Abstract
In this study, we determined the seasonal airborne microbial diversity profiles at SMRT stations by sequencing the 16S rRNA and ITS. Particulate matter samples were collected from air purifiers installed in the platform area of the SMRT subway stations. Three stations that included the most crowded one were selected for the sampling. The sampling was done at each season during 2019. After extracting the total DNA from all seasonal samples, PCR was performed with Illumina overhang adapter primers for the V3–V4 region of the 16S rRNA gene and ITS2 region of the ITS gene. The amplified products were further purified, and sequencing libraries were made. Sequencing was carried with the Illumina Miseq Sequencing system (Illumina, USA) followed by in-depth diversity analyses. The elemental composition of the particulate matter samples collected from the different subway stations were obtained using a WD-XRF spectrometer. The SMRT microbiome showed extensive taxonomic diversity with the most common bacterial genera at the subway stations associated with the skin. Overall, the stations included in this study harbored different phylogenetic communities based on α- and β-diversity comparisons. Microbial assemblages also varied depending upon the season in which the samples were taken and the station. Major elements present at the subway stations were from aerosols generated between wheels and brake cushions and between the catenaries and the pantographs. This study shows that the microbial composition of the SMRT subway stations comes from a diverse combination of environmental and human sources, the season and the lifestyle of commuters.
Similar content being viewed by others
Introduction
Microorganisms are ubiquitous, not limited to any specific environment or human activities, and are reported to have a prodigious impact on human life1. Modern metropolitan residents live in closed environments with prolonged exposure to pollutants. These exposures to pollutants including microbial assemblages of cosmopolitan indoor environments have become significant risk factors to the public health2.
In recent years, investigations have been done of the microbiome of urban environments and public mass transit systems, especially subway systems3,4. As urbanization progresses, popular demands on mass transportation system, the subway transit networks in major cities are became a major part of regular travelling and the dependency on subways is expected to increase in future worldwide5.
Several factors influence the microbiome of the aerosol at subway stations; most important factors are the temperature, humidity and lifestyle of the commuters. However, various other factors are also considered as influential, such as aeration systems and pollution. Several studies investigated the microbial communities of metro systems in highly populous cities including New York City, Boston, Hong Kong, Barcelona, Oslo, Mexico city, Athens and Moscow4,5,6,7,8,9,10,11,12,13. Seoul, the capital of South Korea, is a densely populated metropolitan area accounting for 12% of the total area of Korea and more than 20% of the total population and is still growing14. With an epic increase in the commuters in the Seoul Capital Area (SCA), the subway network is also gaining in reputation and is progressively used as the primary public transportation15. In a short period of 40 years, the SMRT has grown into one of the world’s largest public transportation networks with 9 lines with a total length of 327 km (including 290 km underground). On average, 7.2 million passengers per day and 2.6 billion passengers per year use SMRT16. No extensive studies have been done evaluating the substances in the SMRT which may be related to the health of the commuters17.
A study on personal microbial dispersion reported that individuals can release 106 biological particles per hour and can transmit pathogens to other people and indoor environments18. In this context, Seoul commuters on average spend almost 2 h in public transit networks19 with an intensive exposure to various particles in indoor environments. Though multiple factors such as different magnitudes of particulate matters, soil and plant debris are involved in the horizontal transfer of biological particles, most dominant factor, the subway network’s architecture, especially the ventilation of aerosol particles, has a dominant effect on complex subway environments20,21. In this study, an annual investigation of the SMRT microbiome in 2019 was done with next generation sequencing of 16S rRNA and ITS2 genes and chemical analysis of the aerosols with a wavelength dispersive X-ray fluorescence (WD-XRF) spectrometer. Particulate matter samples were collected from air purifiers installed in the platform area of the SMRT subway stations. Three stations including the most crowded one were selected for the sampling. The average daily passengers of station A, B, and C were about 76, 200 and 52 K, respectively. The microbiome of the SMRT was also compared with other urban microbiomes of the world, showing the unique and common features of the SMRT microbiome.
Results
Bacterial phylum at the different subway stations
We have studied 3 major SMRT subway stations and analyzed the 16 samples collected during the different months of year 2019 for this study. All samples received sufficient coverage (> 20,000 reads after filtering using EZbiocloud). The reads were normalized to 20,000 reads for each sample. Total 13,202 OTUs were recovered. The most abundant bacterial phyla were Actinobacteria, Proteobacteria, Bacteriodetes, Firmicutes, Cyanobacteria, Deinococcus-Thermus and Choloroflexi (Fig S1). Actinobacteria with a relative abundance of 39.6% was the most dominant phylum observed at all three stations, followed by Proteobacteria (33.3%), Firmicutes (11.4%), Cyanobacteria (4.9%), Deinococcus-Thermus (4%), Bacteroidetes (3.9%) and Chloroflexi (0.61%). Cyanobacteria were detected in higher relative abundance at subway station B and C. Chloroflexi was observed at station C while absent at the other stations.
Characterization of the bacterial community at the genus level
Skin-associated bacterial genera with an average abundance including Cutibacterium (4.7%), Micrococcus (3%), Staphylococcus (3.3%), Enhydrobacter (3%), Corynebacterium (2.7%) were detected in the aerosols of the SMRT stations. Cutibacterium (previously known as Propionibacterium) is a nonsporulating, gram-positive anaerobic bacillus that is regarded as commensal bacteria on the skin22. However, an exception was observed in the Athens subway stations where Paracoccus was the most abundant genus in the air with a mean value of (8.0%)9 while the SMRT stations had a mean abundance of Paracoccus (2%). Moreover, some other significant skin-associated bacterial genera were also present at the SMRT Stations including Kocuria (4%), Deinococcus (3.7%), Methylobacterium (2.7%), and Rubellimicrobium (2.3%) (Supplementary Table S2). In addition, the air-associated genus Sphingmonas (3.7%), the fresh-soil-dwelling Blastococcus (4%) and Staphylococcus (3.3%) were the most common genera in the SMRT stations. Although broad-spectrum inclinations are described, distinctive and unique bacterial communities could be observed for some of the samples (Fig S4) similar to interpretations reported by some studies5,12.
Seasonal variations in the bacterial compositions at the SMRT stations
To analyze the seasonal fluctuations in the bacterial communities, we classified the samples into four seasonal groups: Winter, Spring, Summer, Autumn. Abundance plots of the phyla across the seasons showed a comparatively constant distribution (Fig. 1); however, Cyanobacteria was observed to be more abundant during the spring and summer which was in contrast to Cyanobacteria being more prevalent in the summer and autumn at the Oslo, Norway subway11.
Cyanobacteria are a phylum of phototrophic bacteria. The capability to carry out oxygenic photosynthesis (water-oxidizing, oxygen-evolving and plant-like photosynthesis) is the most prominent feature of this phylum23. Firmicutes showed similar seasonal drifts with the Oslo subway. Compared to the season specific microbiome studies of subways on a family level11, Micrococcaceae existed as the major bacterial family, showing simila r prevalence trends for the Oslo subway (Supplementary Table S3). Micrococcaceae are non-motile and non-spore forming bacteria which include aerobic and facultative anaerobic organisms diversely spread throughout nature and a part of the flora of the human skin24. Some abundant bacterial families were found at the Oslo subway but were absent at the SMRT and vice versa (Supplementary Table S3).
A few genera showed the seasonal patterns; for example, Kocuria was strongly related to the summer season with an abundance of 7.1% while Bacillus, Sphingomonas exhibited a seasonal pattern during the winter and Cutibacterium was abundant during autumn and spring. The remaining genera showed no significant seasonal variations.
Bacterial diversity analysis
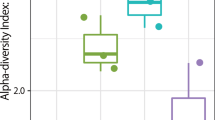
All α-diversity indices showed constant results for certain comparisons (Supplementary Table S5). Collectively, SMRT station B had a higher taxonomic diversity than the other SMRT stations in this study (Fig. 2).
The comparisons were made by a raw contrast of the diversity indices found in other subways. The peak of the Shannon diversity index (H′ = 5.5) was observed during the summer season. A few previous studies have also specified a relatively greater abundance of airborne microbiomes during the summer season which is probably due to the rise in temperature25,26,27,28 and high wind speed that can migrate the microbes29. All alpha diversity estimators showed a contrary outcome with a higher diversity in the summer at the SMRT compared to another indoor environment study with more diversity in the spring and fall30. A significant diversity difference was recognized between the summer diversity with the highest diversity indices and the autumn with the lowest ones (Fig. 3).
(A) α-diversity indices of seasons; Shannon and Simpson Indices showed a significant difference of airborne bacterial communities at SMRT stations during summer and autumn. (B) Principal Coordinate Analysis (PCoA) between SMRT stations and seasons. PCoA plots of phylogenetic dissimilarities between samples of different SMRT stations were constructed on UniFrac distance metric. A significant difference was observed between SMRT stations B and A also in the summer and autumn seasons.
Major fungal taxa present in the SMRT subway stations
We have studied 3 major SMRT subway stations and analyzed the 16 samples collected during the different months of year 2019 for this study. All samples received sufficient coverage (> 10,000 reads after filtering using EZbiocloud). The reads were normalized to 10,000 reads for each sample. The aerosol fungal diversity at Seoul subway stations was mainly attributed to Ascomycota and Basidiomycota which was previously observed in a metro station study9 and an outdoor study31. In our study, within the fungal ITS libraries, the overall most abundant taxa belonged to Ascomycota (Mean = 56.11%) followed by Basidiomycota (Mean = 19.14%) similar to the findings in the Athens metro study9. Remarkably, the third most abundant taxa in the SMRT stations were classified as plants (Mean = 13.91%). These plant taxa may have originated from local centers of diversity or consumption by local population on a regular basis32. Similar interesting findings have also been reported in the Mexico metro and in an urban center in Denver4,28. The composition and diversity of an aerobiome are strongly linked to the coverage of plantation within a given radius of a site33. Other major fungal phyla were soil-associated Mucoromycota (2.1%), a potential candidate for biodiesel production34 and an unclassified Fungal phylum (Fungi_p) with an average abundance of 5.3% observed during the study.
Overall, 15 fungal classes were detected across all stations with Eurotiomycetes having the highest abundance (Mean = 27.4%) compared to the Athens metro (4.3%)9. Other major classes (mean abundance) at the SMRT stations were as follows: Dothideomycetes (14.3%), Agaricomycetes (13.1%), Saccharomycetes (9.3%), and Sordariomycetes (6.7%). A composition difference in the health-related fungi was observed between the SMRT and Athens metro. The most dominant fungal genus detected in this study was Aspergillus (21.9%) which can cause allergic reactions and lung infections called Aspergillosis35,36. Candida (8.3%) is a skin inhabited genus and can cause severe skin infections37. The unclassified genus of Capnodiales (6.5%) was also identified at New York subway platforms12; Capnoidales can cause Chromoblasomycosis-like infection in the human skin38. Nigrospora (2.6%) also was reported to infect the skin in HIV patients39. This outcome suggests that the dominant taxa of the SMRT stations belonged to the skin flora.
Seasonal variation in the fungal composition
Ascomycota was the most abundant phylum throughout all the seasons. It was most abundant during spring (69.7%), autumn (66.5%) and winter (62.6%) and the lowest during summer (39.3%). These results are not similar to the indoor study of swine houses in South Korea40 where Ascomycota was dominant in the summer. The low relative abundance of Ascomycota in the summer may linked with the unclassified Fungal phylum (unclassified Fungi_p) occurrence raised from spring (1.7%) to summer (10%). The prevalence of the unclassified Fungal phylum was also reported in high amounts in a large drinking water source during the summer season41. Basidiomycota was the most dominant in summer (28.2%) and autumn (26.5%) while its abundance was low in winter (10.3%) and spring (14.2%). Interesting findings were drawn by Plantae_p (plant derived), which were in rich abundance during all three seasons except for autumn when the relative abundance decreased to 3%. This finding might be related to more blooms and greenery during the other months. Mucoromycota was found only in the summer with a relative abundance of 5.5% showing a strong seasonal trend. Mucoromycota recently was found as a causal agent of eye diseases (Mucormycosis) in COVID19 patients in India42. The emergence of Mucoromycetes infections may affect the public health in the near future. Most dominant class throughout the samples was Eurotiomycetes. It was highly abundant during all three seasons except in summer when it decreased significantly from the highest in autumn (36.5%) to the lowest in summer (8.3%). This result might be due to the abundance of Dothideomycetes that increased in the summer up to 18.8% from winter (13.2%), autumn and spring (11% each). Agaricomycetes exhibited large proportions in the first half of the year in the autumn and summer seasons (23% and 21%), while it decreased in the winter and spring (8% each) (Fig. 4). All these classes were also reported in a previous indoor environment study of swine houses in South Korea40, but the dominant seasons were different.
The most abundant genus Aspergillus showed a relatively high abundance in spring and autumn (30%) while it remained on the low side during winter (16.8%) and minimal in summer (5.9%). Candida was the second most abundant genus with maximum abundance in the spring (20%), decreasing in the winter (12.2%) and autumn (5.5%) and the lowest in the summer (1.5%). The decrease of Aspergillus and Candida in the summer might be due to some genera having a strong relationship to high temperatures, for example, unclassified Capnodiales_g, Unclassified Endogonales_g and Xylodon were only present in the summer. However, an increase in the plant-derived taxa may also influence the proportions of other genera in the summer season (Fig. 4). Remarkably, traces of Quercus (Oak) (4.3%), one of the most common evergreen trees in South Korea and a dominant member of the Korean peninsula evergreen forests43, was also identified in the winter.
Fungal diversity
Species richness estimators suggest SMRT station B had the highest richness among all three stations (Supplementary Table S7). The significant difference of species richness between station B and station A can be seen in Fig. S8. However, the diversity indices mirrored no significant difference between any of the stations shown in Fig. S8. The Shannon diversity index was lower than the weekday study of the Athens metro9. Although the diversity indices were highest in summer, no significant difference was observed in the seasonal fungal diversity Fig. 5.
Comparison between the SMRT stations and outdoor micorbiomes
Microbiome and mycobiome analysis of the major taxa detected at the SMRT subway stations revealed that these taxa comprised not only normal inhabitants of skin but also of soil, plant debris and water-associated communities. The microbial composition of the major taxa present at the SMRT stations and outdoor environment are shown in Fig. 6a and b. In a previous study, it was revealed that Acinetobacter, Sphingomonas and Pseudomonas in the microbiomes of subway stations originated from outdoor sources5. Similarly, higher abundances of these taxa were observed in the outdoor microbiome while the microbiomes of the SMRT stations were abundant with the skin microflora (Fig. 6a, b).
(A)Comparison of microbiome of SMRT stations in relation to the outdoor microbiome. Some genera including Leucobacter and Luteimonas were observed in the outdoor microbiome during this study. However, the typical skin associated Enhydrobacter and Kocuria were absent in the outdoor microbiome. (B) Principal Coordinate Analysis (PCoA) by UniFrac distance metric.
There were some genera identified in both the indoor and outdoor microbiomes, such as Bacillus, Dermacoccus, Micrococcus, Pseudomonas, Rhodococcus and Sphingomonas, these taxa were more abundant in the outdoor microbiome (Table S8). An interesting observation was the very high abundances of ‘ETC’ (taxa under 1% on average) in the stations compared to those of the outdoor sample. Our results suggest that the outdoor microbiome could also have an important role in shaping the microbiomes of indoor spaces. However, some other significant and relevant factors are necessary to profile the total microbiome of an environment. For example, human activities, lifestyles and ventilation might have an extraordinary role in the making of these microbiome profiles44. A Comparison of β-diversity of bacterial compositions between sample types according to UniFrac distances. *(p < 0.05) is shown in Table 1.
Chemical analysis of the aerosols at the SMRT stations
Bioaerosols consist of diverse biological and chemical sources. The intensities of microbial reactions in atmospheric aerosols may influence human health, ecosystems and atmospheric processes45,46. In total, 31 elements in the bioaerosols from the SMRT stations were measured in this study. The elemental compositions were similar to those of other studies, especially iron. The overall bulk aerosol iron (Fe) concentrations (average of 60.2%) were 45.4% at station B, 65.9% at station C and 69.2% at station A, similar to the results of a previous study47 (Table 2). Iron is the fourth-most abundant element on the earth and required by most organisms including bacteria48. It is also evident that iron (Fe) and selenium (Se) may have an influence in neurodegenerative diseases49. The Fe richness in the PMs of the SMRT stations might be influenced by the friction between the train wheels and rail lines, aerosols generated between the wheels and brake cushions and between the catenaries and the pantographs50. Other major elements were oxygen (average of 13%) with the highest concentration at station B (17.4%), followed by station C (11.0%) and station A (10.5%).
Crustal origin elements Al, Ca, Mg, and Si were also observed at all the SMRT subway stations in this study. Interestingly, the abundance of the crustal elements was high at SMRT station B compared to the other stations. Recently, an increment in the surface particulate matter concentrations was reported in the Seoul Metropolitan area51.
Minor elements Ba, Cl, Cu, Mn and Zn and trace elements Br, Cr, Ti, P and Na were also present. Ba is used as one of the marker species of road dust because Ba is a chemical that is normally applied to lubricating oil to reduce smoke and engine scuff in diesel vehicles52. However, it is well known that Ba and Mn can be rich at subway stations53,54,55.
Sodium, Potassium and Magnesium (Na, K, and Mg) are alkaline and alkaline earth elements, which are used to modify the efficacy of biodiesel production and its stability56, while Ca is enriched in sea spray aerosol particles57. The presence of these elements at the subway stations might be due to the biological activities and ambient sources of the aerosol. Due to direct releases of numerous aerosol particles (carbonaceous aerosols that include black, brown, and organic carbon) or their constituent elements such as sulfur dioxide, nitrogen oxides and volatile organic compounds, human beings have dramatically altered the structure of airborne particles58. One of the possible explanations for the higher abundance of human related elements such as carbon, oxygen, phosphorus and sulfur (C, O, P, and S) at station B might be the result of the large number of commuter’s activities. Human activities can enhance the amount of nitrogen (N) emitted to the aerosol, modifying the N biogeochemical cycle59. Our results showed increased concentrations of N at subway station B, which may involve the greater presence of commuters and their activities at the station. Principal Component Analysis in Fig. 7 shows the major components at the SMRT subway stations.
Discussion
This is the first aerosol microbial study of SMRT subway stations, providing the bases for the further exploration of subway air quality by estimating the microbial diversity and chemical composition of aerosol particles. The current study also included the seasonal dynamics of bacterial and fungal assemblages, which were presented with the elemental composition of the particulate matter (PM) for the first time at subway stations. The most abundant bacterial phyla in the aerosols of Seoul Subway station were Actinobacteria, Proteobacteria, Bacteriodetes, Firmicutes, Cyanobacteria, Deinococcus-Thermus and Choloroflexi. The major bacterial phyla were detected, similar to the microbiomes of the aerosols at the subway stations of New York City (NYC)12, Hong Kong5, and Athens9. Skin-associated bacterial genera with an average abundance including Cutibacterium (4.7%), Micrococcus (3%), Staphylococcus (3.3%), Enhydrobacter (3%), Corynebacterium (2.7%) were detected in the aerosols of the SMRT stations. These bacterial phyla have also been detected in the aerosols of indoor building environments60,61,62 and in urban microbiomes as well63.
Shannon’s diversity index (H′) was larger in the SMRT subway stations (H′ (average) = 4.77 ± 0.54) compared to another studies on the aerosol microbiome of Hong Kong (H′ (average) = 4.13 ± 0.307)5 and the Barcelona subway airborne bacteria (H′ ~ 1.5)13. However, the SMRT bacterial diversity was less than the aerosol bacterial diversity of the Athens subway (H′(average) = 5.75 ± 0.212)9 and the Mexico City metro (H′ (average) = 6.38 ± 0.661)4. Overall, the airborne bacterial diversity found at SMRT stations suggests its source microbes originated mainly from human skin, oral and soil related microbiomes.
Despite having similarities with other subway stations, the SMRT showed a relatively higher diversity than those of NYC, Boston, and Barcelona, however, less than those of Athens and Mexico City. Interestingly, these microbiomes from metro systems with a high number of commuters (SMRT, Hong Kong, and NYC) and metro systems with a lower number of commuters (Boston and Oslo) share almost the same dominant bacterial genera. This result suggests that the microbial community of metro systems can be driven by human activities as most of the genera were related to the skin flora.
Overall, the microbiome of the subway stations was mostly dominated by human skin related taxa and taxa with an environmental origin (soil, water and vegetation). Within the fungal ITS libraries, the overall most abundant taxa belonged to Ascomycota (Mean = 56.11%) followed by Basidiomycota (Mean = 19.14%) similar to the findings in the Athens metro study9. However, the prevalence of Basidiomycota was significantly less at the SMRT compared to Athens (36.4%). The higher abundance of Ascomycota can be explained as follows: the small growth forms of Ascomycota are easier to aerosolize than the large growth forms of Basidiomycota64.
The iron (Fe) was the most abundant element at the subway stations including this study. A previous study on subway particles indicated that Fe-containing particles were the most common subway particle by weight concentration. Most Fe/Si-rich particles were found in the subway particle samples obtained by the portable collectors in London, accounting for 53 percent of the overall particles65. The iron concentrations of New York, Helsinki, Tokyo, and Budapest ranged from 40 to 92%66,67,68,69.
Overall, the stations included in this study harbored different phylogenetic communities based on α- and β-diversity comparisons. Microbial assemblages also varied depending upon the season in which the samples were taken and the station. Major elements present at the subway stations were from aerosols generated between wheels and brake cushions and between the catenaries and the pantographs. Lastly, subway stations and public transport systems generally are regarded as infection hubs for the dissemination of microbial infections and chemical influence on the public health, and our research findings provide fundamental and valuable evidence for the possible role of the public transport system in the spread of infection.
Overall, this study makes progress into the research of microbiomes and chemical components of subway station aerosols. In the future, it can be used to analyze the vulnerability of public transport and commuters to the spread of major airborne diseases in terms of potential chemical and microbial human-health hazards.
Materials and methods
Sample collection and processing
Particulate matter samples were collected from air purifiers installed in the platform area of the SMRT subway stations. Three stations including the most crowded one were selected for the sampling. The average daily passengers of station A, B, and C were about 76, 200 and 52 K, respectively. The sampling was done at each season during 2019 (Table 3) by recovering particulate matters collected in the medium and HEPA filters of the air purifiers operated 24 h a day for a month of period. The medium and HEPA filters were exchanged monthly, and the samples were recovered from used filters according to the maintenance schedule. The rate of air-intake was 900 m3/min for each air purifier. Coarse particles in the indoor air of the platform area were first removed by a pre-filter, and only fine particulate matters were collected in the medium and HEPA filters. The outdoor particulate matters in the same period of 2019 were also collected from medium filters of intake-air purification system of large buildings in the Greater Seoul Area. Each system has pre-filters and medium filters to supply clean air inside the building. The fine particle samples were recovered from the used medium filters several times in 2019 when they were exchanged. Recovered samples during the period of 2019 were merged and processed to remove coarse particles. Then, they were divided into two fractions (PM10 and > PM10) depending on their particle sizes. The separation of the two fractions was carried out using a home-made cyclonic particle separator. Samples with “19” means that the collection year was 2019.
Chemical analysis
The semi-quantitative elemental composition of the particulate matter samples collected from the three different subway stations were obtained using a ZSX Primus II WD-XRF spectrometer (Rigaku, Japan). The X-ray tube was operated in the range of 30–50 kV and 60–100 mA depending on the analyte elements. Two detectors (scintillation and gas flow counters) and eight different analyzing crystals (LiF200, PET, Ge, RX-25, RX-40, RX-45, RX-61F and RX-75) were used for effective detection of various elements. For the semi-quantitative analysis of various elements, the fundamental parameter method70 was applied for samples pressed into discs.
DNA extraction and PCR amplification
Total DNA was extracted from the collected samples using the DNeasy PowerSoil Kit (Qiagen, Germany) in accordance with the manufacturer’s instructions. PCR amplification with the extracted DNA was performed using fusion primers targeting the V3 to V4 regions of the 16S rRNA gene71,72 and the ITS2 region73,74. For bacterial and fungal PCR amplification, Illumina overhang adapter primers were used (Table 4).
The amplifications were carried out under the following conditions: initial denaturation at 95 °C for 3 min, followed by 25 cycles of denaturation at 95 °C for 30 s, primer annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final elongation at 72 °C for 5 min.
The PCR products were confirmed by 1% agarose gel electrophoresis and the Chemi Doc Touch imaging system (BioRad, Hercules, CA, USA).
Library preparation and sequencing
The sequencing libraries were prepared by a sequencing company (Chunlab, Korea). The amplified products were purified with CleanPCR (CleanNA, Netherland). Equal concentrations of purified products were pooled together, and short fragments (non-target products) were removed with CleanPCR (CleanNA, Netherland). The quality and product size were assessed on a Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA) using a DNA 7500 chip (Agilent, Palo Alto, CA, USA). Mixed amplicons were pooled, and the sequencing was carried with an Illumina MiSeq Sequencing system (Illumina, USA) according to the manufacturer’s instructions.
Data analysis
The obtained sequence reads from both the 16S rRNA genes and ITS regions were normalized to 20,000 and 10,000 reads, respectively. The normalized reads were analyzed by microbiome taxonomic profiling (MTP) of EzBioCloud (https://help.ezbiocloud.net/mtp-pipeline/)75. All α-diversity indexes and β-diversity of the samples were determined using MTP. At the species/phylotype and subspecies level, the EzBioCloud database has 66,303 rRNA gene sequences. Principal Coordinate Analysis (PCoA) was done with the Unifrac distance. PERMANOVA test was performed with all MTP sets including this work, it is equivalent to the “beta-group-significance” command in the QIIME2 package (https://help.ezbiocloud.net/mtp-secondary-analysis/#creating-mtp-set/).75 Q-values (adjusted p value) are obtained according to Benjamini and Hochberg76. Indicator value (IndVal%) analysis was done using PAST (Paleontological Statistics Software Package for Education and Data Analysis) 4.05 with default parameters77. Origin(Pro), Version (2022) OriginLab Corporation, Northampton, MA, USA, and RStudio (http://www.rstudio.com/) were used for the visualization78.
Data availability
The datasets generated during and/or analysed during the current study are available in the European Nucleotide Archive (ENA) repository, with the Study Accession Number PRJEB55312.
Abbreviations
- OTU:
-
Operational Taxonomic Unit;
- SMRT:
-
Seoul Metro Rapid Transit;
- PCoA:
-
Principal Coordinate Analysis;
- MTP:
-
Microbiome taxonomic Profiling
References
Kim, K. H., Kabir, E. & Jahan, S. A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. (China) 67, 23–35 (2018).
Klepeis, N. E. et al. The national human activity pattern survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 11, 231–252 (2001).
The Metagenomics and Metadesign of the Subways and Urban Biomes (MetaSUB) International Consortium inaugural meeting report. In Microbiome 4, 24 (BioMed Central, 2016).
Hernández, A. M., Vargas-Robles, D., Alcaraz, L. D. & Peimbert, M. Station and train surface microbiomes of Mexico City’s metro (subway/underground). Sci. Rep. 10, 1–10 (2020).
Leung, M. H. Y., Wilkins, D., Li, E. K. T., Kong, F. K. F. & Lee, P. K. H. Indoor-air microbiome in an urban subway network: Diversity and dynamics. Appl. Environ. Microbiol. 80, 6760–6770 (2014).
Afshinnekoo, E. et al. Geospatial resolution of human and bacterial diversity with city-scale metagenomics. Cell Syst. 1, 72–87 (2015).
Klimenko, N. S. et al. Co-occurrence patterns of bacteria within microbiome of Moscow subway. Comput. Struct. Biotechnol. J. 18, 314–322 (2020).
Walker, A. R., Grimes, T. L., Datta, S. & Datta, S. Unraveling bacterial fingerprints of city subways from microbiome 16S gene profiles. Biol. Direct 13, 10 (2018).
Grydaki, N., Colbeck, I., Mendes, L., Eleftheriadis, K. & Whitby, C. Bioaerosols in the Athens Metro: Metagenetic insights into the PM10 microbiome in a naturally ventilated subway station. Environ. Int. 146, 106186 (2021).
Hsu, T. et al. Urban transit system microbial communities differ by surface type and interaction with humans and the environment. mSystems 1, 18–34 (2016).
Gohli, J. et al. The subway microbiome: Seasonal dynamics and direct comparison of air and surface bacterial communities. Microbiome 7, 160 (2019).
Robertson, C. E. et al. Culture-independent analysis of aerosol microbiology in a metropolitan subway system. Appl. Environ. Microbiol. 79, 3485–3493 (2013).
Triadó-Margarit, X. et al. Bioaerosols in the Barcelona subway system. Indoor Air 27, 564–575 (2017).
KOSIS KOrean Statistical Information Service. Available at: https://kosis.kr/eng/. Accessed 8th April 2021
Kim, K. Y., Kim, Y. S., Kim, D. & Kim, H. T. Exposure level and distribution characteristics of airborne bacteria and fungi in seoul metropolitan subway stations. Ind. Health 49, 242–248 (2011).
Seoul Metropolitan Government. SMRT. Available at: https://www.metropolis.org/sites/default/files/seoul_metropolitan_rapid_transit_english.pdf. Accessed: 7th April 2021.
Choi, S. et al. Characteristics of PM10 levels monitored for more than a decade in subway stations in South Korea. Aerosol Air Qual. Res. 9, 2746–2756 (2019).
Meadow, J. F. et al. Humans differ in their personal microbial cloud. PeerJ 2015, e1258 (2015).
The Korea Times. Seoul commuters spend 2 hours on the road every day: Poll. Available at: http://www.koreatimes.co.kr/www/nation/2019/03/281_264942.html. Accessed: 8th April 2021.
Loxham, M. & Nieuwenhuijsen, M. J. Health effects of particulate matter air pollution in underground railway systems—a critical review of the evidence. Part. Fibre Toxicol. 16, 1–24 (2019).
Wen, Y. et al. Environmental and health effects of ventilation in subway stations: A literature review. Int. J. Environ. Res. Public Health 17, 1084 (2020).
Kadler, B. K., Mehta, S. S. & Funk, L. Propionibacterium acnes infection after shoulder surgery. Int. J. Shoulder Surg. 9, 139–144 (2015).
Whitton, B. A. & Potts, M. Introduction to the Cyanobacteria. Ecol. Cyanobacteria 5, 1–11. https://doi.org/10.1007/0-306-46855-7_1 (2006).
Noble, W. C. Distribution of the micrococcaceae*. Br. J. Dermatol. 81, 27–32 (1969).
Du, P., Du, R., Ren, W., Lu, Z. & Fu, P. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 610–611, 308–315 (2018).
Li, H. et al. Spatial and seasonal variation of the airborne microbiome in a rapidly developing city of China. Sci. Total Environ. 665, 61–68 (2019).
Bowers, R. M. et al. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 47, 12097–12106 (2013).
Xu, A. L., Song, Z. W., Lang, X. L., Chen, X. & Xia, Y. Seasonal variability in bacterial and fungal diversity and community composition of the near-surface atmosphere in coastal megacity. Aerobiologia (Bologna). 33, 555–575 (2017).
Zhong, X., Qi, J., Li, H., Dong, L. & Gao, D. Seasonal distribution of microbial activity in bioaerosols in the outdoor environment of the Qingdao coastal region. Atmos. Environ. 140, 506–513 (2016).
Rintala, H., Pitkäranta, M., Toivola, M., Paulin, L. & Nevalainen, A. Diversity and seasonal dynamics of bacterial community in indoor environment. BMC Microbiol. https://doi.org/10.1186/1471-2180-8-56 (2008).
Pashley, C. H., Fairs, A., Free, R. C. & Wardlaw, A. J. DNA analysis of outdoor air reveals a high degree of fungal diversity, temporal variability, and genera not seen by spore morphology. Fungal Biol. 116, 214–224 (2012).
Xie, W. et al. The source and transport of bioaerosols in the air: A review. Front. Environ. Sci. Eng. 15(3), 1–19 (2020).
Robinson, J. M. et al. Exposure to airborne bacteria depends upon vertical stratification and vegetation complexity. Sci. Rep. 11, 9516 (2021).
Kosa, G. et al. High-throughput screening of Mucoromycota fungi for production of low- and high-value lipids. Biotechnol. Biofuels 11, 1–17 (2018).
Mousavi, B., Hedayati, M. T., Hedayati, N., Ilkit, M. & Syedmousavi, S. Aspergillus species in indoor environments and their possible occupational and public health hazards. Curr. Med. Mycol. 2, 36–42 (2016).
(CDC), C. for D. C. and P. Aspergillosis|Types of Fungal Diseases|Fungal Diseases|CDC. Available at: https://www.cdc.gov/fungal/diseases/aspergillosis/index.html. Accessed 10th May 2021
Kühbacher, A., Burger-Kentischer, A. & Rupp, S. Interaction of candida species with the skin. Microorganisms 5, 32 (2017).
Campolina, S. S., Caligiorne, R. B., Rezende-Silva, S., Hahn, R. C. & De Hoog, G. S. A skin infection mimicking chromoblastomycosis by a Capnodialean fungus. Med. Mycol. 47, 81–85 (2009).
Motswaledi, H. M. & Pillay, R. T. An unusual deep fungal infection with Nigrospora sphaerica in HIV positive patient. Int. J. Dermatol. 58, 333–335 (2019).
Kumari, P., Woo, C., Yamamoto, N. & Choi, H. L. Variations in abundance, diversity and community composition of airborne fungi in swine houses across seasons. Sci. Rep. 6, 1–11 (2016).
Jiang, T. et al. Microbial diversity characteristics and the influence of environmental factors in a large drinking-water source. Sci. Total Environ. 769, 144698 (2021).
News, B. Mucormycosis: The ‘black fungus’ maiming Covid patients in India—BBC News. BBC News 2021. Available at: https://www.bbc.com/news/world-asia-india-57027829. Accessed 22nd May 2021.
Lee, J.-H. et al. The distribution and population status of Quercus myrsinifolia (Fagaceae) on the Korean peninsula. Korean J. Plant Taxon. 44, 165–170 (2014).
Rai, S., Singh, D. K. & Kumar, A. Microbial, environmental and anthropogenic factors influencing the indoor microbiome of the built environment. J. Basic Microbiol. 61, 267–292 (2021).
Wang, B. et al. Characteristics of microbial activity in atmospheric aerosols and its relationship to chemical composition of PM2.5 in Xi’an. China. J. Aerosol. Sci. 146, 105572 (2020).
Kalisa, E. et al. Chemical and biological components of urban aerosols in Africa: Current status and knowledge gaps. Int. J. Environ. Res. Public Health 16, 941 (2019).
Kang, S., Hwang, H., Park, Y., Kim, H. & Ro, C. U. Chemical compositions of subway particles in Seoul, Korea determined by a quantitative single particle analysis. Environ. Sci. Technol. 42, 9051–9057 (2008).
Zughaier, S. M. & Cornelis, P. Editorial: Role of Iron in bacterial pathogenesis. Front. Cell. Infect. Microbiol. 8, 344 (2018).
Billimoria, K., Douglas, D. N., Huelga-Suarez, G., Collingwood, J. F. & Goenaga-Infante, H. Investigating the effect of species-specific calibration on the quantitative imaging of iron at mg kg−1 and selenium at μg kg−1 in tissue using laser ablation with ICP-QQQ-MS. J. Anal. At. Spectrom. 36, 1047–1054 (2021).
Kim, J. B. et al. Status of PM in Seoul metropolitan subway cabins and effectiveness of subway cabin air purifier (SCAP). Clean Technol. Environ. Policy 16, 1193–1200 (2014).
Kim, H. C. et al. Recent increase of surface particulate matter concentrations in the Seoul Metropolitan Area Korea. Sci. Rep. 7, 1–7 (2017).
Monaci, F. & Bargagli, R. Barium and other trace metals as indicators of vehicle emissions. Water. Air. Soil Pollut. 100, 89–98 (1997).
Seaton, A. et al. The London underground: Dust and hazards to health. Occup. Environ. Med. 62, 355–362 (2005).
Boudia, N. et al. Manganese concentrations in the air of the Montreal (Canada) subway in relation to surface automobile traffic density. Sci. Total Environ. 366, 143–147 (2006).
Johansson, C. & Johansson, P. Å. Particulate matter in the underground of Stockholm. Atmos. Environ. 37, 3–9 (2003).
Sánchez, R., Maestre, S., Prats, S. & Todolí, J. L. Aerosol-phase extraction method for determination of Ca, K, Mg, and Na in biodiesel through inductively coupled plasma optical emission spectrometry. Anal. Chem. 89, 13618–13625 (2017).
Salter, M. E. et al. Calcium enrichment in sea spray aerosol particles. Geophys. Res. Lett. 43, 8277–8285 (2016).
Kanakidou, M., Myriokefalitakis, S. & Tsigaridis, K. Aerosols in atmospheric chemistry and biogeochemical cycles of nutrients. Environ. Res. Lett. 13, 063004 (2018).
Galloway, J. N. et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 70, 153–226 (2004).
Chu, W.-L., Tneh, S.-Y. & Ambu, S. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in Kuala Lumpur, Malaysia. Grana 52, 207–220 (2013).
Kembel, S. W. et al. Architectural design drives the biogeography of indoor bacterial communities. PLoS ONE 9, e87093 (2014).
Flores, G. E. et al. Diversity, distribution and sources of bacteria in residential kitchens. Environ. Microbiol. 15, 588–596 (2013).
Danko, D. et al. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell 184, 3376-3393.e17 (2021).
Moore, D. R. G. & T. A. P. 21st century guidebook to fungi with CD. (2011).
Sitzmann, B., Kendall, M., Watt, J. & Williams, I. Characterisation of airborne particles in London by computer-controlled scanning electron microscopy. Sci. Total Environ. 241, 63–73 (1999).
Salma, I., Weidinger, T. & Maenhaut, W. Time-resolved mass concentration, composition and sources of aerosol particles in a metropolitan underground railway station. Atmos. Environ. 41, 8391–8405 (2007).
Furuya, K. et al. Seasonal variation and their characterization of suspended particulate matter in the air of subway stations. J. Trace Microprobe Tech. 19, 469–485 (2001).
Aarnio, P. et al. The concentrations and composition of and exposure to fine particles (PM2.5) in the Helsinki subway system. Atmos. Environ. 39, 5059–5066 (2005).
Chillrud, S. N. et al. Elevated airborne exposures of teenagers to manganese, chromium, and iron from steel dust and New York City’s subway system. Environ. Sci. Technol. 38, 732–737 (2004).
Shiraiwa, T. & Fujino, N. Theoretical calculation of fluorescent X-ray intensities in fluorescent X-ray spectrochemical analysis. Jpn. J. Appl. Phys. 5, 886–899 (1966).
Illumina. 16S Metagenomics Sequencing With the iSeq100 System. Available at: https://sapac.illumina.com/content/dam/illumina-marketing/documents/products/appnotes/iseq100-16s-app-note-770-2018-009.pdf. Accessed: 7th June 2022 (2018)
Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41, 1 (2013).
Illumina. Illumina Adapter Sequences. 2021. Available at: https://support-docs.illumina.com/SHARE/AdapterSeq/illumina-adapter-sequences.pdf. Accessed: 6th June 2022.
Bellemain, E. et al. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 10, 189 (2010).
Yoon, S. H. et al. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 67, 1613–1617 (2017).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Hammer et al. (8) (PDF) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 2001. Available at: https://www.researchgate.net/publication/259640226_PAST_Paleontological_Statistics_Software_Package_for_Education_and_Data_Analysis. Accessed 4th May 2021.
RStudio. RStudio|Open source and professional software for data science teams—RStudio. Available at: https://www.rstudio.com/. Accessed 8th June 2022.
Acknowledgements
This study was supported by the Korea Research Institute of Standards and Science, the National Research Council of Science and Technology, and the University of Science and Technology.
Funding
This work was supported by the “Establishment of measurement standards for microbiology,” grant number GP2021-0003-08, funded by the Korea Research Institute of Standards and Science, and by the National Research Council of Science and Technology grant by the Ministry of Science and ICT (Grant No. CRC-16-01-KRICT). This work was also supported by the UST Young Scientist Research Program (2020YS26), funded by the University of Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Z.U.H. carried out Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Visualization. H.C. and Y.-H.Y. contributed to Chemical Analysis, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft. C.P. provided the Resources, Data Curation. S.K. provided the overall directions for the research, Conceptualization, Resources, Writing—Review and Editing, Supervision, Project administration, and Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, Z.U., Cho, H., Park, C. et al. Seasonal variations of the airborne microbial assemblages of the Seoul subway, South Korea from 16S and ITS gene profiles with chemical analysis. Sci Rep 12, 18456 (2022). https://doi.org/10.1038/s41598-022-21120-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21120-8
- Springer Nature Limited