Abstract
The simian parasite Plasmodium knowlesi is the predominant species causing human malaria infection, including hospitalisations for severe disease and death, in Malaysian Borneo. By contrast, there have been only a few case reports of knowlesi malaria from Indonesian Borneo. This situation seems paradoxical since both regions share the same natural macaque hosts and Anopheles mosquito vectors, and therefore have a similar epidemiologically estimated risk of infection. To determine whether there is a true cross-border disparity in P. knowlesi prevalence, we conducted a community-based malaria screening study using PCR in Kapuas Hulu District, West Kalimantan. Blood samples were taken between April and September 2019 from 1000 people aged 6 months to 85 years attending health care facilities at 27 study sites within or close to jungle areas. There were 16 Plasmodium positive samples by PCR, five human malarias (two Plasmodium vivax, two Plasmodium ovale and one Plasmodium malariae) and 11 in which no species could be definitively identified. These data suggest that, if present, simian malarias including P. knowlesi are rare in the Kapuas Hulu District of West Kalimantan, Indonesian Borneo compared to geographically adjacent areas of Malaysian Borneo. The reason for this discrepancy, if confirmed in other epidemiologically similar regions of Indonesian Borneo, warrants further studies targeting possible cross-border differences in human activities in forested areas, together with more detailed surveys to complement the limited data relating to monkey hosts and Anopheles mosquito vectors in Indonesian Borneo.
Similar content being viewed by others
Introduction
One of the aims of the Global Technical Strategy for malaria 2016–2030 developed by the World Health Organisation (WHO) was for a reduction in malaria case incidence and mortality rate of at least 40% between 2015 and 20201. Malaysia is one of the countries to have achieved this goal, with zero reported indigenous non-zoonotic malaria cases since 2018. However, zoonotic malaria due to Plasmodium knowlesi increased from 1600 cases to > 4000 between 2016 and 2018 before declining to 3213 and 2609 cases in 2019 and 2020, respectively1. Malaysia is the nation with the highest reported incidence of human P. knowlesi infections in South-east Asia2,3 and, in the Malaysian Borneo states of Sabah and Sarawak, the majority of malaria cases and most malaria-associated hospitalisations are caused by P. knowlesi4,5,6. The presence of this parasite in jungle areas harbouring its natural macaque monkey hosts and Anopheles mosquito vectors is a clear impediment to achieving malaria eradication once human malarias have been eliminated7,8.
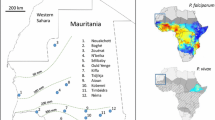
Indonesia is another South-east Asian country that has come a long way in controlling malaria. In the late 1940s, malaria was identified as a major public health problem with substantial implications for economic activity9, and 70 years later there were still large estimated numbers of clinical cases each year (1.3 million Plasmodium falciparum and 1.5 million Plasmodium vivax in 2010)10,11. However, enhanced control measures had reduced the burden of malaria to an estimated 0.3 million clinical cases of P. falciparum and 0.4 million clinical cases of P. vivax by 2021.12 The Indonesian government aims to eliminate malaria in stages by 2030, from the most to the least developed islands13. As in the example of Malaysia, transmission of P. knowlesi is likely to impede eradication efforts in Indonesia. Indeed, three Indonesian provinces (North, East and West Kalimantan) share a border with the Malaysian states of Sabah and Sarawak (see Fig. 1), and the natural hosts (Macaca fascicularis and M. nemestrina)14 and mosquito vectors (including Anopheles leucosphyrus)14,15 of P. knowlesi occur on both sides of the border. The predicted risk of knowlesi malaria is, therefore, similar for both Malaysian and Indonesian Borneo16.
Although there is a significant burden of knowlesi malaria in both Sabah and Sarawak, including severe and fatal cases6,17,18,19,20, data relating to the prevalence and clinical sequelae of this infection in humans in Indonesian Borneo are sparse. Only 12 confirmed cases have been reported to date21,22,23,24,25 and all of these are from South and Central Kalimantan rather than the provinces of West and North Kalimantan bordering Malaysian Borneo. Most data relating to P. knowlesi in Indonesia come from the approximately 400 cases found in Sumatra26 and Aceh27 provinces. The reasons for the disparity in the burden of P. knowlesi between Indonesian and Malaysian Borneo is unclear. Misdiagnosis may be contributory since the microscopic appearances of P. knowlesi can be interpreted as those of Plasmodium malariae or P. falciparum, and the more accurate but expensive nested PCR-based testing is rarely done outside research settings in Indonesia19,28,29. There could be greater awareness of knowlesi malaria as a diagnostic possibility in Malaysian Borneo than in Kalimantan given that it was first discovered as a significant public health problem around 20 years ago in the Kapit Division of Sarawak30. However, there may also be differences between Indonesian and Malaysian Borneo in demographic and environmental conditions impacting transmission.
To investigate the cross-border disparity in P. knowlesi prevalence, we conducted a community-based screening study using PCR in Kapuas Hulu District, West Kalimantan, the closest Indonesian district to the Kapit Division of Sarawak. We hypothesized that there would be previously unrecognised cases of P. knowlesi and perhaps cases of other simian malarias among people attending health care facilities and living and working in areas of the district in close proximity to forested areas.
Results
A total of 1000 individuals were recruited from 27 sites in Kapuas Hulu District of West Kalimantan and, of these, 431 (43.1%) were from government health clinics (GHCs), 143 (14.3%) from palm oil plantation health clinics, 102 (10.2%) from satellite health clinics, 65 (6.5%) from villages, and 53 (5.3%) from longhouses (see Fig. 2 for locations and Supplementary Table 1 for full details of the individual sites selected and case distribution). The characteristics of the sample are summarised in Table 1. Participants ranged from 6 months to 85 years of age but the majority were adults of working age. The proportions of males and females were similar. Approximately one third were plantation workers and farmers from relatively high-risk malarious areas in the district. A third had fever or a recent history of fever. Compared to those who were afebrile, this sub-group was younger with proportionately more children and males. Although they were also less likely to be plantation workers and farmers, this may have reflected the larger numbers of people attending peripheral health care facilities for general health checks rather than because of symptoms.
There were four participants diagnosed with malaria at the recruitment site but with negative PCR (see Table 2), two with a positive RDT (one P. falciparum and one mixed P. falciparum/P. vivax) and the other two with a positive blood slide (one P. falciparum and one P. vivax). All were adults who presented with fever. Five cases, four of whom presented with fever and three of whom had an RDT which was negative, were positive by PCR and the infecting species was identified (two cases of P. vivax and two of P. ovale, and one case of P. malariae). Two of these cases were children and one an infant aged 6 months—all of these cases were febrile when recruited.
There were 11 cases, six of whom had fever, who were Plasmodium-positive by PCR but the species could not be identified by PCR assays with primers specific for P. falciparum. P. vivax, P. malariae, P. ovale, P. knowlesi, P. cynomolgi, P. inui, P. fieldi and P. coatneyi. We further characterized these 11 samples by amplifying the partial small subunit ribosomal RNA (SSU rRNA) genes and generating 233–234 base pair (bp) and 272–294 bp amplicons with two different pairs of Nest-2 PCR primers for direct sequencing. Analysis of the DNA sequences, after the PCR primer sequences were removed, was based on the Basic Local Alignment Search Tool (BLAST) from the National Center for Biotechnology Information (NCBI) database revealed the presence of Plasmodium species, with high sequence similarity to human and simian Plasmodium species (P. knowlesi, P. inui, P. fieldi and P. coatneyi) for seven of the 11 cases (see Table 3). However, the identity of the infecting Plasmodium species could not be inferred through phylogenetic analyses since the DNA sequences generated were relatively short, resulting in phylogenetic trees with low bootstrap values (see Supplementary Fig. 1). None of the 11 cases were diagnosed and treated for malaria at the time of recruitment since RDTs and microscopy were not done or were negative. Of the 16 PCR positive cases, there were four who presented at the Batang Lupar Health Clinic in the north of the survey area and another 11 at sites around Putussibau Hospital in the east, both areas close to the border with Sarawak, with only a single case in the west at the Belian Estate Company Clinic (see Fig. 3).
Discussion
The present data collected over a six-month period show that, in contrast to the epidemiological situation in neighbouring Kapit Division of Sarawak, Malaysian Borneo4,5,6, there were very few cases of malaria in Kapuas Hulu District, West Kalimantan, identified through screening people resident and/or working in theoretically high-risk areas, a third of whom had fever. In addition, there was no definitive evidence of P. knowlesi infection or other simian malarias in our samples. We found PCR evidence of human malarias except P. falciparum but, in most of the PCR-positive participants, the species of Plasmodium could not be accurately identified. This could indicate that there is low level parasitaemia of species such as P. knowlesi in Kapuas Hulu District, but affecting only up to approximately 1% of a population living in relatively close proximity to the natural hosts and mosquito vectors of P. knowlesi16.
One possible difference between Indonesian and Malaysian Borneo in the risk of transmission of P. knowlesi is in ease of access to forested areas. On the Indonesian side, there are few roads penetrating densely vegetated areas compared to the more extensive road network on the Malaysian side (see Fig. 4). This may mean that forest-related occupations such as logging, mining and farming associated with potentially greater vector exposure are more common in Sarawak and Sabah than West Kalimantan. There may also be other ecological and demographic factors at play such as cross-border differences in deforestation, habitat fragmentation, agricultural expansion, socioeconomic changes and wildlife reservoirs that can influence P. knowlesi transmission31. It is not possible to determine whether the lack of zoonotic infections in the Kapuas Hulu District is due to smaller populations of the two macaque hosts of zoonotic malaria parasites compared with the geographically adjacent Kapit Division of Sarawak, Malaysian Borneo, since no data are available on macaque populations in these two regions, but the mosquito vectors are present on both sides of the border29. Greater economic development in Malaysian compared with Indonesian Borneo32,33 could result in an increased risk of zoonoses34. Some relevant Malaysian studies have been conducted35,36,37, including one in the state of Sabah in Malaysian Borneo examining potential associations between environmental variables obtained from satellite-based remote-sensing data and P. knowlesi incidence which showed that deforestation in areas surrounding villages was positively associated with numbers of P. knowlesi cases at a village level38. There is a relative paucity of such data from Kalimantan.
Our failure to identify the species of Plasmodium in a number of participants who were positive for Plasmodium by PCR has been observed in other surveys of malaria parasites in humans, non-human primates and mosquitoes39,40,41. One explanation for this is that relatively small volumes of blood with very low density infections may yield sufficient DNA for the Plasmodium-specific PCR assay targeting both the A- and S-types of the SSU rRNA genes, but not for the species-specific primers directed at only one type of gene41. Another possible reason is that these individuals were infected with a Plasmodium species other than the eight targeted by the species-specific primers, such as P. inui-like or P. simiovale which have recently been found to cause zoonotic infections42,43. Although BLAST searches were suggestive, phylogenetic analyses of the sequencing data were not definitive for species confirmation. It is, therefore, possible that a proportion of these individuals had sub-microscopic P. knowlesi infections that were asymptomatic or not the cause of fever, but the corollary to this is that there should be other people in the same areas with clinically evident knowlesi malaria requiring treatment in local healthcare facilities27,39 with some progressing to severe disease6,17,18,19,20. Even if P. knowlesi cases were misidentified by microscopy as one of the human malarias19,28,29, historical data showing low number of total malaria cases with relatively few hospitalisations in Kapuas Hulu District12 would suggest that the burden of knowlesi malaria in the community, if present at all, is small. By contrast, in the neighbouring region of Kapit Division in Sarawak, Malaysian Borneo, 83 knowlesi malaria patients were admitted to Kapit Hospital during the period during which the present survey was conducted, with 1246 and 973 annual cases of knowlesi malaria reported in 2018 and 2019, respectively, for the state of Sarawak, Malaysian Borneo (unpublished data, Sarawak State Health Department).
There were four cases of malaria (0.4%) diagnosed by microscopy or RDT who were negative by subsequent PCR, and three of the participants (0.3%) were PCR positive with a species identified but who were negative by RDT. False positive and negative microscopy results are well recognised in areas of low and unstable transmission such as Kapuas Hulu District44, and can occur even in the context of clinical trials45. False positive RDTs can result from persistence of antigen well after successful treatment46 or rarely in patients with cross-reacting antibodies47, while false negatives are usually due to parasitaemias below the limit of detection of the test48. The overall numbers of such cases in the present study were, therefore, consistent with other studies.
In conclusion, the results of the present survey are consistent with the very limited number of cases of knowlesi malaria reported to date from Indonesian Borneo21,22,23,24,25 which do not include any from the Kapuas Hulu District of West Kalimantan where the survey was conducted. Although we cannot rule out low-level and largely asymptomatic simian malarias including that due to P. knowlesi, evidence from Malaysian Borneo39,40 and other parts of Indonesia27 suggests that this would be expected to parallel symptomatic cases requiring treatment which are not observed in Kapuas Hulu District. There are likely to be significant differences in sociodemographic, economic and environmental factors that underlie the substantial disparity between the burden of knowlesi malaria either side of the Malaysian-Indonesian border on the island of Borneo. Nevertheless, there is a need to survey other areas along the Kalimantan-Sarawak and Kalimantan-Sabah border to determine whether the low prevalence of malaria, including simian Plasmodium infections, is confirmed.
Methods
Study design, site and approvals
The present study was a cross-sectional survey of malaria prevalence including speciation in people from Kapuas Hulu District in West Kalimantan Province. This district has a total area of 29,842 km2 and a population of 253,740 in the latest census by Indonesian Bureau of Statistics49. It was selected as the study site based on its close proximity to the Kapit division in Sarawak, Malaysia30 with which it shares its northern border (see Fig. 1). Kapuas Hulu has stable low malaria transmission with an Annual Parasite Incidence (API; number of positive cases per 1000 individuals in a year) of 0.1312. It has tropical climate with an average temperature of 26.7 °C and average annual precipitation of 4231 mm. The district comprises mainly forested and plantation areas. The population lives in towns, in villages by rivers or lakes, and in housing inside plantations.
Two district hospitals and 23 sub-district GHCs serve the district. For the purposes of the present study, screening was performed at both district hospitals (Achmad Diponegoro and Badau) and at seven of the GHCs (Putussibau Utara, Putussibau Selatan, Lanjak, Badau, Suhaid, Seberuang and Empanang; see Fig. 2). The sub-district GHCs in the present survey were selected based on ease of access and relatively high malaria prevalence from the previous year’s numbers reported to the Kapuas Hulu Health Department. To capture potentially at-risk individuals in remote areas who may not have been able to access available health care facilities, community-based screening was also performed in six palm oil plantation health clinics, five satellite health clinics (Pos Binaan Terpadu, Posbindu), five villages and two longhouses (see Fig. 2). The study was approved by Ethical Committee for Health Research of Diponegoro University, Indonesia (Ref. No. 608/EC/FK-UNDIP/X/2018) and Human Ethics of The University of Western Australia, Australia (Ref. RA/4/20/4879). The study was performed in accordance with relevant guidelines and regulations. Permits for screening were obtained from palm oil plantation companies and outreach services provided by health clinics.
Sample size
Sample size calculations were based on the assumption that screening in hospital clinics, GHCs and more remote sites would increase the likelihood of identifying P. knowlesi in both symptomatic and asymptomatic individuals compared to that in the general Kapuas Hulu population. In a study from Sabah, a study of an enriched sample of household and community members of clinical cases showed a prevalence of 1.7% by PCR using the P. knowlesi-specific primers39. A sample size of ≥ 1000 would be sufficient to identify a prevalence of 1.0% greater than this with 1% precision and 95% confidence, with fewer numbers of participants required if the true prevalence were less50, as has been shown in some previous community-based surveys in Sabah51 and Sarawak40.
Sample collection
Screening was conducted between April and September 2019. Between April and June, staff at the government health facilities were requested to take a dried blood spot (DBS) on filter paper, in addition to malaria blood film and/or or Rapid Diagnostic Test (RDT), from all patients presenting with fever. Because the numbers of participants recruited in this way was low, and given emerging evidence that asymptomatic cases of knowlesi malaria would be missed40,51, screening between July and September 2019 was extended to all people utilizing outpatient services at the various health facilities provided that they (or their parent/guardian in the case of children) gave witnessed informed consent to sampling and had been resident in the area for at least 6 months. In addition to a DBS for PCR, basic demographic details and a history of symptoms were taken, and testing for malaria by microscopy (single read as negative or positive with speciation but without quantification or semi-quantitative ‘plus’ system grading of parasite density) and/or RDT (CareStart™, Access Bio, Somerset, NJ, USA) was performed depending on the capacity of the local facilities. The same requirements and procedures were followed for community screening in which sample collection took place at the same time as outreach services to villages and longhouses by government health clinics. The outreach service was a routine monthly activity by nurses or health workers for villages with limited access to health care facilities. The individual sites and numbers of samples taken are summarised in Table 1.
Malaria DBS samples were collected from individuals with or without fever who had been resident in the area for a minimum of six months. A total of 1000 blood samples were obtained by finger prick using a spring-loaded lancet which were spotted onto filter papers (Whatman® Schleicher and Schuell® qualitative filter paper) for subsequent molecular analysis. Three separate blood spots per filter paper were collected from the same sampling site for each participant. The spotted filter paper was air dried and stored in an individual sealed plastic bag at room temperature until DNA extraction in the laboratory. Participants diagnosed with malaria by microscopy or RDT were treated according to national guidelines.
DNA extraction and detection of Plasmodium DNA
DNA was extracted from DBS by using Instagene® Matrix (BioRad Laboratories, https://www.bio-rad.com) as described previously52. Nested PCR assays based on the Plasmodium SSU rRNA genes were used to screen DNA extracted from DBS53. Screening for presence of malaria parasites using Plasmodium-genus specific primers for the first amplification Nest-1 (rPLU1 and 5) and Nest-2 amplification (rPLU3 and 4) was first performed on all samples to generate an approximately 1.6 kb amplicon and a 240 base pair amplicon, respectively. For Nest-1, each sample was tested using reaction mixture of 50 μL consisting 0.25 μM of each primer (rPLU1 and rPLU5), PCR buffer (50 mM KCl, 10 mM Tris–HCL), 3 mM MgCl2, 200 mM each deoxynucleotide triphosphate, 1.25 U of Taq DNA polymerase (Promega, USA, https://www.promega.com) and 15 μL of DNA template. PCR amplification was performed using a ProFlex Thermal Cycler (Thermo Fisher, https://www.thermofisher.com) under the following conditions: 94 °C for 4 min, 35 cycles of 94 °C for 30 s, 55 °C for 1 min and 72 °C for 1 min, followed by 72 °C for 4 min. Two microlitres of the Nest-1 products were used as DNA template in the 20μL Nest-2 Plasmodium genus-specific assays under the following conditions: 94 °C for 4 min, 40 cycles of 94 °C for 30 s, 62 °C for 1 min and 72 °C for 30 s, followed by 72 °C for 4 min. Nest-2 PCR products were analysed by gel electrophoresis and SYBR® Safe (Invitrogen, USA) gel staining. For the Plasmodium-positive samples, the Nest-1 amplification products were reused for species-specific Nest-2 amplifications. All genus positive samples were analysed at least twice by two independent laboratory staff. Positive species-specific samples were confirmed by retesting and samples with inconsistent results were re-extracted before being re-analysed.
Molecular detection of human and simian Plasmodium species
Plasmodium-positive samples were further tested for the identification of human and simian Plasmodium species using the species-specific PCR primers for P. falciparum, P. vivax, P. malariae, P. ovale, P. knowlesi, P. inui, P. cynomolgi, P. fieldi, and P. coatneyi in Nest 2 PCR assays as described previously54. The primers were rFAL1 and rFAL2 for P. falciparum, rVIV6 and rVIV7 for P. vivax, rMAL1 and rMAL2 for P. malariae, rOVA1 and rOVA4 for P. ovale, Kn1f. and Kn3r for P. knowlesi, PinF2 and INAR3 for P. inui, CY2F and CY4R for P. cynomolgi, PctF1 and PctR1 for P. coatneyi and PfldF1 and PfldR2 for P. fieldi54. Nest-2 PCR amplification was carried out in a 20μL reaction mixture containing identical amount of species-specific primers and other constituents apart from the addition of 2 mM MgCl2 and 0.5 U Taq DNA polymerase. Nest-2 PCR amplification conditions were akin to the of Nest-2 Plasmodium-genus PCR assay, except for the annealing temperature of 58 °C for three human Plasmodium species-specific primer pairs (P. falciparum, P. ovale and P. malariae)55, 65 °C for P. vivax, 62 °C for P. knowlesi and P.coatneyi, 60 °C for P. cynomolgi and P. inui, and 66 °C for P. fieldi. Similarly, the species-specific Nest-2 products were subjected to gel electrophoresis and were stained using SYBR Safe™. Precautions to prevent cross-contamination in nested PCR assays were taken as described previously30.
DNA sequencing and analyses
PCR amplification of partial SSU rRNA genes was conducted using the Plasmodium-specific primers rPLU3 and rPLU4 as described above. In addition, we also amplified the partial SSU rRNA gene of approximately 252 bp long by independent hemi-nested PCR assays using primers PlasmoM_N1F and PlasmoM_N1R in the first PCR amplification, followed by primers PlasmoM_N2F and PlasmoM_N1R in the second PCR amplification as described previously56. Both amplified products were sent to Apical Scientific Sdn. Bhd. (Selangor, Malaysia) for direct DNA sequencing. The DNA sequences generated were confirmed for Plasmodium DNA sequence using BLAST from the NCBI website. DNA sequences were then aligned using the MegAlign software (DNAStar Lasergene, Madison, USA) together with other reference sequences of primate Plasmodium species (see Supplementary Fig. 1), and the Neighbour-Joining trees were generated using the MEGA X software57 with a bootstrap of 1000 replications.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The DNA datasets generated and/or analysed are available in the GenBank repository (Accession numbers OP020384 to OP020394).
References
World Health Organisation. World Malaria Report 2021 (WHO, Geneva, 2021).
Zaw, M. T. & Lin, Z. Human Plasmodium knowlesi infections in South-East Asian countries. J. Microbiol. Immunol. Infect. 52, 679–684. https://doi.org/10.1016/j.jmii.2019.05.012 (2019).
Karunajeewa, H. & Berman, J. Is the epidemiology of Plasmodium knowlesi changing, and what does this mean for malaria control in Southeast Asia?. Clin Infect Dis 70, 368–369. https://doi.org/10.1093/cid/ciz238 (2019).
Hussin, N. et al. Updates on malaria incidence and profile in Malaysia from 2013 to 2017. Malar. J. 19, 55. https://doi.org/10.1186/s12936-020-3135-x (2020).
Lai, M. Y. et al. High incidence of Plasmodium knowlesi malaria compared to other human malaria species in several hospitals in Malaysia. Trop. Biomed. 38, 248–253. https://doi.org/10.47665/tb.38.3.065 (2021).
William, T. et al. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg. Infect. Dis 17, 1248–1255. https://doi.org/10.3201/eid1707.101017 (2011).
Ng, O. T. et al. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg. Infect. Dis. 14, 814–816. https://doi.org/10.3201/eid1405.070863 (2008).
Cooper, D. J. et al. Plasmodium knowlesi malaria in Sabah, Malaysia, 2015–2017: Ongoing increase in incidence despite near-elimination of the human-only Plasmodium species. Clin Infect Dis 70, 361–367. https://doi.org/10.1093/cid/ciz237 (2020).
Soeparmo, H. & Stoker, W. Malaria control in Indonesia. Madj Kes Ind 2, 253–261 (1952).
Elyazar, I. R. et al. Plasmodium falciparum malaria endemicity in Indonesia in 2010. PLoS ONE 6, e21315. https://doi.org/10.1371/journal.pone.0021315 (2011).
Elyazar, I. R. et al. Plasmodium vivax malaria endemicity in Indonesia in 2010. PLoS ONE 7, e37325. https://doi.org/10.1371/journal.pone.0037325 (2012).
Malaria, S. Direktorat pencegahan dan pengendalian penyakit tular vektor dan zoonotik kementerian kesehatan Republik of Indonesia (Kementrian Kesehatan Republik of Indonesia, Jakarta, 2022).
Kementrian Kesehatan Republic of Indonesia. Profil kesehatan Indonesia tahun 2017. Indonesian Ministry of Health, Jakarta (2017).
Moyes, C. L. et al. Predicting the geographical distributions of the macaque hosts and mosquito vectors of Plasmodium knowlesi malaria in forested and non-forested areas. Parasit. Vectors 9, 242. https://doi.org/10.1186/s13071-016-1527-0 (2016).
Elyazar, I. R. et al. The distribution and bionomics of Anopheles malaria vector mosquitoes in Indonesia. Adv. Parasitol. 83, 173–266. https://doi.org/10.1016/b978-0-12-407705-8.00003-3 (2013).
Shearer, F. M. et al. Estimating geographical variation in the risk of zoonotic Plasmodium knowlesi infection in countries eliminating malaria. PLoS Negl Trop Dis 10, e0004915. https://doi.org/10.1371/journal.pntd.0004915 (2016).
Chin, A. Z. et al. Risk factor of Plasmodium knowlesi infection in Sabah Borneo Malaysia, 2020: A population-based case-control study. PLoS ONE 16, e0257104. https://doi.org/10.1371/journal.pone.0257104 (2021).
Cox-Singh, J. et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46, 165–171. https://doi.org/10.1086/524888 (2008).
Cox-Singh, J. & Singh, B. Knowlesi malaria: Newly emergent and of public health importance?. Trends Parasitol. 24, 406–410. https://doi.org/10.1016/j.pt.2008.06.001 (2008).
Rajahram, G. S. et al. Deaths from Plasmodium knowlesi malaria: Case series and systematic review. Clin. Infect. Dis. 69, 1703–1711. https://doi.org/10.1093/cid/ciz011 (2019).
Berens-Riha, N., Sulistianingsih, E., Fleischmann, E. & Loescher, T. Plasmodium knowlesi found in several samples from Indonesia. https://promedmail.org/promed-post/?id=20090621.2278 (2009). Accessed 18 July 2022.
Figtree, M. R. L. et al. Plasmodium knowlesi in Human, Indonesian Borneo. Emerg. Infect. Dis. 16(4), 672–674 (2010).
Ompusunggu, S. Malaria hutan di Provinsi Kalimantan Tengah dan Kalimantan Selatan, Indonesia tahun 2013. Indones. J. Health Ecol. 14, 145–156 (2015).
Ompusunggu, S. et al. Penemuan baru Plasmodium knowlesi pada manusia di Kalimantan Tengah Indonesian. Bullet. Health Res. 43, 63–76 (2015).
Setiadi, W. et al. A zoonotic human infection with simian malaria, Plasmodium knowlesi, in Central Kalimantan, Indonesia. Malar. J. 15, 218. https://doi.org/10.1186/s12936-016-1272-z (2016).
Lubis, I. N. D. et al. Contribution of Plasmodium knowlesi to multispecies human malaria infections in North Sumatera, Indonesia. J. Infect. Dis. 215, 1148–1155. https://doi.org/10.1093/infdis/jix091 (2017).
Herdiana, H. et al. Malaria risk factor assessment using active and passive surveillance data from Aceh Besar, Indonesia, a low endemic, malaria elimination setting with Plasmodium knowlesi, Plasmodium vivax, and Plasmodium falciparum. Malar. J. 15, 468. https://doi.org/10.1186/s12936-016-1523-z (2016).
Barber, B. E., William, T., Grigg, M. J., Yeo, T. W. & Anstey, N. M. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 12, 8. https://doi.org/10.1186/1475-2875-12-8 (2013).
Moyes, C. L. et al. Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Negl. Trop. Dis. 8, e2780. https://doi.org/10.1371/journal.pntd.0002780 (2014).
Singh, B. et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363, 1017–1024. https://doi.org/10.1016/s0140-6736(04)15836-4 (2004).
Cuenca, P. R. et al. Epidemiology of the zoonotic malaria Plasmodium knowlesi in changing landscapes. Adv. Parasitol. 113, 225–286. https://doi.org/10.1016/bs.apar.2021.08.006 (2021).
Gaveau, D. L. et al. Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in Borneo. Sci. Rep. 6, 32017. https://doi.org/10.1038/srep32017 (2016).
Woo, W. T. & Hong, C. Indonesia’s economic performance in comparative perspective and a new policy framework for 2049. Bullet. Indones. Econ. Stud. 46, 33–64 (2010).
Gibb, R. et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402. https://doi.org/10.1038/s41586-020-2562-8 (2020).
Brock, P. M. et al. Predictive analysis across spatial scales links zoonotic malaria to deforestation. Proc. Biol. Sci. 286, 20182351. https://doi.org/10.1098/rspb.2018.2351 (2019).
Brock, P. M. et al. Plasmodium knowlesi transmission: Integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology 143, 389–400. https://doi.org/10.1017/s0031182015001821 (2016).
Davidson, G., Chua, T. H., Cook, A., Speldewinde, P. & Weinstein, P. The role of ecological linkage mechanisms in Plasmodium knowlesi transmission and spread. EcoHealth 16, 594–610. https://doi.org/10.1007/s10393-019-01395-6 (2019).
Fornace, K. M. et al. Association between landscape factors and spatial patterns of Plasmodium knowlesi infections in Sabah, Malaysia. Emerg. Infect. Dis. 22, 201–208. https://doi.org/10.3201/eid2202.150656 (2016).
Fornace, K. M. et al. Asymptomatic and submicroscopic carriage of Plasmodium knowlesi malaria in household and community members of clinical cases in Sabah, Malaysia. J. Infect. Dis. 213, 784–787. https://doi.org/10.1093/infdis/jiv475 (2016).
Siner, A. et al. Absence of Plasmodium inui and Plasmodium cynomolgi, but detection of Plasmodium knowlesi and Plasmodium vivax infections in asymptomatic humans in the Betong division of Sarawak, Malaysian Borneo. Malar. J 16, 417. https://doi.org/10.1186/s12936-017-2064-9 (2017).
Nada-Raja, T. et al. Macaca fascicularis and Macaca nemestrina infected with zoonotic malaria parasites are widely distributed in Sarawak, Malaysian Borneo. Sci. Rep. 12, 10476. https://doi.org/10.1038/s41598-022-14560-9 (2022).
Yap, N. J. et al. Natural human infections with Plasmodium cynomolgi, P. inui, and 4 other simian malaria parasites, Malaysia. Emerg. Infect. Dis. 27, 2187–2191. https://doi.org/10.3201/eid2708.204502 (2021).
Liew, J. W. K. et al. Natural Plasmodium inui Infections in humans and Anopheles cracens mosquito, Malaysia. Emerg. Infect. Dis. 27, 2700–2703. https://doi.org/10.3201/eid2710.210412 (2021).
Golassa, L. et al. Microscopic and molecular evidence of the presence of asymptomatic Plasmodium falciparum and Plasmodium vivax infections in an area with low, seasonal and unstable malaria transmission in Ethiopia. BMC Infect. Dis. 15, 310. https://doi.org/10.1186/s12879-015-1070-1 (2015).
Ohrt, C., Purnomo, Sutamihardja, M. A., Tang, D. & Kain, K. C. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J. Infect. Dis. 186, 540–546. https://doi.org/10.1086/341938 (2002).
Tjitra, E., Suprianto, S., Dyer, M. E., Currie, B. J. & Anstey, N. M. Detection of histidine rich protein 2 and panmalarial ICT Malaria Pf/Pv test antigens after chloroquine treatment of uncomplicated falciparum malaria does not reliably predict treatment outcome in eastern Indonesia. Am. J. Trop. Med. Hyg. 65, 593–598. https://doi.org/10.4269/ajtmh.2001.65.593 (2001).
Gatton, M. L. et al. An assessment of false positive rates for malaria rapid diagnostic tests caused by non-Plasmodium infectious agents and immunological factors. PLoS ONE 13, e0197395. https://doi.org/10.1371/journal.pone.0197395 (2018).
Watson, O. J. et al. False-negative malaria rapid diagnostic test results and their impact on community-based malaria surveys in sub-Saharan Africa. BMJ Glob. Health 4, e001582. https://doi.org/10.1136/bmjgh-2019-001582 (2019).
Badan Pusat Statistik (BPS). Informasi Statistik Indonesia https://www.bps.go.id/ (2022).
Dhand, N. K. & Khatkar, M. S. Statulator: An online statistical calculator. Sample Size Calculator for estimating a single proportion. http://statulator.com/SampleSize/ss1P.html (2014). Accessed June 2021.
Fornace, K. M. et al. Environmental risk factors and exposure to the zoonotic malaria parasite Plasmodium knowlesi across northern Sabah, Malaysia: A population-based cross-sectional survey. Lancet Planet Health 3, e179–e186. https://doi.org/10.1016/S2542-5196(19)30045-2 (2019).
Cox-Singh, J., Mahayet, S., Abdullah, M. S. & Singh, B. Increased sensitivity of malaria detection by nested polymerase chain reaction using simple sampling and DNA extraction. Int. J. Parasitol. 27, 1575–1577. https://doi.org/10.1016/s0020-7519(97)00147-1 (1997).
Singh, B. et al. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 60, 687–692. https://doi.org/10.4269/ajtmh.1999.60.687 (1999).
Lee, K. S., Cox-Singh, J., Brooke, G., Matusop, A. & Singh, B. Plasmodium knowlesi from archival blood films: Further evidence that human infections are widely distributed and not newly emergent in Malaysian Borneo. Int. J. Parasitol. 39, 1125–1128. https://doi.org/10.1016/j.ijpara.2009.03.003 (2009).
Snounou, G. et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61, 315–320. https://doi.org/10.1016/0166-6851(93)90077-b (1993).
Grignard, L. et al. Natural human infections with Plasmodium cynomolgi and other malaria species in an elimination setting in Sabah, Malaysia. J. Infect. Dis 220, 1946–1949. https://doi.org/10.1093/infdis/jiz397 (2019).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Acknowledgements
We are grateful to staff at the various Kapuas Hulu health facilities for their assistance in identifying and recruiting participants, and collecting data and samples. We also thank Karina Dian Lestari for help with qGIS maps.
Funding
This study was supported by Hadi Soesastro Award from the Australian Department of Foreign Affairs and Trade (DFAT) to SRS. DN was supported by a UNIMAS Postgraduate Student Research Grant (F05/PGRG/2042/2020). TMED is supported by a Medical Research Future Fund Practitioner Fellowship (APP1154192). The molecular studies were funded by a University of Western Australia grant to UNIMAS (04-FA050100-0401-007).
Author information
Authors and Affiliations
Contributions
S.R.S. drafted the proposal, prepared the field study, collected and initially analysed samples, and produced the first draft of the manuscript. D.N. analysed samples, performed DNA amplification, sequencing and sequence analysis, contributed to data interpretation, and reviewed/edited the manuscript. D.S.A.M. and N.R. analysed samples. W.D. contributed to data analysis and interpretation, and reviewed/edited the manuscript. J.K.B. helped the fieldwork preparation, contributed to data interpretation, and reviewed/edited the manuscript. B.S. co-supervised the PCR analysis, contributed to data interpretation, and reviewed/edited the manuscript. I.E. helped the fieldwork preparation, supervised the qGIS mapping development, contributed to data interpretation, and reviewed/edited the manuscript. P.C.S.D. co-supervised the PCR analysis, contributed to data interpretation and reviewed/edited the manuscript. T.M.E.D. conceived the study, verified the results and interpretation, and produced the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sugiarto, S.R., Natalia, D., Mohamad, D.S.A. et al. A survey of simian Plasmodium infections in humans in West Kalimantan, Indonesia. Sci Rep 12, 18546 (2022). https://doi.org/10.1038/s41598-022-21570-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21570-0
- Springer Nature Limited