary chemoreceptor unit. Science 122, 417–418 (1955).
Marion-Poll, F. & Pers, J. V. D. Un-filtered recordings from insect taste sensilla. Entomol. Exp. Appl. 80, 113–115 (1996).
Marion-Poll, F. Display and analysis of electrophysiological data under windowsTM. Entomol. Exp. Appl. 80, 116–119 (1996).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2022). https://www.R-project.org/.
Kaplan, E. L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481 (1958).
Acknowledgements
CA was supported by a Ph.D. fellowship BES-2017-081131 from the Ministerio de Economía, Industria y Competitividad (MINECO, Spain) joint financing by the European Social Fund. This study was supported by research grants AGL2016-77373-C2-2-R MINECO and PID2019-107030RB-C22 MICINN. We thank Carla Gomez for the labial palp 3D models.
Author information
Authors and Affiliations
Contributions
C.A. performed the electrophysiology and statistical analyses. M.A.N.-R. performed the survival experiment. C.A. and C.G. wrote the initial manuscript and all the authors contributed in the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amat, C., Marion-Poll, F., Navarro-Roldán, M.A. et al. Gustatory function of sensilla chaetica on the labial palps and antennae of three tortricid moths (Lepidoptera: Tortricidae). Sci Rep 12, 18882 (2022). https://doi.org/10.1038/s41598-022-21825-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21825-w
- Springer Nature Limited
This article is cited by
-
Ovipositional responses of tortricid moths to sugars, salts and neem oil
Scientific Reports (2024)