Abstract
Tobacco heating products (THPs) have reduced emissions of toxicants compared with cigarette smoke, and as they expose user to lower levels than smoking, have for a role to play in tobacco harm reduction. One key concern of Public Health is that new tobacco and nicotine products should not be more addictive than cigarettes. To assess their abuse liability, we determined nicotine pharmacokinetics and subjective effects of two THPs compared with conventional cigarettes and a nicotine replacement therapy (Nicotine inhaler). In a randomised, controlled, open-label, crossover study healthy adult smokers used a different study product in a 5 min ad libitum use session in each of four study periods. Product liking, overall intent to use again, urge for product and urge to smoke questionnaires were utilised to assess subjective effects. Nicotine uptake was greater for the cigarette (Cmax = 22.7 ng/mL) than for either THP (8.6 and 10.5 ng/mL) and the NRT (2.3 ng/mL). Median Tmax was significantly longer for the NRT (15.03 min) than for the tobacco products (4.05–6.03 min). Product liking and overall intent to use again was highest for the cigarette, and higher for the THPs than the NRT. Urge to smoke was reduced more by the cigarette than by the other three products. Urge to use the THPs was greater than the NRT. These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT.
Similar content being viewed by others
Introduction
Background and objectives
Nicotine, a chemical found naturally in tobacco leaf that is transferred to cigarette smoke during combustion, is primarily responsible for the addictive properties of cigarette smoking1. However, nicotine is not considered to contribute substantially to smoking-related diseases2,3, which generally result from the inhalation of tobacco smoke containing thousands of chemicals4 and numerous toxicants5,6. When a smoker inhales cigarette smoke, nicotine is rapidly transferred to the bloodstream and transported around the body; in the brain, it activates neuronal nicotinic receptors involved in mood and relaxation, which along with the sensorial aspects of smoking, results in the pleasurable and rewarding effects experienced by a smoker7.
Pharmaceutical nicotine products such as nicotine replacement therapies (NRTs) aim to replace the nicotine supplied by conventional cigarettes and thereby assist individuals in stopping smoking by reducing cravings, symptoms of withdrawal, and mood changes8,9,10. In general, however, the delivery of nicotine from NRT products is relatively slow and the pharmacokinetic (PK) profile does not resemble that of cigarettes11,12,13,14. The time to maximum plasma nicotine concentration (Tmax) tends to be longer, while the maximum nicotine concentration (Cmax) is not characterised by the sharp peak seen with cigarettes, but by a lower and flatter peak12,13,14. As a result, smokers do not achieve the same nicotine levels or satisfaction with NRT products that they do when smoking cigarettes. NRT is also sensorially deficient as compared to cigarette smoking in terms of puffing ritual and cues associated with smoking, and as a result of the PK profile, it has a lower abuse liability compared to cigarette15.
A recent review of more than 100 trials concluded that NRTs can increase the rate of successful quit attempts by 50–60% for smokers who want to quit16, but they do not do so for all smokers, potentially due to the slower and reduced delivery of nicotine relative to cigarettes8,9,17, the sensorial deficiencies of NRT and because they do not replace the behavioural activities of smoking [16. Notably, NRTs are considered medicinal products whereas tobacco heating products and other non-combustible products are still considered consumer products and are not approved for smoking cessation. It is therefore important to complement existing cessation initiatives with strategies that attempt to reduce or prevent harm in those who would otherwise continue to smoke.
Tobacco harm reduction, where smokers who are unwilling or unable to quit smoking cigarettes replace cigarette smoking with the use of nicotine and tobacco products with potentially fewer health risks18,19, is a strategy that—if widely adopted—might potentially offer substantial public health gains through avoidance of projected tobacco-related harm19. For many years, tobacco researchers and policy experts have embraced the idea that alternative sources of nicotine that provide rewarding effects similar to those of cigarettes might be used to encourage smokers to switch away from cigarette smoking19. In this regard, tobacco heating products (THPs) are electronic devices that heat, rather than combust, tobacco contained in a consumable “stick”, producing an aerosol that the user inhales20. Owing to the lack of combustion, many of the toxicants found in cigarette smoke are absent or present at significantly reduced levels in the aerosol generated by THPs21,22. Preclinical evidence further indicates that the THP aerosol has reduced in vitro biological activity compared with cigarette smoke21,23,24,25, while several clinical studies have shown significantly reduced toxicant exposure and favourable changes in biomarkers among adults who switch from smoking to using THPs26,27,28,29,30.
Although information on the chemical emissions from THPs and preliminary toxicological data are emerging, relatively little is known about consumer behaviour and/or the abuse liability (dependence potential) of these products, i.e., the likelihood of engaging in persistent and problematic use of alternative nicotine products resulting in undesirable consequences including physical and/or psychological dependence31,32. This information is especially important because any product that fails to deliver nicotine satisfactorily, or conversely demonstrates an abuse liability profile such that non-users may be more likely to initiate and develop sustained product use, may significantly undermine any potential for harm reduction. As a result, the US Food and Drug Administration (FDA) requires an abuse liability assessment, including exposure to nicotine during use and evaluations of misuse potential, as a component of Premarket Tobacco Product Applications for new tobacco products33.
The abuse liability of a tobacco or nicotine product can be determined both by assessing the speed and quantity of nicotine delivery, with higher abuse liability observed for products providing greater delivery, faster absorption and higher plasma concentrations of nicotine, and by assessing subjective effects such as appeal, responses to products and product acceptability31,34,35,36. Subsequently, an approach based on such methods, including an assessment of nicotine PK and subjective effects, has been used to determine the abuse liability of several e-cigarettes (e.g.,13,14,37,38,39,40). However, while nicotine PK data for THPs has previously been reported41,42, there is a paucity of literature concerning the abuse liability of these products coming from studies in which it has been rigorously assessed. In this study, therefore, nicotine PK and various subjective effects indices (product liking, intent to use again, urge to smoke, urge to use the THP, satisfaction, and evaluation of other both positive and negative effects) have been evaluated to determine the abuse liability profile of the glo THP in relation to two products on the extremes of the tobacco and nicotine-containing product risk continuum43,44,45,46, namely combustible cigarettes, which have high abuse liability, and NRT, which has low abuse liability34,45,47.
Methods
Study design
This was an open-label, randomised, crossover, four-treatment, four-period, single-dose clinical study in which healthy adult smokers were recruited to receive one of four investigational products (IPs) during each of four study periods. Blood samples were taken for nicotine PK analysis and participants completed various subjective effects questionnaires. The study was conducted at the Centro Ricerche Cliniche di Verona (Verona, Italy) in July to September 2018. Study authorisation was received from the Italian Medicines Agency (AIFA) based on review by the Italian National Health Institute (reference 1630) and was also approved by the Ethics Committee for Clinical Trials of the Provinces of Verona and Rovigo (reference 1778CESC). The study was conducted in compliance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice (International Conference on Harmonisation E6 Consolidated Guidance, April 1996) and Italian laws, including those relating to the protection of personal data. All participants provided written informed consent prior to any study procedures. The study was registered in the ISRCTN and EudraCT databases (references ISRCTN13439529 (07/08/2018) and 2018-000701-23, respectively).
Participants
The participants were healthy adult smokers (aged 19–60 inclusive) of at least 10 non-menthol, combustible cigarettes per day who had been smoking for at least 1 year and had been smoking their usual brand of cigarette for at least 6 months. Potential participants attended a screening session, where smoking status was confirmed by exhaled carbon monoxide (eCO: ≥ 10 ppm) and urinary cotinine (≥ 200 ng/ml) assessments.
The main exclusion criteria were pregnancy or breastfeeding; non-agreement to using contraception for the duration of the study (female participants); acute illness (e.g., upper respiratory tract or viral infection) requiring treatment within past 4 weeks; use of nicotine or tobacco products other than commercially manufactured cigarettes within the past 14 days; self-reported non-inhalation of cigarette smoke; medical history of asthma or chronic obstructive pulmonary disease (COPD); use of bronchodilator medication within the past 12 months; blood donation of 450 ml or more within past 90 days; and clinically relevant abnormal findings on physical examination, medical history, electrocardiography, lung function tests, or clinical laboratory panel. Individuals were also excluded if they were planning to quit smoking in the next 12 months. All participants were free to quit smoking and withdraw from the study at any time. Any individual who decided to quit smoking was directed to appropriate stop smoking services.
Study products
The THP (glo, British American Tobacco) consists of two components: an electronic heating device, and a tobacco consumable rod (neo stick). Two variants of the Neo THP consumable rod with different nicotine contents were used with an identical heating device: The THP1.0(RT) consumable rod yielded 0.46 mg nicotine/stick and THP1.1(RT) yielded 0.68 mg nicotine/stick, as measured under a modified Health Canada Intense machine smoking regime (55 mL puff volume, 30 s puff interval, 2 s puff duration, 8 puffs, no vent blocking)22,48. Both variants of the THP consumable rod were tobacco flavoured but differed in blend type (1.0 and 1.1).
The first reference product was a NRT (Nicorette Inhalator; 15 mg nicotine; Johnson & Johnson). Nicorette delivers nicotine via the inhaled route and attempts to replicate some of the sensorimotor cues associated with smoking. The second reference product was each participant’s usual brand of combustible cigarette, as reported at screening and supplied by the participants themselves. The THP devices and consumables and NRT were provided free of charge to participants.
Randomisation procedure
Randomisation sequences were prepared by Cromsource Srl (Verona, Italy) with SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC) and using a Williams latin square design composed of 8 blocks of 4 subjects. In ascending order of subject number, enrolled participants were assigned to receive the four study products in accordance with the pre-defined randomisation sequences, with an equal proportion of participants in each sequence.
Study procedures
Individuals who fulfilled the inclusion/exclusion criteria at screening (visit 1) were enrolled in the study and basic demographic characteristics and information on cigarette consumption were recorded. Participants then attended a randomisation visit (visit 2), where the sequence of study product use was assigned as described above. For approximately 1 week before their PK session for THP1.0(RT), THP1.1(RT) or the NRT, each participant was provided with the relevant study product (glo THP device and one pack of 20 of the relevant neo sticks, or one box of NRT inhalator and consumables) and asked to familiarise themselves with its use before attending the session.
Participants were admitted to the clinic on the evening before each PK session and their eligibility was reconfirmed. They abstained from nicotine or tobacco product use overnight (at least 12 h). On the following morning, a baseline blood sample was taken (–5 min) by direct venepuncture and then participants used the assigned study product for 5 min. Puffs were counted during use and blood samples for plasma nicotine analysis were obtained at 1, 3, 4, 5, 6, 7, 9, 15, 45, 90, 180 and 240 min relative to the first puff on the study product. Plasma samples were analysed for nicotine by ABF GmbH (Planegg, Germany) using an Acquity UPLC equipped with a Xevo TQ-S triple quadrupole mass spectrometer. The lower limit of quantification for nicotine using this validated method was 0.1 ng/mL. Heart rate, blood pressure, respiratory rate and body temperature were also measured throughout each PK assessment period.
At various points before, during and/or after the product use session, participants completed five questionnaires. The product liking questionnaire (PLQ) asked “At this moment, how much do you like the product?” (ranging from 0 [strongly dislike] to 5 [neither like nor dislike] to 10 [strongly like]) and was administered at -5, 3, 5, 9, 30, 60, 120 and 240 min. The overall intent to use again (OIUA) questionnaire was administered at a single timepoint (240 min) and asked participants to “Rate the degree to which you would like to use the product again” (ranging from 0 [not at all] to 10 [very much]). The urge to smoke (UTS) questionnaire asked “How strong is your current urge to smoke your usual brand cigarette?” (ranging from 0 [no urge] to 10 [extremely strong urge]) and was administered at -5, 3, 5, 9, 15, 30, 45, 60, 90, 180 and 240 min. The urge for product (UFP) questionnaire was completed only after use of the THPs and NRT (at -5, 15 and 120 min) and asked “How strong is your current urge to use Investigation Product?” (ranging from 0 [no urge] to 10 [extremely strong urge]). The product evaluation scale (PES)48 was also used to assess subjective responses to the study products and was administered at -5, 15 and 240 min.
Participants were discharged from the clinic after the nicotine PK assessment was completed. No later than 1 week after the final clinic visit, they were followed up by telephone to capture any post-study adverse events (AEs). (Fig. 1).
Study endpoints
The primary endpoints were plasma nicotine PK parameters (Cmax, Tmax and AUC0-240 min), PLQ score, OIUA score, UTS score and UFP score. Secondary endpoints were puff count during the 5-min ad libitum product use session and product evaluation as measured by individual item scores in the PES. Safety endpoints included adverse events and vital signs assessments.
Sample size determination
The sample size was determined as 32 based on assumptions concerning all seven endpoints (AUC nicotine, Cmax, Tmax, OIUA, PLQ, UTS and UFP) at 90% power as outlined in Supplementary Table 1.
Statistical methods
Statistical analyses and data processing were performed by using SAS software version 9.3. The PK analysis was performed by KinetAssist Ltd (Quothquan, United Kingdom) using Phoenix WinNonlin version 8.0 (Certara USA, Inc., Princeton, NJ).
AUC0-240 min and Cmax values were log-transformed and an analysis of variance (ANOVA) model with treatment, sequence and period as fixed effects and subject-within-sequence as a random effect was implemented. Ratios of estimated treatment population geometric means and their associated 90% confidence intervals (CI) were calculated from the exponential of the least-squares means difference and the corresponding 90% CI. Non-inferiority of the THPs versus the NRT was confirmed if the lower bound of the one-sided 90% CI for the ratio was at least 0.80. Non-inferiority of the THP versus the usual brand cigarette was confirmed if the upper bound of the one-sided 90% CI for the ratio was 1.25 or lower. For Tmax, Hodges-Lehmann nonparametric pairwise estimate of location shift between the THPs and NRT was performed. Faster nicotine absorption from the THPs compared with the NRT was confirmed if the upper bound of the one-sided 90% CI for the difference (THP – NRT) was less than -4.
PLQ, UTS, UFP and OIUA scores were log-transformed and an ANOVA model including treatment, sequence and period as fixed effects, and subject-within-sequence as a random effect, was implemented. The ratio of the adjusted geometric means between the THP and NRT was calculated with its one-sided 90% CI. For PLQ and UTS, the AUC of values from 3 to 240 min was used; for UFP, the mean of the values from 15 to 120 min was used; and for OIUA, the actual values at 240 min were used. PLQ and OIUA were tested for non-inferiority between the THP and the NRT, with non-inferiority of the THP confirmed if the lower bound of the one-sided 90% CI for the ratio (THP/NRT) was greater than 0.80. UTS was tested for superiority between the THP and Nicorette Inhalator, with superiority of the THP confirmed if the upper bound of the one-sided 90% CI for the ratio (THP/NRT) was less than 1.0. UFP was tested for superiority between the THP and NRT, with superiority of the THP confirmed if the upper bound of the one-sided 90% CI for the ratio (THP/NRT) was greater than 1.0.
The rationale for using a non-inferiority test is that we’re not interested in THP being significantly more liked than NRT, rather, we’re interested in THP not being less liked. We’re trying to show (at least) equivalence to the NRT (or better, which can be higher or lower depending on the parameter of interest). If we provide evidence for non-inferiority, this shows THP is accepted to the same level as NRTs and could thus provide a good alternative to NRTs in reducing cigarette smoking.
Results
Study population
The study enrolled 32 healthy male (n = 23) and female (n = 9) smokers aged between 22 and 57 years, all of whom completed the study and used all study products. Demographic characteristics of the study participants are summarized in Table 1. Among the 32 participants who enrolled, the mean ± SD age was 35 ± 8.9 years for men and 37 ± 11.9 years for women. Participants were current smokers of, on average, 17 cigarettes per day, with mean FTCD score of 6.0 ± 1.5.
Nicotine pharmacokinetics
Regarding PK assessments, the mean plasma nicotine concentration at any time point was higher for subjects’ usual brand cigarette than for any other study product (Fig. 2). Plasma nicotine concentrations were also higher after use of THP1.0(RT) or THP1.1(RT) than after use of the inhalator NRT, while use of THP1.1(RT) resulted in higher plasma nicotine levels as compared with THP1.0(RT), which is consistent with the higher machine yield for nicotine of THP1.1(RT). Geometric mean Cmax for the cigarette (22.7 ng/mL) was twofold higher than that for either of the THP variants (8.6 and 10.5 ng/mL) and tenfold higher than that for the NRT (2.3 ng/mL) (Table 2). Notably, median Tmax was comparable for the cigarette (6.03 min) and the two THPs (4.05 and 4.07 min) but was much longer for the NRT (15.03 min).
Mean plasma nicotine concentrations at each timepoint. Seven subjects were excluded from the PK analysis population due to major protocol deviations (washout problem), therefore n = 30 for usual brand cigarette, n = 31 for glo THP1.0(RT), n = 30 for glo THP1.1(RT) and n = 30 for Nicorette 15 mg Inhalator.
For both AUC0-240 min and Cmax, THP1.0(RT) and THP1.1(RT) were found to be non-inferior to the NRT, and the cigarette non-inferior to either THP, meaning that the amount and maximum concentration of nicotine in the blood following use of either THP were between levels found for the NRT and the cigarette. Both THPs were superior to the NRT for Tmax, i.e. absorption of nicotine was faster after use of the THPs than after use of the NRT (Table 3).
Subjective responses to product use
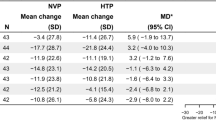
To assess different aspects of abuse liability, four questionnaires assessing product liking (PLQ), overall intent to use again (OIUA), urge to smoke (UTS) and urge for product (UFP) were completed before, during, and at various timepoints after study product use. Regarding product liking the mean PLQ (AUC3-240 min) score was higher for the cigarette than for the other study products, higher for the two THPs than for the NRT, and was slightly higher for THP1.1(RT) than for THP1.0(RT) (Table 4). In the test for non-inferiority, the null hypothesis was rejected and therefore it can be concluded that neither THP was found to be inferior to the NRT in terms of product liking (Table 5).
Regarding participant’s intent to use the product again, the OIUA score was higher for the cigarette than for all other study products, higher for the two THPs than for the NRT, and was slightly higher for THP1.1(RT) than for THP1.0(RT) (Table 4). The null hypothesis was rejected in the non-inferiority test, meaning that neither THP was inferior to the NRT in terms of intent to use the product again (Table 5).
The UTS questionnaire was used to determine whether 5-min use of the study products satisfied the participants urge to smoke. The mean UTS (AUC3-240 min) score was lower for the cigarette than for any of the other three study products (Table 4). The criteria for superiority of the THP variants over the NRT in terms of urge to smoke were not satisfied (Table 5). The participants’ urge to use the study products, assessed by the UFP score, was higher for the THP variants than for the NRT (Table 4). In the test for superiority, the null hypothesis was rejected and thus it can be concluded that both THP1.0(RT) and THP1.1(RT) were found to be superior to the NRT in terms of urge to use the product again (Table 5).
Secondary endpoints
Regarding user behaviour, the number of puffs taken during 5-min product use sessions was similar across the study products. The mean number of puffs during the 5-min use session was 12 ± 5.7 (median 12) for THP1.0(RT), 12 ± 5.2 (12) for THP1.1(RT), 13 ± 5.8 (12) for the NRT, and 14 ± 4.6 (13) for the cigarette.
In terms of product evaluation, the mean scores for single items of the PES indicated better satisfaction both pre-use, and 15 and 240 min post-use, for the cigarette than for the three other study products, and an overall higher level of satisfaction for using both THP1.0(RT) and THP1.1(RT) than for the NRT. Based on the PES scores obtained before each product use session, use of the usual brand cigarette was considered by participants to (1) be more effective in relieving withdrawal symptoms and urge to smoke, (2) contain enough nicotine, (3) cause bothersome side effects, and (4) reduce craving for a cigarette after using the product, as compared with the other study products. Conversely, use of the participant’s usual brand cigarette was perceived to have a higher risk of causing dizziness or dependency. In addition, use of the inhalator NRT was perceived as having a higher risk of causing dizziness and nausea, or containing more nicotine, as compared with either THP1.0(RT) or THP1.1(RT) (Supplementary Table 2).
The most relevant changes reported between pre-use and 240 min after use were (1) a perceived reduction in irritability and urge to smoke for use of the cigarette; (2) a perceived increase in risk of causing dizziness for THP1.1(RT) and the cigarette; and (3) a perceived decrease in risk of causing dizziness for the NRT. The perception of craving for a cigarette decreased between pre-use and 240 min post-use for both THP1.0(RT) and THP1.1(RT).
Safety assessment
A total of 8 treatment-emergent adverse events (TEAEs) were reported in 6 subjects (18.8%) overall (Supplementary Table 3). Two TEAEs were reported in 2 subjects (6.3%) during the use of glo THP1.0(RT), 2 TEAEs were reported in 2 subjects (6.3%) during the use of glo THP1.1(RT), 1 TEAE was reported in 1 subject (3.1%) during the use of the NRT, and 3 TEAEs were reported in 3 subjects (9.4%) during the use of their usual brand cigarette. One TEAE occurring in 1 subject (3.1%) during the use of their usual brand cigarette was considered as treatment-related (cough). There were no serious adverse events, TEAEs of severe intensity or TEAEs leading to discontinuation of the study products. There were no clinically important changes in systolic blood pressure, diastolic blood pressure, pulse rate, body temperature or respiratory rate from pre-dose to any post-dose time point with any of the study products.
Discussion
The last decade has seen an increasing number of non-combustible tobacco and nicotine-containing products introduced to the consumer market. These include electronic cigarettes/vaping devices, Tobacco Heating Products (THPs), and oral products which deliver nicotine absent of tobacco. Furthermore, although they are not marketed to compete directly with consumer products, medicinal nicotine replacement therapies (NRT) such as nicotine gums, lozenges, inhalers and transdermal patches are also accessible as an aid to smoking cessation and freely available over-the-counter in many countries. In addition to capitalising on new technologies, the expansion in the number of new consumer nicotine products has been driven by the recognition that harm reduction may be a viable strategy for those who cannot or will not quit smoking combusted tobacco19. One such product type, THPs, have been shown to be, or evidence is accumulating which suggest that their use may be, less harmful than cigarette smoking, as estimated by reductions in exposure to known toxicants in cigarette smoke or favourable changes in biomarkers for cancer, cardiovascular disease and chronic obstructive pulmonary disorders26,27,28,29,30.
What is less well understood is whether there are differences regarding the abuse liability, and consequently the development of nicotine dependence, associated with the use of THPs, or regarding the abuse liability of THPs relative to combustible cigarettes. Furthermore, the possibility that there will be an indirect effect of alternative nicotine products in elevating nicotine use among non-smokers has been raised in the public health community50. In consequence, determination of abuse liability is now a regulatory requirement to obtain marketing authorisation for tobacco and nicotine-containing products in some countries, with the expectation that this will give an indication as to the proportion of individuals within a population who will become dependent on a new nicotine product. It is also of importance given the potential impact of alternative nicotine products on overall population health for manufacturers to understand the abuse liability of any new product.
The measurement of abuse liability in human subjects is complex. For tobacco and nicotine products, a consensual view is that assessment of abuse liability requires data examining both nicotine PK and subjective effects31,34,35,36. While the specifics of the types of subjective effects measures to assess are less well defined, recent studies on a variety of tobacco and nicotine products have examined measures of product liking, urge to smoke or to use the product again, and intent to use again, as well as assessing indices related to product use such as enjoyment, satisfaction, relief, and other negative and positive effects13,14,39,40,50, in order to generate comparative abuse liability assessments. Integrating these many different inputs into an overall abuse liability assessment commonly also involves comparing abuse liability of control products with high (cigarettes) and low (NRT inhaler) abuse liability34,45,47 and, taking all factors into account, providing a subjective assessment of relative abuse liability.
Overall, utilising such an integrated approach combining both nicotine PK and subjective effects, data obtained in this study demonstrate an abuse liability of the THP variants which is lower than that of participants’ usual brand cigarette yet higher than that of NRT inhaler. In terms of nicotine PK, the cigarette had approximately twofold higher Cmax than the two THPs; however, mean Tmax was similar for the THP compared with the cigarette, suggesting that nicotine absorption was similar between the two products. Notably, the NRT inhaler, a licensed medical product designed to relieve and/or prevent nicotine cravings and withdrawal symptoms associated with tobacco dependence and to promote smoking cessation, had an approximately fourfold lower Cmax and fourfold longer Tmax compared with the THPs. This indicates that because THPs more closely mimic the nicotine delivery of cigarettes than do NRT inhaler’s, smokers may find THP’s more acceptable as alternatives to cigarettes. These findings were supported by the subjective response questionnaires, which showed that the THPs were non-inferior to the NRT inhaler in terms of PLQ and OIUA scores, and superior to the NRT inhaler in terms of UFP score.
In terms of tobacco harm reduction, the abuse liability profile of THPs demonstrated here supports their role reducing in smoking-related harms. A product with low abuse liability likely will not be adopted or used extensively and may not encourage existing smokers to switch from using high-harm cigarettes to lower-harm THPs, and some degree of abuse liability of nicotine products has been proposed to support overall population tobacco harm reduction44,45. The potential trade-off of this abuse liability may be the potential for initiation and sustained use among nicotine non-users, or relapse into tobacco use by former smokers44,45,50. However, studies have shown that, for THPs, use is extremely uncommon (0.1%) among those with no smoking history while use among former smokers is also very uncommon (1%)52.
Until now only one study has examined the abuse liability42, and only one study has reported the nicotine PK41, of THPs. Thus, a major strength of this study is our rigorous assessment of the abuse liability of a THP in comparison to cigarettes and NRT inhaler, which was conducted in accordance with consensus standards for tobacco product abuse liability assessments. A further strength was the use of overnight confinement of participants within the clinic to ensure compliance with PK assessment procedures and abstention from tobacco product use. Limitations include assessment only of a single type of THP, and as such our findings may not be generalisable to other THPs currently marketed in many countries. Furthermore, only a single THP flavour (tobacco) was assessed and although flavour has been shown not to impact abuse liability of e-cigarettes13,14,39,40, we are unable to discern whether non-tobacco flavoured THPs have a different level of abuse liability. Lastly, the study is also limited by a single session which does not necessarily mimic real word product use where THP can have multiple sessions.
In conclusion, we have described findings from a study which rigorously assessed the abuse liability of a THP, demonstrating that the glo THP has a lower abuse liability than cigarettes but a higher abuse liability than NRT inhaler. Since THPs have lower emissions and reduced in vitro biological activity compared to cigarettes, and since switching to using THPs reduces exposure to harmful toxicants, the presence of some degree of abuse liability of the THP supports tobacco harm reduction such that it may provide an appealing and accepting alternative to cigarette smoking in adult smokers and be supportive of their switching away from harmful cigarette smoking.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
27 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-37432-2
References
Benowitz, N. L. Nicotine addiction. N. Engl. J. Med. 362, 2295–2303 (2010).
Farsalinos, K. E. & Polosa, R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Saf. 5, 67–86 (2014).
National Center For Chronic Disease Prevention And Health Promotion (Us) Office On Smoking And Health. The Health Consequences Of Smoking- 50 Years Of Progress: A Report Of The Surgeon General. (Centers For Disease Control And Prevention (Us), 2014).
Rodgman, A. & Perfetti, T. A. The Chemical Components Of Tobacco And Tobacco Smoke, Second Edition. (Taylor & Francis Group, 2013).
Fowles, J. & Dybing, E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 12, 424–430 (2003).
United States & Administration, F. A. D. Harmful and potentially harmful constituents in tobacco products and tobacco smoke established list. Fed. Reg. 77, 20034–20037 (2012).
Polosa, R. & Benowitz, N. L. Treatment of nicotine addiction: Present therapeutic options and pipeline developments. Trends Pharmacol. Sci. 32, 281–289 (2011).
Schneider, N. G., Olmstead, R. E., Franzon, M. A. & Lunell, E. The nicotine inhaler: Clinical pharmacokinetics and comparison with other nicotine treatments. Clin. Pharmacokinet. 40, 661–684 (2001).
Le Houezec, J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: A review. Int. J. Tuberc. Lung Dis. 7, 811–819 (2003).
Wadgave, U. & Nagesh, L. Nicotine Replacement therapy: An overview. Int. J. Health Sci. (Qassim) 10, 425–435 (2016).
Lunell, E., Molander, L., Ekberg, K. & Wahren, J. Site of nicotine absorption from a vapour inhaler—Comparison with cigarette smoking. Eur. J. Clin. Pharmacol. 55, 737–741 (2000).
Digard, H., Proctor, C., Kulasekaran, A., Malmqvist, U. & Richter, A. Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob. Res. 15, 255–261 (2013).
Stiles, M. F. et al. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: Implications for abuse liability. Psychopharmacology 234, 2643–2655 (2017).
Stiles, M. F. et al. Assessment of the abuse liability of three menthol vuse solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology 235, 2077–2086 (2018).
Barbeau, A. M., Burda, J., & Siegel, M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: A qualitative approach. Addict. Sci. Clin. Pract. 8(1), 5. https://doi.org/10.1186/1940-0640-8-5 (2013).
Hartmann-Boyce, J., Chepkin, S. C., Ye, W., Bullen, C. & Lancaster, T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 5, Cd000146. https://doi.org/10.1002/14651858.Cd000146.Pub5 (2018).
Benowitz, N. L., Hukkanen, J. & Jacob, P. 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 192, 29–60 (2009).
Stratton, K., Shetty, P., Wallace, R. & Bondurant, S. Clearing the smoke: the science base for tobacco harm reduction—Executive summary. Tob. Control. 10, 189–195 (2001).
Royal College Of Physicians. Nicotine Without Smoke. Tobacco Harm Reduction. A Report By The Tobacco Advisory Group Of The Royal College Of Physicians. (Rcp, 2016).
Design, P. et al. Assessment of tobacco heating product Thp1.0. Part 2. Regul. Toxicol. Pharmacol. 93, 4–13 (2018).
Schaller, J-P. Et Al. Evaluation of the tobacco heating system 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 81 Suppl 2, S27-S47 (2016).
Aerosol, C. C. C. O. H. A. P. H. et al. Assessment of novel tobacco heating product Thp1.0. Part 3. Regul. Toxicol. Pharmacol. 93, 14–33 (2018).
Jaunky, T. Et Al. Assessment of tobacco heating product Thp1.0. Part 5: In vitro dosimetric and cytotoxic assessment. Regul. Toxicol. Pharmacol. 93, 52–61 (2018).
Taylor, M. Et Al. Assessment of novel tobacco heating product Thp1.0. Part 6: A comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharmacol. 93, 62–70 (2018).
Comparative In Vitro Toxicological Evaluation. Thorne, D., Breheny, D., Proctor, C. & Gaca, M. Assessment of novel tobacco heating product Thp1.0. Part 7. Regul. Toxicol. Pharmacol. 93, 71–83 (2018).
Beatrice, F. & Massaro, G. Exhaled carbon monoxide levels in forty resistant to cessation male smokers after six months of full switch to electronic cigarettes (E-Cigs) or to a tobacco heating systems (Ths). Int. J. Environ. Res. Public Health. 16, 3916. https://doi.org/10.3390/Ijerph16203916 (2019).
Gale, N. et al. Changes In biomarkers of exposure on switching from a conventional cigarette to tobacco heating products: A randomized, controlled study in healthy Japanese subjects. Nicotine Tob. Res. 21, 1220–1227 (2019).
Gale, N. et al. Changes in biomarkers after 180 days of tobacco heating product use: A randomised trial. Intern. Emerg. Med. https://doi.org/10.1007/S11739-021-02798-6 (2021).
Haziza, C. Et Al. Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the menthol tobacco heating system 2.2 for three months (Part 1). Nicotine Tob. Res. 22, 539–548 (2019).
Lüdicke, F. et al. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: A randomized trial. Cancer Epidemiol. Biomarkers Prev. 28, 1934–1943 (2019).
Carter, L. P. et al. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev. 18, 3241–3262 (2009).
Vansickel, A. R., Cobb, C. O., Weaver, M. F. & Eissenberg, T. E. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomarkers Prev. 19, 1945–1953 (2010).
United States, Food and drug administration. Premarket tobacco product applications for electronic nicotine delivery systems (Ends). Guidance for industry. https://www.fda.gov/media/127853/download (2019).
Henningfield, J. E. & Keenan, R. M. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 61, 743–750 (1993).
Benowitz, N. L. Pharmacology of nicotine: addiction, smoking-induced disease and therapeutics. Annu. Rev. Pharmacol. Toxicol. 49, 57–71 (2009).
Henningfield, J. E., Hatsukami, D. K., Zeller, M. & Peters, E. Conference on abuse liability and appeal of tobacco products: conclusions and recommendations. Drug Alcohol Depend. 116, 1–7 (2011).
Effects, S. A. S. Walele, T., Sharma, G., Savioz, R., Martin, C. & Williams, J. A Randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B. Regul. Toxicol. Pharmacol. 74, 193–199 (2016).
Maloney, S. F. et al. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp. Clin. Psychopharmacol. 27, 443–454 (2019).
Goldenson, N. I., Buchhalter, A. R., Augustson, E. M., Rubinstein, M. L. & Henningfield, J. E. Abuse liability assessment of the juul system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 217, 108395. https://doi.org/10.1016/J.Drugalcdep.2020.108395 (2020).
Goldenson, N. I. Et Al. Abuse liability assessment of the juul system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 217, 108441. https://doi.org/10.1016/J.Drugalcdep.2020.108441 (2020).
Picavet, P., Haziza, C., Lama, N., Weitkunat, R. & Lüdicke, F. comparison of the pharmacokinetics of nicotine following single and ad libitum use of a tobacco heating system or combustible cigarettes. Nicotine Tob. Res. 18, 557–563 (2016).
Brossard, P. Et Al. Nicotine pharmacokinetic profiles of the tobacco heating system 2.2, cigarettes and nicotine gum in Japanese smokers. Regul. Toxicol. Pharmacol. 89, 193–199 (2017).
Mcneill, A. & Munafò, M. R. Reducing harm from tobacco use. J. Psychopharmacol. 27, 13–18 (2013).
Abrams, D. B. et al. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu. Rev. Public Health. 39, 193–213 (2018).
Abrams, D. B. et al. Managing nicotine without smoke to save lives now: evidence for harm minimization. Prev. Med. 117, 88–97 (2018).
Zeller, M. The future of nicotine regulation: Key questions and challenges. Nicotine Tob. Res. 21, 331–332 (2019).
Fagerström, K. & Eissenberg, T. Dependence on tobacco and nicotine products: A case for product-specific assessment. Nicotine Tob. Res. 14, 1382–1390 (2012).
Newland, N. et al. Evaluating the effects of switching from cigarette smoking to using a heated tobacco product on health effect indicators in healthy subjects: Study protocol for a randomized controlled trial. Intern. Emerg. Med. 14, 885–898 (2019).
Hatsukami, D. K., Zhang, Y., O’connor, R. J. & Severson, H. H. Subjective responses to oral tobacco products: scale validation. Nicotine Tob. Res. 15, 1259–1264 (2013).
Cahn, Z. et al. Applying the population health standard to the regulation of electronic nicotine delivery systems. Nicotine Tob. Res. 23, 780–789 (2021).
Liu, J. et al. Characterization of the abuse potential in adult smokers of a novel oral tobacco product relative to combustible cigarettes and nicotine polacrilex gum. Clin. Pharmacol. Drug Dev. 10, 241–250 (2021).
Adamson, J. Et Al. Results from a 2018 cross-sectional survey In Tokyo, Osaka and Sendai to assess tobacco and nicotine product usage after the introduction of heated tobacco products (Htps) in Japan. Harm Reduct. J. 17, 32. https://doi.org/10.1186/S12954-020-00374-3 (2020).
Acknowledgements
The authors acknowledge the expert assistance of the study CRO, Cromsource Srl (Verona, Italy); of Neil Sherwood, PhD in the design of the study; of ABF GmbH (Planegg, Germany) in providing bioanalytical services; of Charlie Brindley, PhD of KinetAssist Ltd (Quothquan, UK) in performing the PK analysis; and of Ian Fearon, PhD of whatIF? Consulting Ltd (Harwell, UK) in supporting the writing of this manuscript.
Funding
The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial.
Author information
Authors and Affiliations
Contributions
G.H. contributed substantially to the conception and design of the study and led the drafting of the manuscript. N.G. was the Project Lead on this study and was principally responsible for all aspects of the study design, protocol development and study conduct, and contributed substantially to manuscript drafting and revision. J.M., and C.J.P. contributed substantially to the conception of the study and, along with M.M., contributed to the technical development of the study design and the protocol, as well as reviewing the manuscript. S.M. and L.Z. led the acquisition of study data, provided technical expertise on the study design and contributed significantly to analysis and interpretation of the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, the Supplementary Information file was omitted from the Supplementary Information section.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hardie, G., Gale, N., McEwan, M. et al. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep 12, 14701 (2022). https://doi.org/10.1038/s41598-022-19167-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19167-8
- Springer Nature Limited