Abstract
Snow ecosystems are an important component of polar and mountainous regions, influencing water regime, biogeochemical cycles and supporting snow specific taxa. Although snow is considered to be one of the most unique, and at the same time a disappearing habitat, knowledge of its taxonomic diversity is still limited. It is true especially for micrometazoans appearing in snow algae blooming areas. In this study, we used morphological and molecular approaches to identify two tardigrade species found in green snow patches of Mt. Gassan in Japan. By morphology, light (PCM) and scanning electron microscopy (SEM), and morphometry we described Hypsibius nivalis sp. nov. which differs from other similar species by granular, polygonal sculpture on the dorsal cuticle and by the presence of cuticular bars next to the internal claws. Additionally, phylogenetic multilocus (COI, 18S rRNA, 28S rRNA) analysis of the second taxon, Hypsibius sp. identified by morphology as convergens-pallidus group, showed its affinity to the Hypsibiidae family and it is placed as a sister clade to all species in the Hypsibiinae subfamily. Our study shows that microinvertebrates associated with snow are poorly known and the assumption that snow might be inhabited by snow-requiring tardigrade taxa cannot be ruled out. Furthermore, our study contributes to the understanding subfamily Hypsibiinae showing that on its own the morphology of specimens belonging to convergens-pallidus group is insufficient in establishing a true systematic position of specimens.
Similar content being viewed by others
Introduction
Seasonal snow in mountains is an ephemeral cold environment that melts completely by end of the summer1. Despite harsh conditions such as low temperature and high UV irradiation, many organisms are well-adapted to the snow environment, and they are represented by primary producers (snow algae and cyanobacteria), microbial heterotrophs (ciliates and fungi), and consumers (invertebrates)2,3,4,5. Numerous attempts have been made to recognize and describe the snow ecosystems, but snow’s biodiversity has still been poorly recognised, the best examples of which are minute invertebrates6,7,8,9.
During the melt season, the colour of snow surface changes into red, green, golden-brown or orange due to snow algae blooming. Most common genera for each variety of the coloured snow are Sanguina for red snow, Chloromonas for green and orange snow, and Ochromonas for golden-brown snow. Species composition of snow algae varies across each coloured snow5,6,7, which drives different composition of other heterotrophic organisms4. Although a large number of taxonomic studies have been conducted only on snow algae, being primary producers sustaining ecosystems and affecting the reduction of snow albedo9,10,11,12,13, less attention has been paid to top consumers like tardigrades4. These organisms seem to be a forgotten faunal element in studies on snow ecosystems. Therefore, the description of faunal diversity and understanding whether snow ecosystems support any typical snow metazoans, which might be endangered due to global warming, is a crucial task.
Tardigrada (water bears) are a cosmopolitan phylum of microinvertebrates (mostly < 1 mm) that can live in almost all types of environments such as marine and terrestrial, from polar regions to the tropics14,15,16. Until now, ca. 1400 species of tardigrades have been reported17. However, an increasing number of new taxa descriptions is a robust indicator of many water bears still awaiting discovery across the globe17 (https://www.tardigrada.net). Owing to the ability of cryptobiosis that is a latent state under which tardigrade metabolism is undetectable, many limnoterrestrial species can withstand unfavourable conditions e.g., freezing18,19, high pressure20 and radiation21,22. However, active tardigrade species are found in extreme habitats such as ice or snow4,16. Tardigrades play multitrophic roles in ecosystems i.e. some of them may effectively control the population of other metazoans in soil ecosystems, tardigrades on snow feed on algae4. The question how many species inhabit snow ecosystems and how they differ from other tardigrades remains open.
New taxa of tardigrades in cold environments, like glaciers and ice sheets, have been described in recent years. For example, Cryoconicus with dark-brown pigment and claws of Ramazzotius type were reported from cryoconite granules (dark, oval, biogenic structures on ice; mixture of organic and mineral particles23,24) in glaciers of central Asia25, or Cryobiotus with dark pigment, big eyes and modified claws of Hypsibius type from cryoconite holes (water-filled reservoirs on glacial ice26,27) in mountainous glaciers of Europe and Asia28,29,30,31. These glacier genera have dark-coloured pigment, which is thought to protect from a high dose of UV radiation25,32. Cryoconite holes in the Arctic and Antarctica are also inhabited by transparent tardigrades, some of them representing new species, most probably glacier obligates33. Extensive field sampling revealed that some glacier tardigrades are unique and adapted to live only on glaciers34. Taking into account all findings from glacial ecosystems and recent findings of tardigrades on snow4,35, we decided to identify and provide the description of snow tardigrades to give a baseline for answering a question whether as on glaciers, tardigrades on snow are represented by unique, snow-requiring species.
In this paper, we report two taxa of tardigrades belonging to the Hypsibiidae family from snow ecosystems in Japan, one of which is formally described as a new species. We have analyzed green snow samples collected over two seasons (2019 and 2020) from seasonal snow patches at 750 m a.s.l. in Mt. Gassan in the north of Japan where active tardigrades have already been observed4. The first tardigrade found was described by classical methods combining light and scanning electron microscopy imaging, the second was diagnosed by combing light imaging with sequencing of nuclear and mitochondrial DNA fragments, two conservative (18S rRNA, 28S rRNA) and one more variable (COI).
Results and discussion
We identified two taxa of tardigrades found in the blooming of green algae (Chloromonas spp.) on snow surface in Japan (Fig. 1a,b). Their intestine were green which suggest that they feed on Chloromonas spp. Both taxa belong to the one of the most species-rich tardigrade family of Hybsibiidae. According to morphology, both species belong to the Hypsiibinae subfamily. By morphology alone, we described here Hypsibius nivalis sp. nov., and by morphology and DNA we diagnosed the second taxon Hypsibius sp., which according to its morphology (claws of Hypsibius type, two macroplacoids, the lack of cuticular bars, hook-shaped AISM) belongs to the convergens-pallidus group. However, phylogenetic analysis (Bayesian) based on concatenated mitochondrial (COI) and nuclear (18S rRNA, 28S rRNA) molecular markers placed Hypsibius sp. from Mt. Gassan as a sister clade to Hypsibiinae subfamily (Fig. 2). This taxon was found by DNA during two sampling seasons in 2019 and 2020, which suggests its link with green algae blooming on the snow surface.
Taxonomic account
Phylum: Tardigrada Doyère, 184036
Class: Eutardigrada Richters, 192637
Order: Parachela Schuster et al., 198038
Superfamily: Hypsibioidea Pilato, 196939
Family: Hypsibiidae Pilato, 196939
Subfamily: Hypsibiinae Pilato, 196939
Genus: Hypsibius Ehrenberg, 184840
Hypsibius nivalis sp. nov. (Figs. 3, 4, 5 and 6, Table 1, Supplementary material 1).
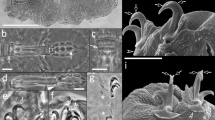
Hypsibius nivalis sp. nov., bucco-pharyngeal structures (PCM): (a) cephalic part, buccal apparatus and eyes, holotype, insert are AISM, (b) buccal apparatus, arrow indicates typical Hypsibius type furcae, arrowhead indicates incision in the first macroplacoid, paratype, (c,d) apophyses (arrowhead) and macroplacoids.
Hypsibius nivalis sp. nov., claws: (a) claws I, arrowheads indicates accessory points, arrow indicates cuticular bar, paratype (PCM), (b) claws II, arrowheads indicates accessory points, arrow indicates cuticular bar, asterix indicates pseudolunules, paratype (PCM), (c) claw III, paratype (PCM), (d) claws IV, arrowhead indicates small cuticular bar, holotype (PCM), (e) claws IV, details of claws and claw bases, paratype (SEM), (f) leg III, external claw, paratype (SEM).
Type locality. Japan, Mt. Gassan (38º 30′ N, 140º 00′ E: altitude 770 m a.s.l.).
Type material. Holotype, slide code: “April, 19, Japan snow no. 1/10” is deposited at the Graduate School of Science and Engineering, Chiba University, Chiba, Japan; 31 paratypes, slide codes: “April, Japan snow no. 1/4, 1/6–1/9, 1/15, 2/1–2/2, R/2, R/4–R6”; SEM stubs codes: “2005GA no. R-1, R” are deposited at the Graduate School of Science and Engineering, Chiba University, Chiba, Japan; and four paratypes, slide codes: “Japan snow 2/1, 2/3, 3/2” are deposited at Department of Animal Taxonomy and Ecology, Adam Mickiewicz University in Poznań.
Etymology. Name nivalis refers to the environment where the species was found –nival in a latin means related to snow.
Description. Body transparent/white, eyes present in all specimens mounted in Hoyer’s medium (Fig. 3a,b). Eyes composed of small granules (Figs. 3a,b,5a). Dorsal cuticle sculptured, covered by polygonal granules, each polygon is separated, polygons form reticular network (Fig. 4a–d). Polygons small in size, do not exceed 2 µm (Fig. 4d). Reticulum covers the legs dorsally. Ventral cuticle smooth. Buccal tube short and rigid (Fig. 5a–d). Teeth in the oral cavity armature absent or not visible under PCM. AISM blunt hook-shaped (Fig. 5a), similar to Mixibius41. Stylet supports located in posterior position of the buccal tube. Typical Hypsibius type stylet furcae (Fig. 5a,b). Pharynx with apophyses and with two rod-shaped macroplacoids. Apophyses are big, triangular or square in shape, clearly separated from macroplacoids. Macroplacoid length sequence 2 < 1. The first macroplacoid with constriction, clearly separated from the second one. Microplacoid and septulum absent (Fig. 5a–d). Claws of the Hypsibius-type, internal and anterior claws smaller than external and posterior claws respectively (Fig. 6a–f). Claws with widened bases and with obvious accessory points on the primary branches. Near the border between accessory points and primary claw branch, a thick line is visible along entire branch length (Fig. 6a–d). Smooth, indistinct pseudolunules under claws more visible on external claws (Fig. 6b). Claw bases smooth. Wide, cuticular bars at the internal claws I–III present, a small bar is present at the posterior claw IV (Fig. 6a–d). Eggs unknown.
Differential diagnosis. By having two macroplacoids, no microplacoid and septulum, and presence of cuticular sculpture, Hypsibius nivalis sp. nov. is the most similar to Hypsibius biscuitiformis Bartoš, 196042, Hypsibius calcaratus Bartoš, 193543, Hypsibius camelopardalis Ramazzotti & Maucci, 198344, Hypsibius giusepperamazzotti Sudzuki, 197545, Hypsibius macrocalcaratus Beasley, 198846, Hypsibius maculatus Iharos, 196947, Hypsibius morikawai Ito, 199548, Hypsibius ragonesei Binda & Pilato, 198549, Hypsibius roanensis Nelson & McGlothlin, 199350, Hypsibius runae Bartoš, 194151 and Hypsibius stiliferus Abe, 200452 but differs from:
-
H. biscuitiformis described from mosses in Hungary by: type of cuticular sculpture (polygonal granules, each polygon is separated, polygons form reticular network in H. nivalis sp. nov. vs. fine and regular granulation in H. biscuitiformis), presence of cuticular bars, and different shape of second macroplacoid (rod shaped in H. nivalis sp. nov. vs. granular macroplacoid in H. biscuitiformis).
-
H. calcaratus described from Slovakia by: presence of cuticular bars, shape of claws (convergens-pallidus type in H. nivalis sp. nov. vs. Ramazzottius type in H. calcaratus (based on original drawings)), and wider buccal tube diameter (2.6–4.6 µm (external wide) in H. nivalis sp. nov. vs. 1–2 µm in H. calcaratus).
-
H. camelopardalis described from Iberian Peninsula by: type of sculpture (polygonal granules, each polygon is separated, polygons form reticular network in H. nivalis sp. nov. vs. plates of various sizes in H. cameopardialis), presence of similar in size granular polygons on entire dorsal side of the body, presence of cuticular bars under the claws.
-
H. giusepperamazzotti described from Tama River in Japan by: different macroplacoid length sequence (2 < 1 in H. nivalis sp. nov. vs. 1 < 2 in H. giusepperamazzotti), presence of cuticular bars under the claws.
-
H. macrocalcaratus described from Mexico by: shape of macroplacoids (rod-shaped in H. nivalis sp. nov. vs. granular in H. macrocalcaratus), smaller cuticular granules (ca. 1–1.5 µm in H. nivalis sp. nov. vs. ca. 2 µm in H. macrocalcaratus), shape of claws (convergens-pallidus type in H. nivalis sp. nov. vs. Ramazzottius type in H. macrocalcaratus, based on original drawings and description in Beasley46).
-
H. maculatus described from Cameroon by: type of cuticular sculpture (polygonal granules, each polygon is separated, polygons form reticular network in H. nivalis sp. nov. vs. hemispherical tubercles, arranged in transverse rows with many dark granules in H. maculatus), presence of cuticular bars, and relatively bigger body size (204–543 µm in H. nivalis sp. nov. vs. 200–225 µm in H. maculatus).
-
H. morikawai described from mosses in Japan by: type of cuticular sculpture (polygonal granules, each polygon is separated, polygons form reticular network, well visible in PCM in H. nivalis sp. nov. vs. very faint rugulae in H. morikawai, according to the original description of Ito48).
Note: Due to lack of detailed description and drawings of cuticular sculpture of H. morikawai, we analyzed holotype (courtesy provided by professor Masamichi Ito), we did not find similar cuticular pattern. We found only very faint shapes resembling cuticular sculpture (Supplementary material 2).
-
H. ragonesei described from Italy by: type of cuticular sculpture (polygonal granules, each polygon is separated, polygons form reticular network in H. nivalis sp. nov. vs. wrinkled cuticle, distributed in bands on the dorsal side of the body), and presence of cuticular bars.
-
H. roanensis described from lichens in Tenneesee (Roan Mountain) by: shape of macroplacoids (rod-shaped in H. nivalis sp. nov. vs. granular in H. roanensis), and presence of cuticular bars.
-
H. runae described from Carpathians by: type of cuticular sculpture (polygonal granules, each polygon is separated, polygons form reticular network in H. nivalis sp. nov. vs. dorsal cuticle covered by papillae in H. runae) and presence of cuticular bars.
-
H. stiliferus described from east Russia by: shape of macroplacoids (rod-shaped in H. nivalis sp. nov. vs. granular in H. stiliferus), and type of cuticular sculpture (polygonal granules, each polygon is separated, polygons form reticular network in H. nivalis sp. nov. vs. polygons of various size sparsely arranges in eight transverse rows in H. stiliferus), size of polygons (ca. 1–1.5 µm in H. nivalis sp. nov. vs. 0.8–4 µm in H. stiliferus), and presence of cuticular bars.
Remarks. Regrettably, the amplification of DNA fragments of Hypsibius nivalis sp. nov. failed (we did not have a fresh material for new analysis).
Hypsibius sp. from Mt. Gassan (Figs. 2, 7–9 , Supplementary material 3).
Diagnosis. The body transparent/white, with eyes present in the examined specimens. The cuticle smooth in the PCM (Fig. 7a,b). The buccal apparatus of the Hypsibius type (Fig. 8a,b). Oral cavity armature either absent or not visible in the PCM (Fig. 8b). The pharyngeal bulb with apophyses, with two rod-shaped macroplacoids (Fig. 8a–d). Stylet supports located in the posterior position. AISM hook-shaped, as presented for Hypsibius in Pilato41 (Fig. 8a). Hypsibius type furcae present. The macroplacoid length sequence 2 < 1, microplacoid and septulum absent (Fig. 8a–d). The apophyses clearly separated from the 1st macroplacoids. All macroplacoids clearly separated (Fig. 8c,d). All main branches with accessory points (Fig. 9a–d). Cuticular bars under and between the claws absent. However the thickening under the claw base IV clearly visible (Fig. 9c,d). Claw basess smooth. Proper lunulae absent, poorly visible pseudolunules present. Eggs unknown.
Hypsibius sp. from Mt. Gassan, claws (PCM): (a) claws I, arrowheads indicates accessory points, (b) claws II, (c) claws IV, arrowhead indicates widened posterior claw base, (d) claw IV, arrowhead indicates widened posterior claw base that form very faint connection between anterior and posterior claws.
Molecular delimitation. The ASAP analysis of 13 COI sequences (11 of tardigrades from snow together with H. dujardini and H. exemplaris) identified 3 putative species at asap-score = 1 (one species of Hypsibius sp. from Mt. Gassan and other two Hypsibius species: H. exemplaris and H. dujardini, Supplementary material 4).
Intraspecific uncorrected pairwise distances for COI marker within 11 specimens of Hypsibius sp. from Mt. Gassan varied between 0 and 2.48% (Supplementary material 5). The p-distances calculated in Mega follow results of ASAP indicating one species of Hypsibius sp. from Mt. Gassan. The sequences of COI, 18S rRNA and 28S rRNA are deposited in GenBank under accession numbers: ON899873–ON899875, ON898549 and ON927924–ON927925, respectively.
Remarks on the species. This species belongs to a large group of hypsibiids with smooth cuticle, two macroplacoids and the lack of cuticular bars under the claws I-III15,33,53. Although phylogenetic analysis placed Hypsibius sp. from Mt. Gassan as a sister lineage to species of Hypsibiinae (Fig. 2), the formal erection of the species as a new taxa without integrative redescription of the most similar by morphology Hypsibius convergens and Hypsibius pallidus, made an exact morphological differential diagnosis dubious. As it has already been shown by using molecular approaches, the genus Hypsibius is polyphyletic, representing similar morphology but distant genetics among its taxon53,54,55. According to DNA, it could be erected as a separate genus, however morphological obstacles do not allow for a proper description in contrast to Cryobiotus or Borealibius which are nested among with other Hypsibius species but differ from them by morphology29,56. Therefore, we decided to only present data on the morphology, morphometry and DNA of a potentially new species from snow.
Tardigrades on snow
Tardigrades have previously been found in snow called “Akashibo” in blooms of algae Hemitoma sp.57, and in red snow consisting of algae Chloromonas spp. and Sanguina spp. in North America5. In spite of that, they have not been identified. Here, we present the taxonomic description of tardigrades from snow for the first time. Our study shows that snow ecosystems are overlooked for studies on the diversity of microinvertebrates. Although tardigrades are most probably wind-blown, delivered to the snow surface in forests from tree canopies or tree trunks58,59, they establish a stable population in snow algae blooming, have persisted for multiple seasons, representing different instars and laying eggs4. Whether specific species of tardigrades need snow (a low-temperature habitat) for their growth and reproduction or they can be only found in a habitat providing them with food (green algae), and without a high number of competitors (compared to mosses) is an open question and requires future findings. Nevertheless, an increasing number of evidence indicates that tardigrades reproduce and feed on snow algae4,5. However, the fate of tardigrades from snow during summer time is unknown and both scenarios, tardigrades are active and reproduce on the snow as well as in mosses after snow melt cannot be ruled out. If these animals need snow ecosystems as a part of their life cycle as was suggested4, and like their glacial counterparts34, the global disappearance of snow ecosystems2,60 may trigger negative changes for snow algae blooming associated metazoans.
Material and methods
Sample processing
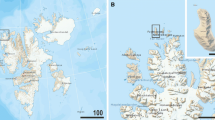
Snow sampling was conducted at Yumiharidaira park (38°30′N, 140°00′E: altitude 770 m above sea level (a.s.l.)) on Mt. Gassan, Yamagata prefecture in Japan (Fig. 1a), details on sampling sites are provided in Ono et al.4. Green snow samples were collected in April and May, 2019 and May, 2020 from seasonal snow in forest area surrounded by beech trees (Fig. 1b). The samples, dimension with 10 × 10 × 2 cm (length × width × depth), were collected using sterile stainless-steel scoop. After sampling, all the samples were kept frozen in Whirl–Pak® bags (Nasco, Fort Atkinson, WI, USA). Tardigrades were isolated from the samples in September 2019 and December 2020, then fixed with 70% ethanol for preservation. Some specimens were mounted on permanent slides for imaging and morphometry in phase contrast light microscopy (PCM), differential interference contrast microscopy (DIC) or for scanning electron microscopy (SEM), remains were used for DNA sequencing.
Microscopy and imaging
Specimens for phase contrast microscopy (PCM) and differential interference contrast microscopy (DIC) were mounted on microscope slides in a drop of Hoyer’s medium61 and examined under a microscopes Olympus BX51 (PCM) and BX53 (DIC). Pictures were taken with a DP21 digital camera (Olympus, Tokyo, Japan), cellSens Entry 1.12 software or Quick PHOTO CAMERA 3.0 software (Promicra, Prague, Czech Republic). Tardigrades for scanning electron microscopy (SEM) were processed following protocol of Sugiura et al.62 with some modifications. Specimens were washed with 0.1 M phosphate buffer, pH 7.0, and fixed with 4% glutaraldehyde, washed in phosphate buffer again and incubated in a 1% OsO4 solution for 1 h. Then specimens were washed with MiliQ water, and dehydrated in ethanol series 30%, 50%, 70%, 80%, 90%, 95%, and three times in 100% for 20 min. After maintained in 100% t-butyl alcohol for 3 h at refrigerator (5℃), specimens were lyophilized by using JFD-320 (JEOL, Tokyo, Japan), then coated with gold and examined using a scanning electron microscope JSM-6010PLUS/LA (JEOL, Tokyo, Japan).
Morphometrics and nomenclature
Sample size for morphometrics was chosen following recommendations by Stec et al.63. All measurements are given in micrometers (μm) and were performed under PCM with Quick PHOTO CAMERA 3.0 software. Structures were measured only when their orientations were suitable. Body length was measured from the anterior to the posterior end of the body, excluding the hind legs. The pt ratio is the ratio of the length of a given structure to the length of the buccal tube, expressed as a percentage64. Macroplacoid length sequence was determined according to Kaczmarek et al.65. Claws were measured following Beasley et al.66. Tardigrade taxonomy and systematics is presented according to Bertolani et al.67 and Degma et al.17. Morphometric data were handled using the “Parachela” ver. 1.2 template available from the Tardigrade Register68. All microscope slides are deposited at the Graduate School of Science and Engineering, Chiba University, Chiba, Japan; and at Department of Animal Taxonomy and Ecology, Adam Mickiewicz University, Poznań, Poland.
DNA extraction and amplification
Total genomic DNA was extracted individually from 11 specimens using the DNAeasy Blood and Tissue Kit (Qiagen GmbH, Hilden, Germany) according to the manufacture instruction. In order to get exoskeletons, after 48 h of digestion and then lysis, 300 ml of a mixture (i.e., ATL buffer—Qiagen, proteinase K, Lysis buffer—Qiagen and 96% ethyl alcohol) with a tardigrade in a 1.5 ml Eppendorf tube was centrifuged at 7000 min−1. Then, from each tube, 290 ml of the above mixture was carefully removed using a pipette remaining the tardigrade specimen on the bottom in 10 ml of the mixture. The exoskeleton was preserved and then mounted in Hoyer’s medium for morphological analysis.
A fragment of the cytochrome c oxidase subunit I (COI) gene of mtDNA was amplified with a bcdF01 forward primer (5'-CATTTTCHACTAAYCATAARGATATTGG-3') and bcdR04 reverse primer (5'-TATAAACYTCDGGATGNCCAAAAAA-3')69,70. A sequence of 18S rRNA gene of nDNA was amplified using the following primers 18s_Tar_1Ff (5'-AGGCGAAACCGCGAATGGCTC-3') and 18s_Tar_1Rr (5'-GCCGCAGGCTCCACTCCTGG-3')71. D1-D3 region of 28S rRNA gene nDNA was amplified with 28sEUTAR_F (5'-ACCCGCTGAACTTAAGCATAT-3')53 or 28sF0001 (5'-ACCCVCYNAATTTAAGCATAT-3')72 forward and 28sR0990 (5'-CCTTGGTCCGTGTTTCAAGAC-3')72 reverse primers.
Amplification of 18S rRNA and 28S rRNA nucleotide genes fragments was conducted in a total volume of 10 ml including 5 ml Type-it Microsatellite PCR Kit (Qiagen), 0.5 ml of each primer (10 ng ml−1), 0.5 ml Q-Solution (Qiagen) and 3.5 ml of the DNA template. For the COI gene, a total volume of 5 ml was prepared, including 3 ml Type-it Microsatellite PCR Kit (Qiagen), 0.5 ml of each primer (10 ng ml−1) and 1 ml of the DNA template. For amplification 18S rRNA and 28S rRNA gene fragments, a thermocycling profile with one cycle of 5 min at 95 °C followed by 38 steps of 30 s each at 95 °C, 60 s at 60 °C, 1 min at 72 °C, and with a final elongation of 5 min at 72 °C was used. While for COI gene fragment amplification, a hybridization was done at 50 °C for 1 min. After amplification, the PCR products were diluted double-fold with RNase-Free water, after that the diluted PCR product was analysed by electrophoresis on 1% agarose gel. Samples containing visible uniform bands with the expected length of the product were purified with Exonuclease I and Fast alkaline phosphatase (Fermentas). The samples were sequenced using the BigDye Terminator v3.1 kit and the ABI Prism 3130xl Genetic Analyzer (Applied Biosystems), following the manufacturer’s instructions.
Phylogeny
The phylogenetic analyses were conducted using concatenated nuclear (18S rRNA, 28S rRNA) and mitochondrial (COI) sequences of 35 Hypsibiidae taxa, one representative of Incerta subfamilia (similar in morphology to Hypsibius sp. from Mt. Gassan genus Acutuncus) with Calohypsibius ornatus73 as the outgroup to hypsibiids. The phylogenetic pipline follows that of recently published robust phylogenies74,75,76,77. Sequences were downloaded from GenBank and the full list of accession numbers is given within Supplementary Material 6.
The sequences were aligned using the AUTO method (in the case of COI) and the Q-INS-I method (18S rRNA and 28S rRNA) in MAFFT version 778,79. Then, the aligned sequences were trimmed to: 657 (18S rRNA), 335 (28S rRNA), 490 (COI) bp. All COI sequences were translated into protein sequences in MEGA7 version 7.080 to check against pseudogenes. The sequences were then concatenated in SequenceMatrix81. Using PartitionFinder version 2.1.182 under the Akaike Information Criterion (AIC), and with greedy algorithm83 implemented within the software we chose the best scheme of partitioning and substitution models for posterior phylogenetic analysis. As the COI is a protein coding gene, before partitioning, we divided our alignment of this marker into 3 data blocks constituting separated three codon positions. Best-fit partitioning schemes and models suggested by PartitionFinder are given within Supplementary Material 7.
Bayesian inference (BI) marginal posterior probabilities were calculated using MrBayes v3.284. Random starting trees were used and the analysis was run for fifteen million generations, sampling the Markov chain every thousand generations. An average standard deviation of split frequencies of < 0.01 was used as a guide to ensure the two independent analyses had converged. The program Tracer v1.685 was then used to ensure Markov chains had reached stationarity and to determine the correct ‘burn-in’ for the analysis, which was the first 10% of generations. The ESS values were greater than 200 and a consensus tree was obtained after summarising the resulting topologies and discarding the ‘burn-in’. All final consensus trees were viewed and visualised with FigTree v.1.4.3 available from (http://tree.bio.ed.ac.uk/software/figtree), then, the tree was modified in Adobe Illustrator, version 25.4.1.
Species delimitation
The species were identified and compared with other taxa based on the previous descriptions42,43,46,48,49,52. If the information on the cuticular bars at the claws was not available either in original descriptions or drawings we assumed these structures were absent.
Using data sets for COI which includes sequences newly generated in this study, as well as COI sequences from Hypsibius dujardini36 and Hypsibius exemplaris53 we performed a genetic species delimitation analyses named the Assemble Species by Automatic Partitioning (ASAP)86. The analyses were run on the respective server (https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html) with default settings. Additionally, we run analysis of p-distance in MEGA7 version 7.080 for COI of Hypsibius sp. from Mt. Gassan.
Data availability
All morphometric data are available in the supplementary materials. The sequences of COI, 18S rRNA and 28S rRNA are deposited in GenBank under accession numbers: ON899873–ON899875, ON898549 and ON927924–ON927925, respectively. The slides are available at the Department of Animal Taxonomy and Ecology at Adam Mickiewicz University in Poznań and at the Graduate School of Science and Engineering, Chiba University, Chiba, Japan.
References
Blahušiaková, A. et al. Snow and climate trends and their impact on seasonal runoff and hydrological drought types in selected mountain catchments in Central Europe. Hydrol. Sci. J. 65, 2083–2096 (2020).
Domine, F. Should we not further study the impact of microbial activity on snow and polar atmospheric chemistry? Microorganisms 7, 260 (2019).
Hoham, R. W., Laursen, A. E., Clive, S. O. & Duval, B. Snow algae and other microbes in several alpine areas in New England. in 50th Eastern Snow Conference. 165–173 (1993).
Ono, M., Takeuchi, N. & Zawierucha, K. Snow algae blooms are beneficial for microinvertebrates assemblages (Tardigrada and Rotifera) on seasonal snow patches in Japan. Sci. Rep. 11, 5973 (2021).
Yakimovich, K. M., Engstrom, C. B. & Quarmby, L. M. Alpine snow algae microbiome diversity in the Coast Range of British Columbia. Front. Microbiol. 11, 1721 (2020).
Engstrom, C. B., Yakimovich, K. M. & Quarmby, L. M. Variation in snow algae blooms in the Coast Range of British Columbia. Front. Microbiol. 11, 569 (2020).
Terashima, M., Umezawa, K., Mori, S., Kojima, H. & Fukui, M. Microbial community analysis of Colored Snow from an Alpine snowfield in Northern Japan reveals the prevalence of Betaproteobacteria with snow algae. Front. Microbiol. 8, 1481 (2017).
Fukushima, H. Studies on Cryophytes in Japan. Yokohama Municipal Univ. 43, 1–146 (1963).
Hoham, R. W. & Duval, B. Microbial Ecology of Snow and Freshwater ice with Emphasis on Snow Algae (Cambridge University Press, 2001).
Dial, R. J., Ganey, G. Q. & Skiles, S. M. What color should glacier algae be? An ecological role for red carbon in the cryosphere. FEMS Microbiol. Ecol. 94, 007 (2018).
Hotaling, S. et al. Biological albedo reduction on ice sheets, glaciers, and snowfields. Earth Sci. Rev. 220, 103728 (2021).
Lutz, S. et al. The biogeography of red snow microbiomes and their role in melting Arctic glaciers. Nat. Commun. 7, 11968 (2016).
Takeuchi, N., Dial, R., Kohshima, S., Segawa, T. & Uetake, J. Spatial distribution and abundance of red snow algae on the Harding Icefield, Alaska derived from a satellite image. Geophys. Res. Lett. 33, L21502 (2006).
Nelson, D. R., Guidetti, R. & Rebecchi, L. Phylum Tardigrada. in Thorp and Covich’s Freshwater Invertebrates. 347–380. https://doi.org/10.1016/B978-0-12-385026-3.00017-6 (Elsevier, 2015).
Kaczmarek, Ł, Michalczyk, Ł & Mcinnes, S. J. Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland. Zootaxa 4203, 1 (2016).
Zawierucha, K. et al. A hole in the nematosphere: Tardigrades and rotifers dominate the cryoconite hole environment, whereas nematodes are missing. J. Zool. 313, 18–36 (2021).
Degma, P., Bertolani, R. & Guidetti, R. Actual Checklist of Tardigrada Species. 41th edn. (2009–2022).
Hengherr, S., Worland, M. R., Reuner, A., Brümmer, F. & Schill, R. O. Freeze tolerance, supercooling points and ice formation: Comparative studies on the subzero temperature survival of limno-terrestrial tardigrades. J. Exp. Biol. 212, 802–807 (2009).
Wright, J. C. Cryptobiosis 300 years on from van Leuwenhoek: What have we learned about Tardigrades? Zool. Anzeiger J. Comp. Zool. 240, 563–582 (2001).
Ono, F. et al. Effect of high hydrostatic pressure on to life of the tiny animal tardigrade. J. Phys. Chem. Solids 69, 2297–2300 (2008).
Horikawa, D. D. et al. Radiation tolerance in the tardigrade Milnesium tardigradum. Int. J. Radiat. Biol. 82, 843–848 (2006).
May, R. M. Action différentielle des rayons x et ultraviolets sur le tardigrade Macrobiotus areolatus, a l’état actif et desséché. Bull. Biol. France Belgique 98, 349–367 (1964).
Rozwalak, P. et al. Cryoconite—From minerals and organic matter to bioengineered sediments on glacier’s surfaces. Sci. Total Environ. 807, 150874 (2022).
Takeuchi, N., Kohshima, S. & Seko, K. Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a himalayan glacier: A granular algal mat growing on the glacier. Arct. Antarct. Alp. Res. 33, 115 (2001).
Zawierucha, K. et al. High mitochondrial diversity in a new water bear species (Tardigrada: Eutardigrada) from mountain glaciers in Central Asia, with the errection of a new genus Cryoconicus. Ann. Zool. 68, 179–201 (2018).
Cook, J., Edwards, A., Takeuchi, N. & Irvine-Fynn, T. Cryoconite: The dark biological secret of the cryosphere. Prog. Phys. Geogr. Earth Environ. 40, 66–111 (2016).
Takeuchi, N., Khoshima, S., Kumiko, G.-A. & Roy M. K. Biological characteristics of dark colored material (cryoconite) on Canadian Arctic glaciers (Devon and Penny ice caps). Mem. Natl Inst. Polar Res. Spec. Issue. 54, 495–505 (2001).
Dastych, H. Redescription of glacier tardigrade Hypsibius janetscheki Ramazzotti, 1968 (Tardigrada) from the Nepal Himalayas. Entomol. Mitt. zool. Mus. Hamburg 14, 181–194 (2004).
Dastych, H. Cryobiotus roswithae gen. n., sp. n., a new genus and species of glacier-dwelling tardigrades from Northern Norway (Tardigrada, Panarthropoda). Entomologie heute 31, 95–111 (2019).
Dastych, H., Kraus, H. & Thaler, K. Redescription and notes on the biology of the glacier tardigrade Hypsibius klebelsbergi Mihelcic, 1959 (Tardigrada), based on material from the Otztal Alps, Austria. Entomol. Mitt. zool. Mus. Hamburg 100, 73–100 (2003).
Dastych, H., Hamburg, U., Grindcl, B. & Museum, Z. Hypsihius thaleri sp. nov., a New Species of a Glacier-Dwelling Tardigrade from the Himalayas, Nepal (Tardigrada). Entomol. Mitt. zool. Mus. Hamburg 101, 169–183 (2004).
Greven, H. & Dastych, H. Notes on the integument of the glacier-dwelling tardigrade Hypsibius klebelsbergi Mihelcic, 1959 (Tardigrada). Entomol. Mitt. zool. Mus. Hamburg 102, 11–20 (2005).
Zawierucha, K., Buda, J., Jaroměřská, T., Janko, K. & Gąsiorek, P. Integrative approach reveals new species of water bears (Pilatobius, Grevenius, and Acutuncus) from Arctic cryoconite holes, with the discovery of hidden lineages of Hypsibius. Zool. Anzeiger 25, 141 (2020).
Zawierucha, K. et al. Water bears dominated cryoconite hole ecosystems: Densities, habitat preferences and physiological adaptations of Tardigrada on an alpine glacier. Aquat. Ecol. https://doi.org/10.1007/s10452-019-09707-2 (2019).
Hanzelová, M., Vido, J., Škvarenina, J., Nalevanková, P. & Perháčová, Z. Microorganisms in summer snow patches in selected high mountain ranges of Slovakia. Biologia 73, 1177–1186 (2018).
Doyère, M. L. Mémoire sur les Tardigrades. Ann. Sci. Nat. Paris 14, 269–362 (1840).
Richters, F. Tardigrada. Handbuch Zool. 3, 1–68 (1926).
Schuster, R. O., Nelson, D. R., Grigarick, A. A. & Christenberry, D. Systematic criteria of the Eutardigrada. Trans. Am. Microsc. Soc. 99, 284 (1980).
Pilato, G. Evoluzione e nuova sistemazione degli Eutardigrada. Bollettino Zool. 36, 327–345 (1969).
Ehrenberg, C. G. Fortgestze Beobachtungen über jetzt herreschende atmospärische mikroscopische etc. mit Nachtrag und Novarum specierum diagnosis. in Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Königlichen Preussischen Akademie der Wissenschaften zu Berlin. Vol. 13. 370–381 (1848).
Pilato, G. The taxonomic value of the structures for the insertion of the stylet muscles in the Eutardigrada, and description of a new genus. Zootaxa 3721, 365 (2013).
Bartos, E. Ergänzungen zur der Tardigradenfauna Böhmens. Acta Univ. Carolina-Biol. 41, 1–5 (1960).
Bartos, E. Vier neue Hypsibiusarten aus der Tschecho-slowakei. Zool. Anz. 110, 257–260 (1935).
Ramazzoti, G. & Maucci, W. The Phylum Tardigrade. Mem. Ist. Ital. Idrobiol. 41, 1–1012 (1983).
Sudzuki, M. Lotic tardigrade from the Tama river with special reference to water saprobity. Mem. Ist. Ital. Idrobiol. 1975, 377–391 (1975).
Beasley, C. W. Altitudinal distribution of Tardigrada of New Mexico with the description of a new species. Am. Midl. Nat. 120, 436–440 (1988).
Iharos, G. Tardigraden aus Mittelwestafrika. Opusc. Zool. (Budap.) 9, 115–120 (1969).
Ito, M. Taxonomic study on the Eutardigrada from the northern Slope of Mt. Fuji, Central Japan, II. Family Hypsibiide. Proc. Jpn. Soc. Syst. Zool. 53, 18–39 (1995).
Binda, M. G. & Pilato, G. Hypsibius ragonesei, nuova specie di Eutardigrado di Sicilia. Animalia 12, 245–248 (1985).
Nelson, D. R. & McGlothlin, K. L. A new species of Hypsibius (phylum Tardigrada) from Roan Mountain, Tennessee, USA. Trans. Am. Microsc. Soc. 112, 140–144 (1993).
Bartos, E. Studien über die Tardigraden des Karpathengebietes. Zool. Jahrb. Abt. Syst. 5, 435–472 (1941).
Abe, W. A new species of the genus Hypsibius (Tardigrada: Parachela: Hypsibiidae) from Sakhalin Island, Far East Russia. Zool. Sci. 21, 957–962 (2004).
Gąsiorek, P., Stec, D., Morek, W. & Michalczyk, Ł. An integrative redescription of Hypsibius dujardini (Doyère, 1840), the nominal taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa 4415, 45 (2018).
Dabert, M., Dastych, H., Hohberg, K. & Dabert, J. Phylogenetic position of the enigmatic clawless eutardigrade genus Apodibius Dastych, 1983 (Tardigrada), based on 18S and 28S rRNA sequence data from its type species A. confusus. Mol. Phylogenet. Evolut. 70, 70–75 (2014).
Tumanov, D. V. & Avdeeva, G. S. Integrative description of Hypsibius repentinus sp. nov. (Eutardigrada: Hypsibiidae) from Sweden. Zoosyst. Rossica 30, 101–115 (2021).
Pilato, G. et al. Geonemy, ecology, reproductive biology and morphology of the tardigrade Hypsibius zetlandicus (Eutardigrada: Hypsibiidae) with erection of Borealibius gen. n. Polar Biol. 29, 595–603 (2006).
Fukuhara, H. et al. Spring red snow phenomenon ‘Akashibo’ in the Ozegahara mire, Central Japan, with special reference to the distribution of invertebrates in red snow. SIL Proc. 1922–2010(28), 1645–1652 (2002).
Villella, J. et al. Tardigrades in the forest canopy: Associations with red tree vole nests in Southwest Oregon. Northwest Sci. 94, 24 (2020).
Young, A. R., Miller, J. E. D., Villella, J., Carey, G. & Miller, W. R. Epiphyte type and sampling height impact mesofauna communities in Douglas-fir trees. PeerJ 6, e5699 (2018).
Hågvar, S. et al. Ecosystem birth near melting glaciers: A review on the pioneer role of ground-dwelling Arthropods. Insects 11, 644 (2020).
Degma, P. Field and laboratory methods. in Water Bears: The Biology of Tardigrades (ed. Schill, R. O.). Vol. 2. 349–369 (Springer, 2018).
Sugiura, K., Minato, H., Matsumoto, M. & Suzuki, A. C. Milnesium (Tardigrada: Apochela) in Japan: The first confirmed record of Milnesium tardigradum s.s. and description of Milnesium pacificum sp. nov. Zool. Sci. 37, 1 (2020).
Stec, D. et al. Estimating optimal sample size for tardigrade morphometry. Zool. J. Linn. Soc. 178, 776–784 (2016).
Pilato, G. Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia 8, 51–57 (1981).
Kaczmarek, Ł, Cytan, J., Zawierucha, K., Diduszko, D. & Michalczyk, Ł. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 3790, 357 (2014).
Beasley, C. W., Kaczmarek, Ł. & Michalczyk, Ł. Doryphoribius mexicanus, a new species of Tardigrada (Eutardigrada: Hypsibiidae) from Mexico (North America). Proc. Biol. Soc. Wash. 121, 34–40 (2008).
Bertolani, R. et al. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Phylogenet. Evol. 76, 110–126 (2014).
Michalczyk, Ł & Kaczmarek, Ł. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. J. Limnol. 72, e22 (2013).
Dabert, M., Witalinski, W., Kazmierski, A., Olszanowski, Z. & Dabert, J. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol. Phylogenet. Evol. 56, 222–241 (2010).
Dabert, J., Ehrnsberger, R. & Dabert, M. Glaucalges tytonis sp. n. (Analgoidea, Xolalgidae) from the Barn Owl Tyto alba (Strigiformes, Tytonidae): Compiling Morphology with DNA Barcode Data for Taxon Descriptions in Mites (Acari). Vol. 12 (2008).
Stec, D., Zawierucha, K. & Michalczyk, Ł. An integrative description of Ramazzottius subanomalus (Biserov, 1985 (Tardigrada) from Poland. Zootaxa 4300, 403 (2017).
Mironov, S. V., Dabert, J. & Dabert, M. A new feather mite species of the genus Proctophyllodes Robin, 1877 (Astigmata: Proctophyllodidae) from the long-tailed tit Aegithalos caudatus (Passeriformes: Aegithalidae)—Morphological description with DNA barcode data. Zootaxa 3253, 54 (2012).
Richters, F. Beiträge zur Kenntnis der Fauna der Umgebung von Frankfurt a. M. in Bericht der Senckenbergischen Naturforschenden gesellschaft in Frankfurt am Main. 21–44 (1900).
Stec, D., Vecchi, M., Calhim, S. & Michalczyk, Ł. New multilocus phylogeny reorganises the family Macrobiotidae (Eutardigrada) and unveils complex morphological evolution of the Macrobiotus hufelandi group. Mol. Phylogenet. Evol. 160, 106987 (2021).
Stec, D., Vecchi, M., Maciejowski, W. & Michalczyk, Ł. Resolving the systematics of Richtersiidae by multilocus phylogeny and an integrative redescription of the nominal species for the genus Crenubiotus (Tardigrada). Sci. Rep. 10, 19418 (2020).
Stec, D., Vončina, K., Møbjerg Kristensen, R. & Michalczyk, Ł. The Macrobiotus ariekammensis species complex provides evidence for parallel evolution of claw elongation in macrobiotid tardigrades. Zool. J. Linn. Soc. https://doi.org/10.1093/zoolinnean/zlab101 (2022).
Stec, D. & Morek, W. Reaching the monophyly: Re-evaluation of the enigmatic species Tenuibiotus hyperonyx (Maucci, 1983) and the genus Tenuibiotus (Eutardigrada). Animals 12, 404 (2022).
Katoh, K. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Katoh, K. & Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evolut. 33, 1870–1874 (2016).
Vaidya, G., Lohman, D. J. & Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27, 171–180 (2011).
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T. & Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. https://doi.org/10.1093/molbev/msw260 (2016).
Lanfear, R., Calcott, B., Ho, S. Y. W. & Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701 (2012).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 67, 901–904 (2018).
Puillandre, N., Brouillet, S. & Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 21, 609–620 (2021).
Acknowledgements
The studies on the diversity and biology of snow invertebrates in Japan are supported by a part of the Program for Overseas Visits by Young Researchers, Arctic Challenge for Sustainability (ArCS) Project for MO, JSPS KAKENHI (19H01143 and 20K21840) and the Arctic Challenge for Sustainability II (ArCS II), Program Grant Number JPMXD1420318865 for NT. Studies on the identification and morphology of snow tardigrades were supported within DARWIN project supported by National Agnecy of Academic Exchange in Poland (Bekker program no. PPN/BEK/2020/1/00321) for KZ. We would like to thanks Daniel Stec for helpful comments on phylogenetic analysis and molecular species delimitataion and Jakub Buda for isolation DNA and barcoding.
Author information
Authors and Affiliations
Contributions
Collection of material: M.O. and N.T.; Conceptualization: M.O., N.T. and K.Z.; Microscopy: M.O. and K.Z.; Molecular and phylogenetic analysis: K.Z. All authors edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ono, M., Takeuchi, N. & Zawierucha, K. Description of a new species of Tardigrada Hypsibius nivalis sp. nov. and new phylogenetic line in Hypsibiidae from snow ecosystem in Japan. Sci Rep 12, 14995 (2022). https://doi.org/10.1038/s41598-022-19183-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19183-8
- Springer Nature Limited