Abstract
Critically ill intubated patients are routinely provided with chlorhexidine (CHX) for their mouth washing, but CHX mouthwash induces several complications. In this study, we aimed to evaluate the efficacy and safety of Moraceae with CHX mouthwash in the reduction of oral bacterial count in critically ill patients and to compare it with CHX-alone mouthwash. This double-blind, randomized, controlled trial included critically ill patients receiving mechanical ventilation. The patients were randomly divided into two groups based on the Modified Beck oral assessment score. The primary endpoint was a reduction in oral bacterial counts after mouth washing on day 1 and day 4. Thirty patients were included in this study; 15 patients received Moraceae with CHX mouthwash and 15 patients received CHX-alone mouthwash. The oral bacterial counts in the Moraceae with CHX group did not differ from the CHX group after mouth washing on day 1 and day 4 of admission. The patients in the CHX group experienced more intolerable taste than those in the Moraceae group (60% vs. 13.3%, P = 0.008). Moraceae with CHX mouthwash had the same effectiveness as CHX alone on bacterial flora but exhibited less intolerable side effects than CHX alone.
Trial registration: TCTR20190530003; 30/05/2019.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is one of the most critical problems in intensive care units (ICU) and is the most common nosocomial infection among patients receiving mechanical ventilation. The incidence of VAP is approximately 8–28%1,2. The mechanism includes bacterial oropharyngeal colonization that progresses into tracheal colonization that subsequently leads to pneumonia. A previous study reported that the causative pathogen was found in the oral cavity in the first 24 h3. When patients are intubated, the normal flora in the oral cavity is replaced by pathogens, thus causing VAP. The number of pathogens increases from the first day to the fourth day and continues to increase till 7 days4,5. In a Thai study, the major organisms were gram-negative bacteria such as Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa and the most common gram-positive pathogen was Staphylococcus aureus6. The mortality rate of patients with VAP is two times the mortality rate of patients without VAP7. The estimated attributable mortality of VAP is around 10% and the disease also increases hospitalization duration and cost8.
Antiseptic oral mouthwash such as chlorhexidine (CHX) was proposed as one intervention to reduce VAP. As CHX exhibits antiseptic effects against oral pathogens causing VAP, CHX mouthwash is becoming popular in ICU patients9. In a meta-analysis, CHX mouthwash decreased bacterial colonization and VAP10. However, the results of CHX are debatable and found to be dependent on the concentration of CHX11,12. At higher concentrations and extended periods of usage, CHX causes mucositis and oral ulcer, as well as changes in taste13.
An in-vitro study reported that Artocarpus lakoocha belonging to the family Moraceae exhibits oral antibacterial and antibiofilm effects14. This herb is commonly found in tropical regions such as Thailand and India. The oxyresveratrol as the major constituent of aqueous extraction of Artocarpus lakoocha is suggested to affect the integrity of bacterial cells14. A pilot study in healthy volunteers revealed that mouthwash products containing 0.02% Moraceae with 0.0005% CHX exhibit antimicrobial and antiplaque activities similar to 0.12% CHX mouthwash. Moreover, the CHX mouthwash group developed a burning sensation and distaste (unpublished data).
In this study, we aimed to evaluate the efficacy and safety of Moraceae with CHX mouthwash in reducing oral bacteria count in critically ill patients and to compare it with CHX alone. We hypothesized that Moraceae with CHX mouthwash has comparable antimicrobial activities with CHX mouthwash alone and is safe for use in critically ill intubated patients.
Methods
Study design
We performed a double-blinded, randomized clinical study of patients between May 2019 and October 2021 in the 10-bed medical ICU of Songklanagarind Hospital, Hat Yai, Thailand.
The study protocol was approved by the Institutional Review Board (REC 61-434-14-1) and registered in the Thai Clinical Trial Registry (TCTR20190530003; 30/05/2019). An independent data safety monitoring board monitored the study for complications with predetermined discontinuation criteria (presence of erythema of the mucosa or grade I of oral mucositis score according to the Radiation Therapy Oncology Group15 or conscious patients felt uncomfortable or patients denied). The study complies with all principles of the Declaration of Helsinki (1964) and its subsequent versions. Informed consent to participate was obtained from participants who met eligibility criteria before the initiation of the study or from their proxies.
Patient selection
Patients aged 18 years or older who were admitted to the medical ICU within 24 h and expected to require mechanical ventilation for at least more than 72 h were included in the study. Exclusion criteria are shown as follows: (1). Intubation for more than 24 h, (2). Pregnancy or sensitivity of anaphylaxis to herbal preparation and CHX, and (3). Individuals with orthodontic appliances or prosthetic appliances that would interfere with evaluation.
Randomization
The investigators evaluated patients for eligibility, obtained informed consent, and enrolled the eligible participants. After inclusion, the patients were randomly assigned without restriction in the block of 4 with a 1:1 ratio stratified by the Modified Beck oral assessment score (BOAS)16 (normal to mild (scores 5–10), moderate (scores 11–15), and severe dysfunction (scores 16–20); supplementary appendix) according to a computer-generated randomization table derived from www.randomization.com by a research nurse assistant, who had no role in patient management. The research nurse assistants who were not otherwise involved in the study administered the study mouthwash and CHX. The attending physicians, nursing care teams, research investigators, and participants and their family members were blinded to treatment allocation. The mouthwash was prepared by a pharmacist who had no other role in the trial. The mouthwashes were packaged in non-identical 10 mL bottles labeled with sequential numbers.
Ethical approval and consent to participate
This study was performed according to the Helsinki Declaration and was approved by the Institutional Review Board (REC 61-434-14-1), the ethics committee of the Faculty of Medicine, Prince of Songkla University. Informed consent to participate was obtained from participants who met eligibility before study initiation or from their proxies.
Study intervention
Mouthwash preparation
For Moraceae mouthwash preparation, we used 0.02% Artocarpus lakoocha extract, 0.0005% CHX, and cremophor RH40 in the mouthwash base, which was prepared in the standard laboratory of the Faculty of Dentistry, Prince of Songkla University (Patent no. 9519). CHX mouthwash of 0.12% was prepared in the pharmaceutical unit of Songklanagarind hospital.
The two types of mouthwashes were straw-yellow colored and filled in non-identical, 10 mL bottles.
Oral care
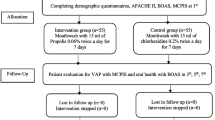
Before oral hygiene intervention, The Modified BOAS was recorded, which ranged between 5 and 20, with 5–10 representing normal to mild dysfunction, 11–15 representing moderate dysfunction, and 16–20 representing severe dysfunction. The first oropharyngeal suction (T0) in the lower gingivobuccal sulcus to quantify bacterial inoculum was performed after randomization. Subsequently, tooth brushing (Colgate) with toothpaste (Colgate) was performed by bedside nurses. The mouth washing of the first group was performed with 10 mL of Moraceae with CHX solution every 8 h. The mouth washing of the second group was performed with 0.12% CHX solution alone every 8 h. After mouth washing, the second sample (T1) was collected by oropharyngeal suction. Oral care with intervention mouthwashes was performed for 7 days or until the patients were extubated. The third sample (T2) was collected on the morning of day 4 before the oral care procedure.
The samples were collected immediately after mouth washing to avoid a decrease in the mouthwash salivary concentration and bacterial colonization11. Considering that the oral microflora changes to VAP pathogen at 48 to 72 h after hospitalization5,17, the samples were also collected on day 4. The Modified BOAS was recorded at 3–4 p.m. daily by the first author (PS). Patient satisfaction was surveyed at the end of the intervention in conscious patients.
Microbiological study
The saliva samples were transferred to 1.5 mL sterile microcentrifuge tubes and stored on ice for a few hours for transportation to the research laboratories. The saliva samples were immediately stored at − 80 °C until used for real-time PCR. Saliva samples were collected at baseline (T0), after mouthwash (T1), and day 4 (T2), and evaluated for Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae using real-time PCR.
Microbial evaluation using real-time PCR
The whole salivary samples were collected from individual subjects at baseline, immediately after using mouthwash, and 4 days after using mouthwash by having them spit into sterile plasticware. DNA was extracted from the salivary samples using a PureDirex Genomic DNA Isolation kit (Bio-Helix Co., LTD., Keelung City, Taiwan) following the manufacturer’s protocol for salivary bacteria, and bacterial DNA was stored at − 20 ºC until further use. The quantities of targeted bacteria in the saliva at baseline (T0), immediately after using mouthwash (T1), and 4 days after using mouthwash (T2) were evaluated by performing real-time PCR. Total bacterial DNA (5 μL) was added to a Sensi-FAST™SYBR kit (Bioline Reagent Ltd., California, USA). The sequences of primers used were as follows: total bacteria (5′-TCCTACGGGAGGCAGCAGT-3′ and 5′-GGACTACCAGGGTATCTAATCCTGTT-3′)18, A. baumannii (5′-CATTATCACGGTAATTAGTG-3′ and 5′-AGAGCACTGTGCACTTAAG-3′)19, K. pneumoniae (5′-AGAGTATTGGTTGACTGCAGGATTT-3′ and 5′- AAACATCAAGCCATATCCATTGG-3′)20, P. aeruginosa (5′- CCTGACCATCCGTCGCCACAAC-3′ and 5′-CGCAGCAGGATGCCGACGCC-3′)21, and S. aureus (5′-GAAATCGATGGTGACAGTAATAA-3′ and 5′- CTACGTCATTTGCACCYGATAA-3′)22. The PCR cycles consisted of an initial step of 10 min at 95 °C followed by 2 min at 50 °C and 40 cycles at 95 °C for 20 s with different annealing temperatures, including 60 °C for total bacteria and P. aeruginosa, 59 °C for K. pneumoniae, 57 °C for S. aureus, and 52 °C for A. baumannii for 20 s. The polymerization temperature was 72 °C for 25 s. Amplification, detection, and data analysis were performed using the CFX96™ Real time system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each sample was run in duplicate and the number of targeted bacteria in the saliva samples was quantified using a standard curve.
The standard curve of each target bacteria has been previously reported23. The bacterial pellets of the target bacteria were collected after centrifugation at 3000 g for 5 min, washed twice with phosphate-buffered saline (pH 7.0), and adjusted to OD600nm = 2.0 using a UV/VIS spectrophotometer (Biochrom Ltd., Cambridge, UK). The bacterial cell suspensions were two-fold diluted, which were then divided into two aliquots. To determine the bacterial number as colony-forming units (CFU)/mL, the first aliquot was measured using the cultivation method. The second aliquot was used for DNA extraction, as mentioned above, to determine the quantification cycle (Cq) of real-time PCR using the CFX96 Touch™ Real-Time PCR detection system (Bio-Rad Laboratories). A linear standard curve was plotted for each bacterial species from log CFU/mL against the corresponding Cq, showing a high correlation coefficient (R2 > 0.99).
Study endpoints and data collection
The primary endpoint of the study was a reduction in oral bacterial total counts as indicated by CFU after mouthwash exposure. We used the following common pathogens: A. baumannii, K. pneumoniae, P. aeruginosa, and S. aureus.
The secondary endpoints included oral health using the Modified BOAS, patients’ satisfaction, and VAP during admission. Patients’ satisfaction evaluation was performed according to the clinical assessment reported by Sarvizadeh et al.24. The conscious patients were asked whether they experienced pain or discomfort during and after mouth washing and graded as tolerable and intolerable. VAP diagnosis was based on the Centers for Disease Control and Prevention criteria25.
Baseline data included age, gender, comorbidities, the reason for ICU admission, SOFA score, antibiotic use, mechanical ventilator days, and length of ICU stay. For adverse events related to intervention mouthwash, we monitored the signs of mucositis, gingivitis, or anaphylaxis.
All patients were followed up until discharge or death, whichever occurred first.
Statistical analysis
The primary outcome was a reduction in total CFU over time after mouth washing in each group. An a priori effect size was difficult to determine because of the lack of appropriately precise previous data on which the calculation was based. According to the report by Julious SA26, the appropriate sample size for a pilot study is 12 participants per group. With a 20% dropout rate consideration, we planned to enroll a total of 30 patients. There was no planned interim analysis.
The study was analyzed on an intention-to-treat basis. No imputation was performed. The Shapiro–Wilk test was used to assess the normal distribution of continuous variables. Continuous data are expressed as the mean and standard deviation or median and interquartile range, depending on data distribution. Numbers and percentages were used to describe the categorical variables. The differences in patient characteristics and outcomes between the two groups were compared using the Wilcoxon-rank sum test, Fisher’s exact test, and χ2 test, as appropriate.
Repeated-measures outcomes such as bacterial counts from patients’ saliva and Modified BOAS at different times were compared between the groups by the GEE with the population-average model. The working correlation matrix structure for GEE analysis was guided by the lowest quasi-likelihood under the independence model criterion (QIC). Finally, the interaction of the group with time was reported as the β-coefficient and 95% Wald confidence interval (CI).
A P value of < 0.05 was considered statistically significant for all comparisons. All statistical analyses were performed using STATA version 16 (StataCorp, College Station, TX, USA) and SigmaPlot (Systat Software, San Jose, CA).
Results
Patients’ flow and recruitment
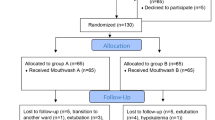
A total of 30 patients were recruited randomly for this study (Fig. 1). Fifteen patients were assigned to the Moraceae group and 15 patients were assigned to the CHX group.
Baseline characteristics
The baseline characteristics of patients were comparable between the two groups Table 1. In all groups, the majority of patients were male; the median age ranged from 56 to 75 years. The two groups did not differ significantly in terms of comorbidities, sequential organ failure assessment (SOFA) score on admission, or reason for ICU admission. The majority of patients (73.3%) had initial normal to mild oral health status. Most patients received at least one antibiotic. Both the median mechanical ventilator and length of ICU stay were 5.5 days.
Outcomes
Oral microflora
Based on the real-time polymerase chain reaction (PCR) analysis, the value of bacterial counts was reported in log CFU/mL. Average total bacterial count and specific pathogens (A. baumannii, K. pneumoniae, P. aeruginosa, and S. aureus) at different times with the pairwise comparison between the groups are shown in Table 2.
For the repeated-measures analysis by generalized estimating equations (GEE), the independent correlation matrix was eventually selected into the equation indicated by the lowest QIC. The results showed that total bacterial count and specific pathogen counts between the two groups were comparable (P > 0.05).
The bacterial count significantly decreased from baseline (T0) to immediately after mouthwash (T1) and increased on day 4 (T2) but was lower than baseline. In the CHX group, P. aeruginosa and A. baumannii counts in T2 significantly increased from those in T1 Fig. 2.
Bacterial counts in the mouthwash-treated patients’ saliva at the baseline, immediately after, and on day 4 a. Moraceae with CHX group b. CHX group. *Statistically significant difference between T0 and T1 or T2 (P < 0.01). **Statistically significant difference between T1 and T2 (P < 0.01). CHX, chlorhexidine; T0, baseline; T1, after using mouthwash; T2, after 4 days.
Oral health
No significant difference was observed in the oral health status according to Modified BOAS for 7 days (β-coefficient of 0.41 (95% Wald CI: − 0.06 to 0.88)) Table 3. However, the patients in the Moraceae group had better oral health than that in the CHX group on days 5–7.
Patients’ satisfaction, VAP, and adverse events
Patients in the CHX group reported more intolerable taste (9/15, 60%) than those in the Moraceae group (2/15, 13.3%) with a significant difference (P = 0.008). The incidence of VAP was 20% overall. Table 4.
A patient in the Moraceae group had a hyperresponsive airway without definite cause during mouth washing and weaning from the ventilator and was withdrawn from the study for safety reasons. No major adverse events were reported related to the intervention.
Discussion
This clinical study showed that mouth washing using Moraceae with CHX mouthwash reduced the bacterial load in the saliva, similar to CHX alone. The patients in the CHX group experienced more intolerable pain than those in the Moraceae group.
The mechanism by which Moraceae with CHX mouthwash reduced oral microbial count needs to be elucidated. Oxyresveratrol, the major constituent of A. lakoocha extract, might affect the cell wall integrity of bacterial cells. In a previous study, Moraceae revealed good antibacterial activity against both cariogenic bacteria and periodontopathogens with more susceptibility toward gram-negative bacteria than gram-positive bacteria14. These properties support our results that the total bacterial count decreased significantly after using mouthwash in the Moraceae with CHX group, which was, however, not observed in the CHX group. Moreover, CHX alone showed an increased count of P. aeruginosa and A. baumannii from day 1 to 4, which was not seen in the Moraceae with CHX group.
Oral chlorhexidine is widely used in critically ill patients to prevent the incidence of VAP based on the previous meta-analysis27. However, two subsequent meta-analyses revealed that CHX might cause excess mortality in ICU patients while failing to prevent VAP12,28. Moreover, a meta-analysis of 11 randomized controlled trials revealed that herbal mouthwashes have potential benefits in controlling plaque and inflammation, which was similar to the effects of CHX29. The most frequently used herb- or plant-derived constituent reported in the studies was green tea extract, followed by neem and marigold. Nevertheless, all studies focused on healthy participants having gingivitis, and no study intervention used mouthwash containing constituents from A. lakoocha. A systematic review of a herbal oral care product in critically ill patients involved 18 studies with more than 10 major natural products that showed the effects of reduction of the oral microbial flora comparable to CHX30.
Complications associated with CHX included the change of taste, dryness, and burning of the mouth leading to an intolerable feeling. The oral health status of patients in the Moraceae group showed a better trend than that in the CHX-alone group based on the Modified BOAS score. In the Moraceae group, we diluted CHX approximately 240 times, which could reduce unacceptable complications caused by the high concentrations of CHX. Two possible reasons behind no significant results are: (1). The baseline oral health status of the majority of patients was normal or mild dysfunction. (2). The median time of mouthwash usage was 5 days. On the other hand, the median time from using CHX to the onset of oral lesions was 8 days in another study13.
Our intervention did not affect VAP prevention. However, the incidence of VAP was nearly 20% in both groups. The possible explanation for the slightly high VAP incidence despite daily mouthwash and systemic antibiotics usage might be from non-strictly VAP prevention bundles, especially handwashing. The majority of pathogens of VAP were gram-negative bacteria. These results are in line with a study performed in Northern Thailand, which reported that the most common VAP pathogens are gram-negative bacteria6. The increase in oral flora on day 4 indicated that the critical care team needed to pay more attention to VAP prevention.
To the best of our knowledge, this is the first clinical study of Moraceae mouthwash intervention in critically ill patients. We collected data on both microbiological and clinical outcomes. Although this study was a pilot study, our randomized, concealed, double-blinded trial had rigid inclusion and exclusion criteria, high protocol adherence, and no loss to follow-up.
This study has some limitations. First, the study is single-centered and includes a small sample size. The latter is the main drawback of our study since only 7 and 8 patients remained for the microbial study on day 4 in the two groups, respectively. Further clinical investigations with a larger study population would provide a better positive correlation among Moraceae mouth washing benefits, bacterial flora reduction, and VAP incidence. Second, we studied only VAP pathogens; hence, we had no data on other types of bacteria. Third, we did not collect data on the sufficient dose of antibiotic used. If antibiotics of sufficient doses for gram-negative bacteria are applied in the treatment, the efficacy of mouthwash is possibly hidden or weakened. However, the types of antibiotics used did not differ between the two groups.
Conclusions
Moraceae with CHX mouthwash had the same effectiveness as CHX alone on bacterial flora but exhibited less intolerable side effects than CHX alone in critically ill patients with mechanical ventilation.
Data availability
The data from this study are available from the corresponding author upon request.
Abbreviations
- VAP:
-
Ventilator-associated pneumonia
- ICUs:
-
Intensive care units
- CHX:
-
Chlorhexidine
- PCR:
-
Polymerase chain reaction
- CFUs:
-
Colony-forming units
- Cq:
-
Quantification cycle
- BOAS:
-
Beck oral assessment score
- SOFA:
-
Sequential organ failure assessment
- GEE:
-
Generalized estimating equations
- QIC:
-
Quasi-likelihood under the independence model criterion
- CI:
-
Confidence interval
References
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 63, e61–e111. https://doi.org/10.1093/cid/ciw353 (2016).
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European respiratory society (ERS), European Society of Intensive care medicine (ESICM), European society of clinical microbiology and infectious diseases (ESCMID) and Asociación latinoamericana del Tórax (ALAT).. Eur. Respir. J. https://doi.org/10.1183/13993003.00582-2017 (2017).
Scannapieco, F. A., Stewart, E. M. & Mylotte, J. M. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit. Care Med. 20, 740–745 (1992).
Pedreira, M. L., Kusahara, D. M., de Carvalho, W. B., Núñez, S. C. & Peterlini, M. A. Oral care interventions and oropharyngeal colonization in children receiving mechanical ventilation. Am. J. Crit. Care. 18, 319–328. https://doi.org/10.4037/ajcc2009121 (2009).
Baradari, A. G., Khezri, H. D. & Arabi, S. Comparison of antibacterial effects of oral rinses chlorhexidine and herbal mouth wash in patients admitted to intensive care unit. Bratisl. Lek. Listy. 113, 556–560 (2012).
Chittawatanarat, K., Jaipakdee, W., Chotirosniramit, N., Chandacham, K. & Jirapongcharoenlap, T. Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a Northern Thai tertiary-care university based general surgical intensive care unit. Infect. Drug Resist. 7, 203–210 (2014).
Safdar, N., Dezfulian, C., Collard, H. R. & Saint, S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit. Care Med. 33, 2184–2193 (2005).
Papazian, L., Klompas, M. & Luyt, C. E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 46, 888–906 (2020).
Rello, J. et al. Oral care practices in intensive care units: A survey of 59 European ICUs. Intensive Care Med. 33, 1066–1070 (2007).
Villar, C. C. et al. Effectiveness of intraoral chlorhexidine protocols in the prevention of ventilator-associated pneumonia: Meta-analysis and systematic review. Respir. Care. 61, 1245–1259 (2016).
La Combe, B. et al. Oropharyngeal bacterial colonization after chlorhexidine mouthwash in mechanically ventilated critically Ill patients. Anesthesiology 129, 1140–1148 (2018).
Klompas, M., Speck, K., Howell, M. D., Greene, L. R. & Berenholtz, S. M. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta-analysis. JAMA Intern. Med. 174, 751–761 (2014).
Plantinga, N. L. et al. Oral mucosal adverse events with chlorhexidine 2% mouthwash in ICU. Intensive Care Med. 42, 620–621 (2016).
Teanpaisan, R., Senapong, S. & Puripattanavong, J. In vitro antimicrobial and antibiofilm activity of Artocarpus lakoocha (Moraceae) extract against some oral pathogens. Trop. J. Pharm. Res. 13, 1149–1155 (2014).
Maria, O. M., Eliopoulos, N. & Muanza, T. Radiation-induced oral mucositis. Front Oncol. 7, 89 (2017).
Ames, N. J. et al. Effects of systematic oral care in critically ill patients: A multicenter study. Am. J. Crit. Care. 20, e103–e114. https://doi.org/10.4037/ajcc2011359 (2011).
Abidia, R. F. Oral care in the intensive care unit: A review. J. Contemp. Dent. Pract. 8, 76–82 (2007).
Nadkarni, M. A., Martin, F. E., Jacques, N. A. & Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148, 257–266 (2002).
Yang, Q. & Rui, Y. Two multiplex real-time PCR assays to detect and differentiate Acinetobacter baumannii and non- baumannii Acinetobacter spp carrying blaNDM, blaOXA-23-Like, blaOXA-40-Like, blaOXA-51-Like, and blaOXA-58-Like genes. PLoS ONE 11, e0158958. https://doi.org/10.1371/journal.pone.0158958 (2016).
Hartman, L. J. et al. Rapid real-time PCR assays for detection of Klebsiella pneumoniae with the rmpA or magA genes associated with the hypermucoviscosity phenotype: Screening of nonhuman primates. J. Mol. Diagn. 11, 464–471 (2009).
Qin, X. et al. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli from patients with cystic fibrosis. J. Clin. Microbiol. 41, 4312–4317 (2003).
Sabat, A. et al. Distribution of the serine-aspartate repeat protein-encoding sdr genes among nasal-carriage and invasive Staphylococcus aureus strains. J. Clin. Microbiol. 44, 1135–1138 (2006).
Pahumunto, N., Sophatha, B., Piwat, S. & Teanpaisan, R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: A double-blind, randomized, controlled study. J. Dent. Sci. 14, 178–184 (2019).
Sarvizadeh, M. et al. Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv. Biomed. Res. 4, 44. https://doi.org/10.4103/2277-9175.151254 (2015).
Horan, T. C., Andrus, M. & Dudeck, M. A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 36, 309–332 (2008).
Julious, S. A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 4, 287–291 (2005).
Labeau, S. O., Van de Vyver, K., Brusselaers, N., Vogelaers, D. & Blot, S. I. Prevention of ventilator-associated pneumonia with oral antiseptics: A systematic review and meta-analysis. Lancet. Infect. Dis. 11, 845–854 (2011).
Price, R., MacLennan, G., Glen, J. & Collaboration, Su. D. D. I. C. U. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: Systematic review and network meta-analysis. BMJ 348, g2197. https://doi.org/10.1136/bmj.g2197 (2014).
Cai, H., Chen, J., Panagodage Perera, N. K. & Liang, X. Effects of herbal mouthwashes on plaque and inflammation control for patients with gingivitis: A systematic review and meta-analysis of randomised controlled trials. Evid. Based Complement Alternat. Med. https://doi.org/10.1155/2020/2829854 (2020).
Mojtahedzadeh, M. et al. Systematic review: Effectiveness of herbal oral care products on ventilator-associated pneumonia. Phytother. Res. 35, 3665–3672 (2021).
Acknowledgements
The authors gratefully acknowledge the clinical research center, Faculty of Medicine, Prince of Songkla University, for providing facilities for this work.
Funding
This study was funded by a research grant from the Faculty of Medicine, Prince of Songkla University (grant number 61-434-14-1).
Author information
Authors and Affiliations
Contributions
P.S., R.T., and V.V. contributed to the conception and design of the study. P.S., S.U., N.P., and V.V. performed the acquisition of data. P.S., N.P., R.T., and V.V. contributed to the analysis and interpretation of the data as well as drafted the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siriyanyongwong, P., Teanpaisan, R., Pahumunto, N. et al. Efficacy of Moraceae with chlorhexidine mouthwash on the microbial flora of critically ill intubated patients: a randomized controlled pilot study. Sci Rep 12, 17261 (2022). https://doi.org/10.1038/s41598-022-21556-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21556-y
- Springer Nature Limited