Abstract
Gender specific all-cause mortality risk associated with a high somatic symptom burden (SSB) in a population-based cohort was investigated. The study population included 5679 women and 5861 men aged 25–74 years from the population-based MONICA/KORA Cohort. SSB was assessed following the Somatic Symptom Scale-8 and categorized as very high (≥ 95th percentile), high (60–95th percentile), moderate (30–60th percentile), and low (≤ 30th percentile). The impact of SSB on all-cause mortality risk within a mean follow-up period of 22.6 years (SD 7.1; 267,278 person years) was estimated by gender-specific Cox regression models adjusted for sociodemographic, lifestyle, somatic and psychosocial risk factors, as well as pre-existing medical conditions. Approximately 5.7% of men and 7.3% of women had very high SSB. During follow-up, 3638 (30.6%) mortality cases were observed. Men with a very-high SSB had 48% increased relative risk of mortality in comparison to men with a low SSB after adjustment for concurrent risk factors (1.48, 95% CI 1.20–1.81, p < .0001), corresponding to 2% increased risk of mortality for each 1-point increment in SSB (1.02; 95% CI 1.01–1.03; p = 0.03). In contrast, women with a very high SSB had a 22% lower risk of mortality (0.78, 95% CI 0.61–1.00, p = 0.05) and women with high SSB had an 18% lower risk of mortality (0.82; 95% CI 0.68–0.98, p = 0.03) following adjustment for concurrent risk factors. The current findings indicate that an increasing SSB is an independent risk factor for mortality in men but not in women, pointing in the direction of critical gender differences in the management of SSB, including women’s earlier health care utilization than men.
Similar content being viewed by others
Introduction
All individuals experience some extent of somatic symptoms during their lifetime, often manifesting as pain, fatigue, dizziness, shortness of breath, or perceived disturbances of organ functions. However, in up to 20% of the general population1 and a third of clinical populations2 somatic symptoms remain an ongoing source of distress3, particularly among women4,5,6,7. Despite the considerable prevalence of a high SSB, the nature and long-term outcomes of somatic symptoms remain poorly understood. Current explanatory models are integrative in nature, attributing perceived symptoms to an interplay of gender, socioeconomic, biological and psychological factors, as well as prior expectations of the individual8. So far, there is substantial evidence that SSB follows the the course of underlying diseases9,10 as well as deteriorating health associated with aging4,6,7,9,10,11,12,13,14.
Nevertheless, increasing evidence suggests that the severity of somatic symptoms can be applied as an independent measure of health in the general population12. According to a population-based review of 9 studies including 28,377 participants, a score of somatic symptom burden can predict general health status, disability and healthcare use beyond that of important confounders including depression, anxiety and the presence or absence of a medical condition14. Most importantly, this review further demonstrates that the effect of somatic symptom burden on health status or health care costs are similar whether the symptoms are driven by organic disease or are medically unexplained15,16,17,18,19, highlighting that it is the degree of somatic symptom burden that needs to be considered as an independent risk factor of ill health.
Early evidence from prospective studies indicate that a high somatic symptom burden is additionally predictive of mortality among community dwelling older adults20,21. In 1583 participants aged between 75 and 95 years, 28% of participants who had a high somatic symptom burden had 2.08 (1.49–2.90) higher odds of mortality during 5 years of follow-up, even following adjustment for age, gender, and medical comorbidities20. Similarly, in 3498 participants with a mean age of 69 years, the total physical symptom count was predictive of 1-year death even after controlling for clinical characteristics, chronic medical conditions, self-rated health, and affective symptoms (OR 1.10, 95% CI 1.07–1.13)21. However, as these studies are limited to older populations with relatively short follow-up periods, and do not include gender-specific differences, the effect of a high somatic symptom burden on the relative risk of mortality in men and women from the general population remains unknown.
In the current investigation, using a representative sample of participants from the general population, followed for a mean of approximately 22 years, we will assess whether somatic symptom burden is associated with an increased risk of all-cause mortality while considering a wide range of sociodemographic, lifestyle, somatic, and psychosocial confounders derived from a priori research on somatic symptom burden. Furthermore, it is well accepted in psychosomatic clinical settings that somatic symptoms have a substantial gender component which cannot be overlooked22—gender differences in somatic symptoms are so prominent that an eventual diagnosis of somatic symptom disorder is estimated to affect more women than men at a ratio of 10:123. Hence, the ensuing analyses will be gender-specific to address the gender discrepancies in somatic symptom reporting3,4,5,6, namely, to examine whether the higher prevalence of increased somatic symptom burden in women leads to a higher risk for mortality in women in comparison to men.
Methods
Participants
The study population was taken from three independent baseline surveys including 13,426 participants aged between 25 and 74 years-old who participated in the Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA)/Cooperative Health Research in the Region of Augsburg (KORA) cohort study. The three baseline surveys were conducted in 1984/1985, 1989/1990, and 1994/1995 as part of the multinational World Health Organization (WHO) MONICA project24. The follow-up mortality data was obtained from the KORA GEFU 4 study (Cooperative Health Research in the Region of Augsburg, Health Follow-up 4) in 2016. Detailed information on the study design, recruitment process and data collection of the KORA studies have been described elsewhere25. All procedures contributing to this work comply with the ethical standards of the relevant committees and comply with the Helsinki Declaration of 1975, as revised in 201326. In the current analysis, missing follow up data (n = 102), and missing covariates at baseline (n = 1784) led to a pooled sample 11,540 participants. Drop out analyses revealed that participants with missing data were more likely to be older (p < 0.001), have higher levels of depression (p < 0.001).
Socio-demographic and lifestyle factors
Gender (woman/man) was self-reported, without further differentiation of biological sex or gender identity. Low educational level was considered as having < 12 years of schooling24. Employment status was based on self-reported information from the participants24. Physical activity was considered as engaging in physical activity on average of ≥ 1 h/week throughout the year27. Smoking was based on current smoking of ≥ 1 cigarette/day28. Alcohol consumption was based on three categories including ‘none-low’, ‘moderate’ and ‘high’ (categorical men: 0 g/day, 0.1–39.9 g/day, ≥ 40 g/day; categorical women: 0 g/day, 0.1–19.9 g/day, ≥ 20 g/day)29. Living arrangement was assessed by whether the individual currently lives alone, irrespectively of the current relationship status30.
Somatic factors
Blood pressure was measured on the right arm in a sitting position using a Hawksley random-zero sphygmomanometer, and three were taken half an hour after the clinical interview in 3-min intervals. Blood pressure was assessed by obtaining the average of the latter two repeated-blood pressure measurements, and hypertension was defined as ≥ 140/90 mmHg and/or use of antihypertensive medication31. Total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were measured as mg/dL in serum by enzymatic methods (CHOD-PAP, Boehringer Mannheim, Germany) and dyslipidemia was defined as the ratio of total cholesterol to high-density lipoprotein cholesterol ≥ 5.032. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared and obesity was defined as having a BMI ≥ 30 kg/m2 33.
Assessment of pre-existing medical conditions and recent health care use
The participants were asked for the following pre-existing medical conditions, diagnosed and treated by a physician within the last 12 months: any form of cancer, cardiac insufficiency, angina pectoris, coronary heart disease, myocardial infarction, stroke, circulatory disorder in arms or legs, thrombosis/phlebitis/varicosis, diabetes,hyperlipidemia/hypercholesterolemia, hyperuricemia/gout, metabolic syndrome, chronic bronchitis, lung/bronchial asthma, arthrosis, rheumatoid arthritis, gastrointestinal illness/ulcer, kidney disease, liver disease, goiter or another thyroid disease12. Participants were categorized accordingly into ‘no pre-existing medical conditions,’ ‘1 pre-existing medical condition’ or ‘ ≥ 2 pre-existing conditions (multimorbidity)’. Recent health care was considered as having had a health care visit at least once within the last 4 weeks12.
Psychosocial risk factors
Depressed-mood was assessed by the depression and exhaustion subscale (DEEX) of the von Zerssen symptom checklist34, whereby participants in the top tertile were considered to have ‘high depressed-mood’, participants in the middle tertile were considered as having ‘moderate depressed mood’ and participants in the lowest tertile were considered as having ‘no/low depressed mood’. Social network was assessed by the Berkman-Syme's Social Network Index, and the components of the index are weighted in an algorithm resulting in four categories35, whereby social isolation was defined as low intimate contacts—not married, fewer than six friends or relatives, and no membership in either church or community groups.
Assessment of somatic symptom burden
Somatic symptom burden was assessed by creating a symptom severity score, based on to the previously established Somatic Symptom Scale-8 (SSS-8)19. Eight somatic symptoms comprising the same categories as the SSS-8 were derived from the von Zerssen symptom checklist36, including bowel pain, back pain, pain in the joints, headaches or pressure in the head, temporary shortness of breath, dizziness, feeling tired and insomnia37. Each item was measured on a four-point scale ranging from 0 (not present) to 3 (strong) leading to a somatic symptom score ranging from 0 to 24. The distribution was approximately normal and Cronbach’s α was estimated as 0.75 in the present study indicating a good reliability37. The somatic symptom burden score was analyzed as a continuous variable, and additionally categorized as low (up to 30th percentile), moderate (30–60th percentile), high (60–95th percentile) and very high (> 95th percentile), which is interpreted as the clinical cut-off in practice19.
Follow-up and mortality endpoints
Death certificates were obtained from local health departments and coded for the underlying cause of death by trained personnel using the 9th revision of the International Classification of Diseases (ICD-9). In the mean 22.6-year follow-up (SD ± 7.1 years; max: 32 years; 267,278.508 person years), there were 3638 fatal events. For mortality analyses, event times were calculated as time to death. Subjects without events or with loss to follow-up were censored at the time point of the last follow-up31.
Statistical analyses
Baseline characteristics of the study population were gender-stratified and according to the severity of somatic symptom burden. Significance of differences between groups were compared using Post Hoc Anova for continuous variables and Multivariate Logistic regression for categorical variables.
Gender stratified mortality rates were calculated according to the severity of somatic symptom burden using Poisson regression with offset38. Gender stratified Cox proportional-hazards models were computed to assess the association of somatic symptom burden with all-cause mortality in men and women, where low somatic symptom burden was considered as the reference group39. The confounding risk factors in the models were chosen based on a priori evidence of factors that are associated with SSB and fitted in a forward stepwise regression. Three multivariate models stratified by gender and adjusted for (1) sociodemographic (age, employment) and lifestyle factors (smoking, alcohol consumption, physical activity) (2) somatic factors (hypertension, hypercholesterolemia, recent health care, 1 pre-existing medical condition or multimorbidity) and (3) psychosocial factors (depressed mood, lives alone, social isolation) were calculated. Model 3 included all primary risk factors. All models included adjustment for ‘survey’, as there were 3 waves of data collection. The magnitude of confounding was computed by comparisons of the estimated measures of associations for the crude and each consecutive model. If the difference of between measures of association were more than 10%, then confounding was considered present. In order to assess potential protopathic bias, a time lag was introduced by excluding the outcome variable in the first year following data collection40,41. Sensitivity analyses included multiplicative interaction testing for modification with the risk factors in the fully adjusted model, first in the total population, followed by in men and women separately. Proportional hazards could be estimated by fitting models stratified by the risk factor categories and plotting the log–log survival curves for each risk factor, which were assessed for parallelism by visual inspection and statistical testing of the Schoenfeld residuals42,43. As deviations from parallelism were not observed for any SSB categories and the global p-values for Schoenfeld residuals did not reach statistical significance in any models, proportional hazards were assumed44. Sensitivity analyses included assessment of modification by all risk factors in the total sample using multiplicative interaction in Model 3.
A p value < 0.05 was considered as statistically significant for main analyses and interactions. All statistical evaluations were performed using SAS 9.4. The analysis and the description in this manuscript follow the STROBE guidelines for cohort studies45.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
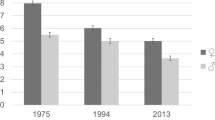
The present data were derived from a total of 5679 women and 5861 men with a mean age of 46.6 (SD 13.2) and 47.8 (SD 13.5) years, respectively. As shown in Fig. 1, men had a higher prevalence of a high somatic symptom burden (29.0% vs. 25.9%), whereas women had a higher prevalence of a very high (7.3% vs. 5.7%) somatic symptom burden (test for trend p < 0.0001).
The baseline characteristics presented in Table 1 revealed a dose–response relationship between the severity of somatic symptom burden and increasing concurrent risk factors in both men and women—participants with an increasing severity of somatic burden were older, unemployed, or retired, less educated, physically inactive, more frequently had hypertension, hypercholesterolaemia, pre-existing medical conditions, and more frequent visits to a physician in comparison to participants with a low somatic symptom burden. The exception to this trend was for smoking and alcohol consumption, which decreased with increasing somatic symptom burden. Furthermore, a significant difference between men and women was found for the use of recent health care—women with high somatic symptoms were more likely than men to have had used recent health care, whereas men with very high symptoms had used more recent health care than women.
As further shown in Table 1, there was a strong link between the severity of somatic symptom burden and psychosocial risk factors. Namely, up to 80% of men and 70% of women with a very high symptom burden had severe depressed-mood—in stark contrast to 4.3% of women and 3.9% of men with a low symptom burden. However, gender differences were found in the associations between social relationships and somatic symptom burden, whereby women who lived alone and/or were socially isolated had a substantially higher somatic symptom burden, but men did not experience an association between their social relationships and somatic symptom burden.
All-cause mortality risk
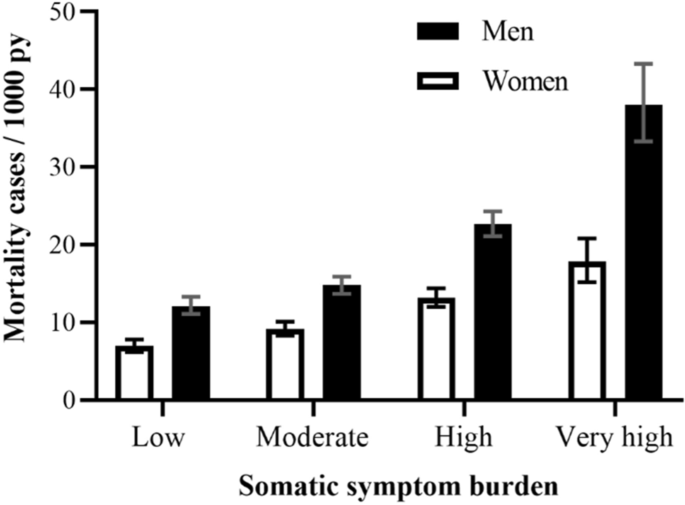
During a mean follow-up period of 22.6 years (SD 7.1), 3531 (30.6%) mortality cases were observed. As displayed by the survey-adjusted absolute mortality rates in Fig. 2, men, and women with increasing SSB levels experienced higher mortality rates / 1000 person-years in comparison to those with low SSB, but statistical significance was only reached in men (p < 0.0001) and not in women (p = 0.21).
Nevertheless, following adjustment for concurrent risk factors, the relative risk of all-cause mortality according to somatic symptom burden led to opposing yet significant results for men and women (Table 2). Men who reported a very high somatic symptom burden had a 48% higher risk of mortality than men with a low somatic symptom burden that remained significant even after adjustment for sociodemographic, lifestyle, somatic, and psychosocial risk factors, as well as pre-existing medical conditions (HR 1.48; 95% CI 1.12–1.81, p = 0.0003). Likewise, using the continuous somatic symptom burden score, the fully adjusted model showed that men had a 2% increased risk of mortality for each 1-point increment in somatic symptom burden (HR 1.02; 95% CI 1.01–1.03; p = 0.03). In contrast, women who presented a high somatic symptom had an 18% lower risk of mortality (HR 0.82; 95% CI 0.68–0.98, p = 0.03), and women with a very high somatic symptom burden had a 22% lower risk or mortality (HR 0.78; 95% CI 0.61–1.00, p = 0.05) in comparison to women with a low somatic symptom burden. The lower risk of all-cause mortality in women with an increasing somatic symptom burden was also evident when using the continuous somatic symptom score in the fully adjusted model, although statistical significance was not reached (HR 0.99; 95% CI 0.97–1.00, p = 0.18).
The magnitude of confounding in the somatic symptom burden and all-cause mortality link showed significant confounding effects by concurrent risk factors (Table 3). Socioeconomic factors including age, education and employment had the largest confounding effect, substantially decreasing the crude all-cause mortality risk of SSB in men and women. The lowest level of confounding was by concurrent psychosocial risk factors, including depressed mood, living alone and social isolation, whereby men had a negative magnitude of confounding, indicating that the association is underestimated in men.
Lastly, although multimorbidity as a concurrent risk factor was substantially significant within the somatic symptom burden and mortality link for women (1.32, 95% CI 1.14–1.53, p < 0.0001) and men (1.27, 95% CI 1.13–1.42, p < 0.0001), there was no statistical interaction between pre-existing medical conditions and severity of somatic symptom burden (women p = 0.17, men p = 0.37), indicating that pre-existing medical conditions does not modify the effect of somatic symptom burden on mortality.
Introduction of a time lag excluding mortality in the first year after data collection (n = 60), attenuated the long-term relative risk between SSB and all-cause mortality to non-significance in women with a very high SSB (Model 3: HR 0.79, 95% CI 0.62–1.01, p = 0.06) but remained unchanged in women with a high SSB (Model 3: HR 0.82; 95% CI 0.68–0.99; p = 0.03). Furthermore, the significant relative risk of all-cause mortality in men with a very high SSB did not considerably differ following the introduction of a time lag (Model 3: HR 1.46; 1.19–1.79, p = 0.0003).
Sensitivity analyses
The modification effect by each concurrent risk factor was computed in the association between somatic symptom burden and all-cause mortality (Model 3). Gender (p < 0.0001) was the only significant modified in the total sample. Gender specific analyses revealed that depressed-mood (p < 0.0001) and physical inactivity (p = 0.05) were significant effect modifiers for men, whereas no significant modifiers were observed for women.
Discussion
In the present investigation including 5679 women and 5861 men from the general population, 29.1% of men and 25.9% women reported high, while 5.7% of men and 7.3% of women reported very high somatic symptoms. Although both men and women with increasing somatic symptom burden suffered from psychosocial and somatic risk factors7,14, men generally had worse health, but women were more likely to report a ‘very high’ somatic symptom burden3,4,5,6. Despite this, women with a ‘high’ somatic symptom burden were more likely to have received recent health care, whereas men were more likely to wait until their symptoms advanced to ‘very high’. Correspondingly, very high somatic symptom burden was independently associated a 48% increased risk of mortality in men during a mean of 22.6 years of follow-up, even following adjustment confounding risk factors, and significant modification by depressed-mood and physical inactivity. In contrast, somatic symptom burden had a protective role against mortality in women—the relative risk of mortality was 18% lower for women with high and 22% lower for women with very high symptom burden following adjustment, potentially due to their readiness to seek health care earlier than men46.
The present findings confirmed and extended the prospective association between somatic symptom burden and all-cause mortality in an existing study. Namely, in the MONICA/KORA population-based study including 11,895 participants aged 24–75 years-old and followed for 12 years, excessive symptom reporting (ESR) in men with no chronic diseases demonstrated a measurable increased survival benefit compared to chronic disease participants with high symptom reporting (HR 0.68; 95% CI 0.48–0.97), however, in women, the most favorable outcome emerged in the population with high symptom reporting and no chronic disease (HR 0.68; 95% CI 0.36–1.31; P = 0.25)12. This finding, although not statistically significant, was attributed to ‘greater female-specific awareness of interoceptive cues and readiness to seek medical help may have led to more prevention-oriented behavior in women’12, comparably to the current findings. However, we have found further evidence that somatic symptom burden may have a significant protective effect against mortality in women when concurrent risk factors are considered, and an independent risk factor mortality in men, even in the presence or absence of pre-existing medical conditions. Specifically, the magnitude of confounding revealed that the most significant risk factors to consider are socioeconomic factors including age, education, and employment status. Although additional studies were also in line with our findings for the somatic symptom burden and mortality link in men15,21, these studies were not gender-specific and included a substantially older study population, hence direct comparison to the current study is not feasible.
The prospective findings herein can be elucidated in light of the male–female health survival paradox47, that is, although females are more often ill48,49 they tend to have a longer life expectancy than males47. The underlying reasons for this paradox are not fully understood, but ‘feminine gender characteristics’50, including women’s processing and handling of somatic symptoms by readiness to seek medical help may contribute46. In addition, neurobiological mechanisms involving the female sex hormone, estrogen, are thought to increase the pain sensation in women, partially due to presence of estrogen receptors on nerve cells51,52,53,54. In turn, the amplification of pain mechanisms can activate an inflammatory response55, inducing more ‘sickness behaviour’56,57. However, emerging findings show that estrogen may also down regulate systematic inflammation—leading to an accelerated and more efficient inflammatory (and perhaps secondary anti-inflammatory) response compared with males58. Essentially, although females may experience somatic symptoms before males22, they may also develop protective mechanisms sooner and more effectively. Hence, the current findings suggest that somatic symptoms may indirectly increase women’s lifespan by adaptive behavioural or biological mechanisms, although these longer years may not necessarily be healthy years49,59.
The limitation of the current observational study is that direct cause and effect conclusions between somatic symptom burden and mortality cannot be discerned. Drop out analyses revealed that excluded participants with missing data were more likely to be older and have higher depressed mood in comparison to the current sample, hence our results might have underestimated the effect of SSB on all-cause mortality. Furthermore, although we have adjusted for a comprehensive set of confounding variables, we cannot exclude that risk factors not included herein may have biased the current results. For instance, the contrasting association between SSB and mortality in men and women may be linked to different comorbidity patterns and differences in severity of disease in men and women. Even though we have adjusted our analyses for somatic factors (hypertension, hypercholesterolemia and multimorbidity), other diseases/comorbidities and/or more severe disease in men that were not specifically included into the analysis might have an influence on all-cause mortality60. The current analyses did not indicate a substantial protopathic bias, however, the protective association between a very high SSB and all-cause mortality risk in women was attenuated to non-significance (p = 0.06) following the introduction of a time lag. This finding suggests that additional measures taken by women with a very high SSB (e.g., increased use of mental health services in women61) could have led to reverse causality. However, the heterogeneity of a large sample of participants randomly drawn from the population, the comprehensive set of variables, and the long follow-up period is expected to increase the validity of the current study.
In conclusion, the current findings have provided a real-world perspective of men and women suffering from somatic symptom burden in the community. An increasing somatic symptom burden, assessed by a brief somatic symptom inventory, has confirmed the exceeding concurrent cluster of risk factors in men and women14. Furthermore, following adjustment for these risk factors, somatic symptom burden was associated with an increased risk of mortality in men, yet had a protective role against mortality in women, potentially due to women’s sooner use of health care. Hence, efforts to improve somatic symptom burden at a population level must begin in primary care settings by increasing effective communication and encouragement for patients to overcome ‘white-coat silence’ and voice their concerns, with a particular focus on men62. For instance, public health initiatives aiming to improve men’s negative attitudes towards seeking health care such as ‘MENtion it to a doctor’ campaign by the Cleveland Clinic are endorsed63. Lastly, with respect to therapeutic interventions in managing somatic symptom burden3, a multidisciplinary health care approach that effectively addresses the exceedingly high comorbidity between somatic symptom burden, depressed-mood64 and unhealthy behaviors are recommended.
Data availability
The data could be requested from the MONICA/KORA-Myocardial Infarction Registry Augsburg, Germany.
Change history
29 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-25157-7
References
Hilderink, P. H., Collard, R., Rosmalen, J. G. M. & Oude Voshaar, R. C. Prevalence of somatoform disorders and medically unexplained symptoms in old age populations in comparison with younger age groups: A systematic review. Ageing Res. Rev. 12(1), 151–156 (2013).
Haller, H., Cramer, H., Lauche, R. & Dobos, G. Somatoform disorders and medically unexplained symptoms in primary care: A systematic review and meta-analysis of prevalence. Dtsch. Arztebl. Int. 112(16), 279 (2015).
Henningsen, P., Zipfel, S., Sattel, H. & Creed, F. Management of functional somatic syndromes and bodily distress. Psychother. Psychosom. 87(1), 12–31 (2018).
Beutel, M. E. et al. Somatic Symptoms in the German General Population from 1975 to 2013. Sci. Rep. 10(1), 1595 (2020).
Kroenke, K. & Spitzer, R. L. Gender differences in the reporting of physical and somatoform symptoms. Psychosom. Med. 60(2), 150–155 (1998).
Beutel, M. E. et al. Somatic symptom load in men and women from middle to high age in the Gutenberg Health Study: Association with psychosocial and somatic factors. Sci. Rep. 9(1), 4610 (2019).
Ladwig, K.-H., Marten-Mittag, B., Formanek, B. & Dammann, G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur. J. Epidemiol. 16(6), 511–518 (2000).
Henningsen, P. et al. Persistent physical symptoms as perceptual dysregulation: A neuropsychobehavioral model and its clinical implications. Psychosom. Med. 80(5), 422–431 (2018).
Creed, F. H. et al. The epidemiology of multiple somatic symptoms. J. Psychosom. Res. 72(4), 311–317 (2012).
Creed, F., Tomenson, B., Chew-Graham, C., Macfarlane, G. & McBeth, J. The associated features of multiple somatic symptom complexes. J. Psychosom. Res. 112, 1–8 (2018).
Molarius, A. & Janson, S. Self-rated health, chronic diseases, and symptoms among middle-aged and elderly men and women. J. Clin. Epidemiol. 55(4), 364–370 (2002).
Ladwig, K. H. et al. Screening for multiple somatic complaints in a population-based survey: Does excessive symptom reporting capture the concept of somatic symptom disorders?: Findings from the MONICA-KORA Cohort Study. J. Psychosom. Res. 68(5), 427–437 (2010).
McAteer, A., Elliott, A. M. & Hannaford, P. C. Ascertaining the size of the symptom iceberg in a UK-wide community-based survey. Br. J. Gen. Pract. 61(582), e1–e11 (2011).
Tomenson, B. et al. Total somatic symptom score as a predictor of health outcome in somatic symptom disorders. Br. J. Psychiatry 203(5), 373–380 (2013).
Jackson, J. et al. Number of bodily symptoms predicts outcome more accurately than health anxiety in patients attending neurology, cardiology, and gastroenterology clinics. J. Psychosom. Res. 60(4), 357–363 (2006).
Kroenke, K. et al. Somatic symptoms in patients with cancer experiencing pain or depression: Prevalence, disability, and health care use. Arch. Intern. Med. 170(18), 1686–1694 (2010).
Sogutlu, A., Levenson, J. L., McClish, D. K., Rosef, S. D. & Smith, W. R. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: The PiSCES project. Psychosomatics 52(3), 272–279 (2011).
Creed, F. The relationship between somatic symptoms, health anxiety, and outcome in medical out-patients. Psychiatric Clin. 34(3), 545–564 (2011).
Gierk, B. et al. The somatic symptom scale-8 (SSS-8): A brief measure of somatic symptom burden. JAMA Intern. Med. 174(3), 399–407 (2014).
Lehti, T. E. et al. Symptom Burden is associated with psychological wellbeing and mortality in older adults. J. Nutr. Health Aging 25, 1–5 (2020).
Michael, C. S. et al. Physical symptoms as a predictor of health care use and mortality among older adults. Am. J. Med. 118(3), 301–306 (2005).
Barsky, A. J., Peekna, H. M. & Borus, J. F. Somatic symptom reporting in women and men. J. Gen. Intern. Med. 16(4), 266–275 (2001).
Kurlansik, S. L. & Maffei, M. S. Somatic symptom disorder. Am. Fam. Physician 93(1), 49–54 (2016).
Lowel, H. et al. The MONICA Augsburg surveys–basis for prospective cohort studies. Gesundheitswesen 67(Suppl 1), S13–S18 (2005).
Holle, R., Happich, M., Löwel, H. & Wichmann, H.-E. KORA-a research platform for population based health research. Das Gesundheitswesen. 67(S 01), 19–25 (2005).
World Medical A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20), 2191–2194 (2013).
Emeny, R. et al. Job strain associated CRP is mediated by leisure time physical activity: Results from the MONICA/KORA study. Brain Behav. Immun. 26(7), 1077–1084 (2012).
Meisinger, C., Döring, A., Thorand, B. & Löwel, H. Association of cigarette smoking and tar and nicotine intake with development of type 2 diabetes mellitus in men and women from the general population: The MONICA/KORA Augsburg Cohort Study. Diabetologia 49(8), 1770–1776 (2006).
Schneider, B. et al. The effect of risky alcohol use and smoking on suicide risk: Findings from the German MONICA/KORA-Augsburg Cohort Study. Soc. Psychiatry Psychiatr. Epidemiol. 46(11), 1127–1132 (2011).
Kandler, U., Meisinger, C., Baumert, J., Löwel, H. & Group, K. S. Living alone is a risk factor for mortality in men but not women from the general population: A prospective cohort study. BMC Public Health 7, 335 (2007).
Atasoy, S., Johar, H., Peters, A. & Ladwig, K. H. Association of hypertension cut-off values with 10-year cardiovascular mortality and clinical consequences: A real-world perspective from the prospective MONICA/KORA study. Eur. Heart J. 40(9), 732–738 (2019).
Löwel, H. et al. The MONICA Augsburg surveys–basis for prospective cohort studies. Gesundheitswesen 67(Suppl 1), S13–S18 (2005).
Atasoy, S., Johar, H., Fang, X. Y., Kruse, J. & Ladwig, K. H. Cumulative effect of depressed mood and obesity on type II diabetes incidence: Findings from the MONICA/KORA cohort study. J. Psychosom. Res. 115, 66–70 (2018).
Zerssen, Dv. & Koeller, D. M. Klinische Selbstbeurteilungs-Skalen (KSb-S) aus dem Münchner Psychiatrischen Informationssystem (PSYCHIS München). Die Beschwerdenliste. Parallelformen BL, B-L’und Ergänzungsbogen B-Lo. Manual. Weinheim: Beltz Test Gesellschaft (1976).
Berkman, L. F. & Syme, S. L. Social networks, host resistance, and mortality: A nine-year follow-up study of alameda county residents. Am. J. Epidemiol. 185(11), 1070–1088 (2017).
Zerssen, D. Klinische Selbstbeurteilungs-Skalen (KSB-S) aus dem Münchener Psychiatrischen Informations-System (Beltz, 1976).
Baumert, J. et al. A pattern of unspecific somatic symptoms as long-term premonitory signs of type 2 diabetes: Findings from the population-based MONICA/KORA cohort study, 1984–2009. BMC Endocr. Disord. 14, 87 (2014).
Austin, P. C., Stryhn, H., Leckie, G. & Merlo, J. Measures of clustering and heterogeneity in multilevel P oisson regression analyses of rates/count data. Stat. Med. 37(4), 572–589 (2018).
Cox, D. R. Regression models and life-tables. J. R. Stat. Soc.: Ser. B (Methodol.) 34(2), 187–202 (1972).
Tamim, H., Monfared, A. A. T. & LeLorier, J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol. Drug Saf. 16(3), 250–258 (2007).
Faillie, J.-L. Indication bias or protopathic bias?. Br. J. Clin. Pharmacol. 80(4), 779 (2015).
Bouliotis, G. & Billingham, L. Crossing survival curves: Alternatives to the log-rank test. Trials 12(1), 1 (2011).
Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 69(1), 239–241 (1982).
Emmert-Streib, F. & Dehmer, M. Introduction to survival analysis in practice. Mach. Learn. Knowl. Extract. 1(3), 1013–1038 (2019).
Cuschieri, S. The STROBE guidelines. (1658–354X (Print)).
Thompson, A. E. et al. The influence of gender and other patient characteristics on health care-seeking behaviour: A QUALICOPC study. BMC Fam. Pract. 17(1), 38 (2016).
Alberts, S. C. et al. The Male-Female Health-Survival Paradox: A Comparative Perspective on Sex Differences in Aging and Mortality: Sociality, hierarchy, Health: Comparative Biodemography: A Collection of Papers (National Academies Press (US), 2014).
Turabian, J. L. Is the meaning of symptoms the same in women and men. J. Women’s Health Care. 6(376), 2167–2420 (2017).
Freedman, V. A., Wolf, D. A. & Spillman, B. C. Disability-free life expectancy over 30 years: A growing female disadvantage in the US population. Am. J. Public Health 106(6), 1079–1085 (2016).
Ballering, A. V., Bonvanie, I. J., Hartman, T. C. O., Monden, R. & Rosmalen, J. G. M. Gender and sex independently associate with common somatic symptoms and lifetime prevalence of chronic disease. Soc. Sci. Med. 253, 112968 (2020).
Chrousos, G. P. Stress and sex versus immunity and inflammation. Sci. Signal. 3(143), pe36 (2010).
Kozlowska, K., Scher, S. & Helgeland, H. The Immune-Inflammatory System and Functional Somatic Symptoms: Functional Somatic Symptoms in Children and Adolescents 175–201 (Springer, 2020).
Sun, L. et al. Estrogen modulation of visceral pain. J. Zhejiang Univ.-Sci. B. 20(8), 628–636 (2019).
Traub, R. J. & Ji, Y. Sex differences and hormonal modulation of deep tissue pain. Front. Neuroendocrinol. 34(4), 350–366 (2013).
Derry, H. M., Padin, A. C., Kuo, J. L., Hughes, S. & Kiecolt-Glaser, J. K. Sex differences in depression: Does inflammation play a role?. Curr. Psychiatry Rep. 17(10), 78 (2015).
Capuron, L. & Miller, A. H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 130(2), 226–238 (2011).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 9(1), 46–56 (2008).
Casimir, G. J. A. & Duchateau, J. Gender differences in inflammatory processes could explain poorer prognosis for males. J. Clin. Microbiol. 49(1), 478–479 (2011).
Stephan, A.-J. et al. Living longer but less healthy: The female disadvantage in health expectancy: Results from the KORA-Age study. Exp. Gerontol. 145, 111196 (2021).
Corrao, S. et al. Sex-differences in the pattern of comorbidities, functional independence, and mortality in elderly inpatients: Evidence from the RePoSI register. J. Clin. Med. 8(1), 81 (2019).
Pattyn, E., Verhaeghe, M. & Bracke, P. The gender gap in mental health service use. Soc. Psychiatry Psychiatr. Epidemiol. 50(7), 1089–1095 (2015).
Baker, P. Men’s health: Time for a new approach. Phys. Ther. Rev. 23(2), 144–150 (2018).
Cleveland Clinic. Guys: Got a Nagging Health Concern? MENtion It! 2019 [Available from: https://health.clevelandclinic.org/mention-it-mens-health/.
Simon, G. E., VonKorff, M., Piccinelli, M., Fullerton, C. & Ormel, J. An international study of the relation between somatic symptoms and depression. N. Engl. J. Med. 341(18), 1329–1335 (1999).
Funding
Open Access funding enabled and organized by Projekt DEAL. The KORA research platform and the KORA Augsburg studies are financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria.
Author information
Authors and Affiliations
Contributions
S.A., C.H.W., C.R., H.S. and P.H. designed the study. S.A. and C.H.W. wrote the main manuscript text. S.A., H.J. and H.S. conducted statistical analyses. P.H. supervised the project. A.P. and K.H.L. helped supervise the project and provided data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Hamimatunnisa Johar, which was incorrectly given as Hamimatunissa Johar.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atasoy, S., Hausteiner-Wiehle, C., Sattel, H. et al. Gender specific somatic symptom burden and mortality risk in the general population. Sci Rep 12, 15049 (2022). https://doi.org/10.1038/s41598-022-18814-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18814-4
- Springer Nature Limited