Abstract
Familial Mediterranean fever (FMF) patients may have hepatic cytolysis, although its origin is not formally elucidated. We aimed to evaluate liver involvement in familial Mediterranean fever (FMF) using non-invasive methods. All adult FMF patients harboring two non-ambiguous mutations of the MEFV gene with hepatic cytolysis were identified in a French tertiary adult center for FMF. Liver impairment was explored with FibroMax (a non-invasive method to estimate hepatic steatosis, necrosis, inflammation and fibrosis) and liver ultrasound. Among 520 FMF adult patients, 43 had persistent hepatic cytolysis and 20 patients were included (11 women, median age at inclusion: 49.5 years). According to the FibroMax results, patients were classified as having steatosis, fibrosis, and possible or definite nonalcoholic steato-hepatitis in 10 (50%), 9 (45%) and 7 (35%) of cases, respectively. The score of steatosis did not seem associated with the usual metabolic risk factors. No significant association was found between the cumulated dose of colchicine and any of the scores included in FibroMax. In adult FMF patients with persistent hepatic cytolysis, steatosis is the first cause to consider even in the absence of usual metabolic risk factors, suggesting other mechanisms. Colchicine did not seem to be involved in this toxicity.
Similar content being viewed by others
Introduction
Familial Mediterranean Fever (FMF) is the most frequent monogenic auto-inflammatory disease, secondary to mutations in the MEFV gene. Patients usually originate from the Mediterranean area and have recurrent self-limited episodes of fever accompanied by abdominal, chest and/or joint pain. Daily treatment with colchicine reduces the risks of crisis and inflammatory AA amyloidosis1.
Some FMF patients exhibit abnormal liver tests, but it is not clear whether these abnormalities are secondary to acute or chronic inflammation, FMF treatment, AA amyloidosis, or comorbidities. Only few small case series have focused on these patients, and found a probable association of FMF with nonalcoholic fatty liver disease (NAFLD) and cryptogenic cirrhosis2. However, the prevalence of obesity and type II diabetes or impaired fasting glycemia, two major risk factors for NAFLD3, among FMF patients with NAFLD is lower than in NAFLD patients without FMF4. These data suggest that recurrent or chronic inflammation, through pro-inflammatory cytokines including interleukin-1, may promote NAFLD in FMF patients2.
The aim of this study was to evaluate liver involvement in a cohort of adult FMF patients using non-invasive methods with persistent elevated transaminase levels, and to correlate their results to the presence of metabolic syndrome and treatment with colchicine.
Methods
Patients
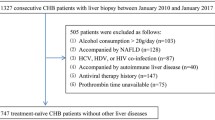
All adult patients followed in the French national reference center for FMF who previously had elevated transaminase levels (aspartate aminotransferase ASAT > 32 UI/L and/or alanine aminotransferase ALAT > 43 UI/L) checked twice in the two preceding years were contacted for clinical examination and blood tests along with liver ultrasound to explore their liver abnormalities. We excluded patients with biopsy-proven AA amyloidosis or with impaired renal function and proteinuria, as well as patients who did not fulfill Livneh FMF criteria5 or who did not harbor two validated pathogenic MEFV mutations as defined in the Infevers online registry (https://infevers.umai-montpellier.fr/web/). We also excluded those with a daily alcohol consumption above 30 g per day or who had another cause of elevated liver tests (see below). During clinical examination, the height was precisely measured without shoes by a stadiometer and the weight without clothes by a scale to the nearest kilogram. Waist circumference was then evaluated from mid-point between the navel and the tenth rib. Body mass index (BMI) was calculated by dividing the weight in kg by the height squared (m2). Patients were questioned about hypertension or the use of antihypertensive, antidiabetic or antilipidemic drugs. Metabolic syndrome in its latest definition6 was diagnosed if the patient fulfilled at least 3 of the following criteria: elevated waist circumference adjusted in the concerned population, elevated triglyceride (TG) level above 150 mg/dL, elevated fasting glycemia above 100 mg/L, reduced high-density lipoprotein (HDL)-cholesterol level less than 40 mg/dL in men or less than 50 mg/dL in women, any treatment for hypertension along with a history of hypertension. No patient was in crisis on the day of the assessment.
Biological parameters
We excluded another cause for elevated liver tests by looking for: hepatitis B; hepatitis C; Epstein-Barr virus; cytomegalovirus; creatine phosphokinase; alpha 1 anti-trypsin; ceruloplasmin; ferritin; transferrin saturation coefficient; anti-transglutaminase and -endomysium antibodies; anti-nuclear antibodies; anti-smooth muscle antibodies and anti-liver-kidney microsomal antibodies.
After overnight fasting, blood samples were taken and analyzed for the following biological parameters: ASAT; ALAT; gamma-glutamyl-transpeptidase (GGT); bilirubin; alkaline phosphatase (ALP); glycemia; glycated hemoglobin (HbA1c); total cholesterol; HDL; low-density lipoprotein (LDL); TG; insulin; high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA).
hsCRP and SAA were measured by nephelometry on an IMMAGE analyzer (Beckman-Coulter, Villepinte, France). Insulinemia was assayed by chemiluminescence (ARCHITECT Insulin Abbott, Rungis, France). ASAT, ALAT, GGT, bilirubin, glucose, total cholesterol, HDL, LDL and TG levels were routinely assayed on ARCHITECT Ci8200 (Abbott, Rungis, France). HbA1c was performed on CAPILLARYS (SEBIA, Lisses, France).
We also used the FibroMax (Biopredictive, Paris, France), following the recommended pre‐analytical and analytical conditions, that combines different validated scores in NAFLD to non-invasively estimate liver involvement, in particular hepatic steatosis (SteatoTest), necrosis, inflammation (NashTest) and fibrosis (FibroTest) at the same time7,8. It relies on the combination of the following parameters: serum α2-macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, GGT, ALAT, ASAT, total cholesterol, TG, fasting glucose, BMI, age and sex. The SteatoTest can quantitatively assess liver steatosis and the score estimates liver steatosis percentage according to the following scale: score less than 0.37: S0 (no steatosis); score between 0.38 and 0.56: S1 (1 to 5% of steatosis); score between 0.57 and 0.68: S2 (6 to 32% of steatosis); score above 0.69: S3 (> 32% of steatosis)9,10. We decided to combine S1 and S2 categories. The FibroTest provided a quantitative estimation of liver fibrosis, and the score can estimate the hepatic fibrosis according to the METAVIR scoring system: score less than 0.27: F0 or F0–F1 (no fibrosis), score between 0.27 and 0.48: F1 or F1–F2 (minimal fibrosis), score between 0.48 and 0.58: F2 (moderate fibrosis), score between 0.58 and 0.74: F3 or F3–F4 (advanced fibrosis), score above 0.74: F4 (severe fibrosis)11. We decided to combine the F1 or F1–F2 and F2 categories, and the F3 or F3–F4 and F4 categories. The NashTest has been validated in the detection of NASH among NAFLD patients7,12. Results are expressed as: score at 0.25: N0 (absence of NASH); score at 0.5: N1 (possible NASH); score at 0.75: N2 (NASH)12. We decided to combine N1 and N2 categories.
Liver ultrasound
Patients had a liver ultrasound performed by experienced radiologists who were blinded to the clinical and biological status of the patients. In order to improve the interpretation of liver ultrasound, results were given as presence or absence of steatosis. All examinations were made with a Hitachi ARIETTA V70A ultrasound machine first used in January 2016. We chose this type of imaging because of its accessibility and its low cost with a reasonable reliability in detecting steatosis13.
MEFV sequencing
All patients had MEFV exon 10 sequencing by Sanger technique as previously described14.
Ethics
The study was conducted in accordance with the recommendations of the Declaration of Helsinki. Patients were included in the JIR-cohort, an international multicenter data repository established by the National Commission on Informatics and Liberty (CNIL authorization number N°: 914677), and were informed that data collected in medical records might be used for research study in accordance with privacy rules. The experimental protocols were approved by the IRB (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891, n°2014-04) and the written consent was waived for the study. The work-up performed for each patient was part of routine care, due to the presence of liver abnormalities.
Statistical analyses
Continuous variables were reported as medians [quartile 1–quartile 3] and analyzed using the nonparametric Mann–Whitney test when two groups were compared, or the non-parametric Kruskal–Wallis test when more than two groups were compared. Categorical variables were expressed as percentages. The Spearman correlation coefficient was calculated to determine correlations between two continuous variables, and the R package “corrplot” was used to visualize the correlation matrix15. Associations were considered significant if the p value was < 0.05 and the q-value (i.e., the false discovery rate using the Benjamini–Hochberg correction method) was < 0.1. Statistical analyses were done using R 4.0.4.16.
Results
Main features of the patients
In our cohort of 520 FMF adult patients followed in the French national reference center for FMF, 234 patients previously had liver biochemical tests. Among them, 43 with elevated transaminase levels checked twice in the two preceding years were contacted for clinical examination and blood tests along with liver ultrasound to explore their liver abnormalities. Overall, 20 adult FMF patients agreed to participate and were included to have all the assessments, consisting in 11 women and 9 men with a median age of 49.5 [29.75–56.25] years (Table 1), and with 5 patients (25% of the cohort) who no longer had an elevated ASAT and ALAT levels. Eighteen patients (90%) were homozygous for the M694V mutation of the MEFV gene and 2 were compound heterozygous for the M694V/V726A mutation (Table 2). All of them received colchicine for a median duration of 24.5 [17.25–36] years with a median cumulative dose of 15.5 [9.85–23.42] g and at a current median daily dose of 1.75 [1.37–2] mg. In addition, two of them were also treated by anti-IL-1 therapy. The median BMI was 23.75 [22.3–25.77] kg/m2 and the median waist circumference was 78 [72–97.5] cm (data available for 19 patients). All but 2 patients had liver ultrasound, and was normal in 10 (55%) patients, found hepatomegaly in 4 (22%) and steatosis in the remaining 4 (22%) patients.
Liver test abnormalities
GGT, ALP and total bilirubin levels were moderately elevated (< 3 times the upper limit of normal) in 8 (40%), 4 (20%) and 0 patients, respectively, with median values of 29.5 [22–79.5] UI/L (normal values < 32), 79 [66.25–95.25] UI/L (normal values < 115) and 8 [7–12] µmol/L (normal values < 17), respectively. Eleven patients (55%) still had elevated ASAT levels with a median value of 34 [29.5–48] UI/L, and 14 (70%) still had elevated ALAT levels with a median value of 53 [38.5–65] UI/L. The search for differential diagnosis of liver involvement (see Methods) was negative in all patients. Of note, patients who no longer had an elevated ASAT and ALAT levels (n = 5, 25%) had normal FibroMax results. Only the 4 patients with a SteatoTest above 0.69 had steatosis on liver ultrasound, and 3 of them had a FibroTest above 0.58 (advanced to severe fibrosis).
Correlations
Correlations between clinical and biological parameters are depicted on Fig. 1. The cumulated colchicine dose was not significantly correlated to any other parameter. As expected, each score was mainly correlated to its components. Therefore, the score at the SteatoTest was significantly correlated with ALAT, ASAT, GGT levels, waist circumference, BMI and results at the NashTest. The score at the NashTest was significantly correlated to the results at the SteatoTest, BMI and triglyceridemia. The score at the FibroTest was only correlated to fasting glycemia.
Correlations between clinical and biological parameters. The size and color intensity of the circles are proportional to the correlation coefficient, calculated with the nonparametric Spearman correlation test. Only statistically significant correlations (p < 0.05 and q-value, i.e., the false discovery rate using the Benjamini–Hochberg correction was < 0.1) are indicated. ALAT Alanine aminotransferase, ASAT aspartate aminotransferase, GGT Gamma-glutamyl-transpeptidase, HbA1c Glycated hemoglobin, BMI Body mass index, CRP C-reactive protein, SAA Serum amyloid A, LDL Low-density lipoprotein, HDL High-density lipoprotein, ALP Alkaline phosphatase.
FibroMax
In addition to significant differences between the parameters included in the calculation of SteatoTest as expected (i.e., GGT, ALAT, TG, BMI), there were significant differences in the number of criteria for metabolic syndrome, waist circumferences, glycated hemoglobin, alkaline phosphatase and NashTest according to the results of the SteatoTest (Supplementary Table 1). Ten patients (50%) were classified as having steatosis (S1 to S3), including 4 (20%) with a score suggestive of severe steatosis (S3).
Regarding FibroTest (Supplementary Table 2), no significant differences were observed after adjustment for multiple comparisons. Nine patients (45%) were classified as having fibrosis (F1 to F4), including 4 (20%) with a score suggestive of advanced to severe fibrosis (F3 and F4). Results of the liver biopsies performed in a patient with a score suggestive of severe fibrosis (F4) are shown in the Fig. 2.
Histological appearance of liver biopsies from a patient suffering from familial Mediterranean fever with a FibroTest score of F4, suggestive of severe fibrosis. Fibrous septa seen on Sirius red (A) and Hematoxylin–eosin-saffron (B) histological sections, magnification × 5, confirming liver cirrhosis.
For NashTest (Supplementary Table 3), in addition to significant differences between the parameters included in the calculation of this score (i.e., GGT, BMI, TG), significant differences were observed between ALAT and SteatoTest according to the results of the NashTest. Seven patients (35%) were classified as having possible or definite NASH (N1 or N2).
Of note, no significant association was found between the cumulated dose of colchicine and any of these 3 scores.
Discussion
In this study of 20 adult FMF patients with persistent hepatic cytolysis, we found that 10 (50%) had steatosis, 9 (45%) had fibrosis and 7 (35%) had steatohepatitis, according to the results of SteatoTest, FibroTest and NashTest, respectively. The FibroTest did not seem associated with the usual metabolic risk factors, suggesting other mechanisms leading to hepatic fibrosis in FMF patients.
A quarter of our FMF patients (5/20), who were included because of previously elevated transaminase levels, no longer had elevated transaminase levels at inclusion. In these patients, FibroMax results were normal. Therefore, in case of elevated transaminase levels in FMF, biological monitoring should be repeated before further explorations.
Even if a part of the high prevalence of liver involvement in our cohort was probably due to a selection bias since our patients were selected based on elevated transaminase levels, half (10/20) of our FMF patients had steatosis according to the results of the SteatoTest. This prevalence is higher than reported in the general population, evaluated between 20 and 30%17,18. The prevalence of steato-hepatitis as suggested by the results of the NashTest was 7/20 (35%), whereas the prevalence in the general population is about 15%, but very few studies have explored this issue because liver biopsy is mandatory for the diagnosis19,20. Similarly, the prevalence of liver fibrosis, as suggested by the results of the FibroTest, was 9/20 (45%) in our population, higher than the prevalence in the general population estimated between 5 and 15%18,21,22.
On the other hand, the prevalence of metabolic syndrome, a major risk factor for NAFLD, in our cohort (5/20, 25%) is similar to the prevalence previously reported in the French general population (21%)23. Therefore, our results suggest another pathway for the occurrence of liver impairment in FMF patients, as it has been hypothesized before4. Of note, interleukin-1, an upregulated cytokine in FMF, promotes hepatic steatosis and liver inflammation24, and may suggest the role of uncontrolled inflammation in the development of NAFLD. Thus, 18/20 (90%) of our patients were homozygous for the M694V MEFV mutation, which is known to be associated with more severe forms of FMF25,26, and has been previously found more frequently in patients with liver impairment2. On the contrary, colchicine did not seem to be associated with FibroMax results. Liver damage has been described in colchicine intoxication with more than 5 mg/day, a higher dose than prescribed in FMF27. In their study, Tweezer-Zaks et al.28 reported that the mean cumulative colchicine dose before the first appearance of liver abnormalities in 9 FMF patients with cryptogenic cirrhosis was 8.2 ± 6.3 g, which is equivalent to ten years of treatment with a daily dose of 2 mg, as compared to more than 2000 patients in their cohort with a similar or higher cumulative dose of colchicine but without any liver abnormality. In addition, no significant increased risk of liver toxicity was found in a recent meta-analysis of randomized clinical trials29. Colchicine is also an antifibrotic drug, although its efficacy in liver fibrosis and cirrhosis remains to be proven30. Therefore, we agree on the latest EULAR guidelines regarding cytolysis in FMF patients stating that patients with elevated liver enzymes should be investigated for causes other than an adverse effect of colchicine31.
Our study has several limits. We have a small number of patients included, but a large work-up was performed in each patient to exclude other cause of liver abnormalities. To our knowledge, this is the first study assessing FMF patients with elevated transaminase levels with FibroMax that combines different non-invasive tests to evaluate liver steatosis, necrosis, inflammation and fibrosis, and liver ultrasound, which is the currently recommended screening method for NAFLD in at-risk patients20. However, non-invasive methods for assessment of liver involvement have several limitations, including different diagnostic performances according to the studied population and FibroMax has not been validated in FMF patients, and we did not compare these results to a liver biopsy32,33,34. In addition, it would be very informative to include a control group consisting of FMF patients with normal liver enzymes. However, all included patients who no longer have elevated transaminase levels had normal FibroMax results.
Conclusion
In adult FMF patients with persistent hepatic cytolysis, NAFLD is the first cause to consider even in the absence of usual metabolic risk factors, suggesting other mechanisms leading to NAFLD in these patients. As no significant association was found between the cumulated dose of colchicine and FibroMax, colchicine did not seem to be involved in this disorder. Therefore, we believe that the reduction of colchicine dosage, as recommended by the latest EULAR guidelines regarding FMF in case of significant hepatic cytolysis31, should be as short as possible and the dosage reincreased if there is no improvement or if another cause is found. FMF should probably be considered as a disease at risk of developing NAFLD. We think that transaminase levels should be regularly checked in FMF patients and, in case of persistent elevation, steatosis and/or fibrosis biomarkers and a liver ultrasound should be performed.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hentgen, V., Vinit, C., Fayand, A. & Georgin-Lavialle, S. The use of interleukine-1 inhibitors in familial Mediterranean fever patients: A narrative review. Front. Immunol. 11, 971 (2020).
Fraisse, T. et al. Non-amyloid liver involvement in familial Mediterranean fever: A systematic literature review. Liver Int. 40, 1269–1277 (2020).
Thandra, K. C. et al. Epidemiology of non-alcoholic fatty liver disease and risk of hepatocellular carcinoma progression. Clin. Exp. Hepatol. 6, 289–294 (2020).
Rimar, D., Rosner, I., Rozenbaum, M. & Zuckerman, E. Familial Mediterranean fever: An association with non-alcoholic fatty liver disease. Clin. Rheumatol. 30, 987–991 (2011).
Livneh, A. et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 40, 1879–1885 (1997).
Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
Munteanu, M. et al. Noninvasive biomarkers for the screening of fibrosis, steatosis and steatohepatitis in patients with metabolic risk factors: FibroTest-FibroMax experience. J. Gastrointest. Liver Dis. 17, 187–191 (2008).
Grattagliano, I. et al. Utility of noninvasive methods for the characterization of nonalcoholic liver steatosis in the family practice. The ‘VARES’ Italian multicenter study. Ann. Hepatol. 12, 70–77 (2013).
Poynard, T. et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp. Hepatol. 4, 10 (2005).
Ratziu, V. et al. Screening for liver disease using non-invasive biomarkers (FibroTest, SteatoTest and NashTest) in patients with hyperlipidaemia. Aliment. Pharmacol. Ther. 25, 207–218 (2007).
Poynard, T. et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 7, 40 (2007).
Poynard, T. et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 6, 34 (2006).
Hernaez, R. et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 54, 1082–1090 (2011).
Deshayes, S. et al. Specific changes in faecal microbiota are associated with familial Mediterranean fever. Ann. Rheum. Dis. 78, 1398–1404 (2019).
Wei, T. & Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.84). (2017).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org (2020).
Vernon, G., Baranova, A. & Younossi, Z. M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285 (2011).
Poynard, T. et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol. 10, 40 (2010).
Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 140, 124–131 (2011).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Poynard, T. et al. The diagnostic performance of a simplified blood test (SteatoTest-2) for the prediction of liver steatosis. Eur. J. Gastroenterol. Hepatol. 31, 393–402 (2019).
Harris, R., Harman, D. J., Card, T. R., Aithal, G. P. & Guha, I. N. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: A systematic review. Lancet Gastroenterol. Hepatol. 2, 288–297 (2017).
Vernay, M. et al. Metabolic syndrome and socioeconomic status in France: The French Nutrition and Health Survey (ENNS, 2006–2007). Int. J. Public Health 58, 855–864 (2013).
Barbier, L. et al. Interleukin-1 family cytokines: Keystones in liver inflammatory diseases. Front. Immunol. 10, 2014 (2019).
Grossman, C., Kassel, Y., Livneh, A. & Ben-Zvi, I. Familial Mediterranean fever (FMF) phenotype in patients homozygous to the MEFV M694V mutation. Eur. J. Med. Genet. 62, 103532 (2019).
Ayaz, N. A. et al. Comorbidities and phenotype-genotype correlation in children with familial Mediterranean fever. Rheumatol. Int. 41, 113–120 (2021).
Finkelstein, Y. et al. Colchicine poisoning: the dark side of an ancient drug. Clin. Toxicol. (Phila) 48, 407–414 (2010).
Tweezer-Zaks, N. et al. Familial Mediterranean fever and cryptogenic cirrhosis. Medicine 86, 355–362 (2007).
Stewart, S., Yang, K. C. K., Atkins, K., Dalbeth, N. & Robinson, P. C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 22, 28 (2020).
Rambaldi, A. & Gluud, C. Colchicine for alcoholic and non-alcoholic liver fibrosis and cirrhosis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002148.pub2 (2005).
Ozen, S. et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 75, 644–651 (2016).
Patel, K. & Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2, 100067 (2020).
Vali, Y. et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 73, 252–262 (2020).
Lardi, L. L. et al. Fibromax and inflamatory markers cannot replace liver biopsy in the evaluation of non-alcoholic fatty liver disease. Minerva Gastroenterol. Dietol. 68(1), 85–90. https://doi.org/10.23736/S1121-421X.20.02746-4 (2020).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the acquisition of data, revised the article critically and gave the final approval of the manuscript to be submitted. Study conception and design: S.D., T.F., S.F., O.S., J.P.B., S.G.L. Acquisition of data: S.D., T.F., S.F., L.S., B.T., M.M., J.M.F., I.G., G.G., J.P.B., S.G.L. Analysis and interpretation of data: S.D., T.F., S.F., O.S., L.S., B.T., M.M., A.A., R.B., V.H., J.M.F., I.G., G.G., J.P.B., S.G.L.
Corresponding author
Ethics declarations
Competing interests
Mona Munteanu received honoraria from Biopredictive. The other authors declare no conflict of interest regarding this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deshayes, S., Fraisse, T., Fellahi, S. et al. Role of non-invasive methods in detecting liver impairment in familial Mediterranean fever adult patients with persistent hepatic cytolysis. Sci Rep 12, 16644 (2022). https://doi.org/10.1038/s41598-022-17358-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17358-x
- Springer Nature Limited