Abstract
Heightened motor variability is a prominent impairment after stroke. During walking, stroke survivors show increased spatial and temporal variability; however, the functional implications of increased gait variability are not well understood. Here, we determine the effect of gait variability on the coordination between lower limbs during overground walking in stroke survivors. Ambulatory stroke survivors and controls walked at a preferred pace. We measured stride length and stride time variability, and accuracy and consistency of anti-phase gait coordination with phase coordination index (PCI). Stroke survivors showed increased stride length variability, stride time variability, and PCI compared with controls. Stride time variability but not stride length variability predicted 43% of the variance in PCI in the stroke group. Stride time variability emerged as a significant predictor of error and consistency of phase. Despite impaired spatial and temporal gait variability following stroke, increased temporal variability contributes to disrupted accuracy and consistency of gait coordination. We provide novel evidence that decline in gait coordination after stroke is associated with exacerbated stride time variability, but not stride length variability. Temporal gait variability may be a robust indicator of the decline in locomotor function and an ideal target for motor interventions that promote stable walking after stroke.
Similar content being viewed by others
Introduction
Variability in motor output is a fundamental characteristic of human motor performance. Motor variability is evident in discrete tasks such as kicking a ball as well as in continuous tasks such as walking1,2. Human gait represents a highly skilled form of movement that is characterized by relatively low fluctuations in timing and positioning of consecutive steps in healthy adults. It is theorized that a lower level of gait variability enables better adaptability of the walking pattern to the environmental changes3,4. However, heightened gait variability during minimal physical or task constraints is linked with poor behavioral outcomes such as accidental falls, decline in cognition, and mental health4,5,6,7,8,9,10. As such, a growing body of literature identifies gait variability as a clinical biomarker of ageing or pathology. Individuals with gait dysfunction due to stroke have a greater amount of spatial and temporal gait variability relative to controls11,12. However, unlike the ageing literature, the implication of increased gait variability after stroke is not well understood. Our study focuses on the consequence of increased gait variability in stroke on the coordination between lower limbs, an important aspect of safe mobility.
Safe mobility relies on continuous and rhythmical coordination between lower extremities such that both legs alternate in swing and stance phase during walking13,14,15. Poor gait coordination may limit the ability to adapt walking to different environments or to sudden perturbations16. Consequently, older adults with poor gait coordination report higher incidence of limitations in walking and transfers17,18,19. Even though age-related changes in gait coordination have been well studied, few studies have directly investigated deficits in lower limb coordination during walking after stroke. Some evidence suggests that individuals with stroke exhibit lower limb incoordination during voluntary ankle movement tasks and treadmill walking20,21. To date, the influence of stroke on lower limb coordination during overground walking remains unclear.
Given that human gait is a highly repeatable form of movement, a higher amount of variability in gait parameters may reflect an error in selection or execution of motor programs, interfering with skilled walking performance22. As gait coordination involves simultaneous, synchronous movement of each lower extremity, increased fluctuations in the timing and positioning of individual foot may affect coordination between the two lower limbs. However, empirical evidence in healthy young and older adults suggests that temporal gait variability is not related to gait coordination, pointing to the possibility of different control mechanisms for these variables in the absence of gait disorders13,14. In contrast, in individuals with gait dysfunctions such as Parkinson’s Disease, multiple sclerosis, and fibromyalgia, poor gait coordination between lower limbs and increased stride-to-stride variability seem to coexist13,23,24. A previous study in individuals with Parkinson’s disease (PD) reported that increased stride time variability was related to gait incoordination in PD13. Likewise, in people with multiple sclerosis, gait incoordination was related to increased temporal gait variability regardless of the severity of disease23. These studies point to the possibility that higher levels of fluctuations in timing and positioning of individual limbs may potentially impact coordination between the two limbs during walking. However, the empirical evidence supporting this hypothesis in stroke survivors is lacking.
Here, we investigate the effect of spatial and temporal gait variability on coordination between lower limbs during overground walking in stroke survivors. To assess lower limb coordination during walking, we measured phase coordination index (PCI) that quantifies the accuracy and consistency of anti-phase leg movements during walking13. Individuals with stroke often modulate their gait by altering spatial or temporal strategies to compensate for sensorimotor impairments of the affected limb. Evidence suggests that the deficits in spatial and temporal gait parameters may exist independent of each other in stroke. For example, stroke survivors with mild-moderate impairments showed swing time asymmetry but no step length asymmetry25. Another study showed that after body-weight support treadmill training, stroke survivors modulated stride length (spatial gait outcome) but not cadence (temporal gait outcome) to improve gait speed26. These studies suggest that stroke survivors may differentially modulate spatial and temporal gait parameters to alter the overall walking outcome. Likewise, gait coordination after stroke may be affected by inconsistency in stride time or stride length.
The purpose of our study is determine the relationship between gait variability and gait coordination, and the relative contribution of stride time (temporal) variability versus stride length (spatial) variability to gait coordination in stroke survivors during overground walking. We hypothesized that increase in temporal and spatial gait variability will relate to poor gait coordination in stroke; however, the relative contribution of spatial versus temporal gait variability to gait coordination will differ. Prior work suggests that individuals with stroke show an increased risk for falls during activities that require coordination between the lower extremities such as walking, turning, and position transfer27,28,29. Recent work from others and our group suggests that gait variability can be improved in people with gait dysfunction30,31. Thus, identifying potentially modifiable biomechanical factors that contribute to deficits in gait coordination can facilitate the development of effective gait interventions that promote safe mobility after stroke.
Results
Gait variability
The stroke group showed significantly greater stride length variability relative to the control group (t|40|= 2.31, p = 0.02; Cohen’s d = 0.71, Fig. 1A). The stroke group also showed greater stride time variability compared with the control group (t|40|= 2.80, p < 0.01; Cohen’s d = 0.86 Fig. 1B). The stride length variability was increased by 21.33% and the stride time variability was increased by 53.17% in the stroke group. The stroke group showed reduced gait speed compared with the controls (t|40|= −3.04, p = 0.004, Cohen’s d = 0.95; Table 1). Gait speed was reduced by 18.26% in the stroke group.
Lower limb coordination during walking
Figure 2A,B shows the phase values for consecutive strides from a representative walking trial of a stroke and a control participant. The stroke group had significantly greater PCI compared with the control group (t|40|= 3.95, p < 0.001; Cohen’s d = 1.22; Fig. 2C). The PCI was higher by 127.65% in the stroke group. Likewise, the stroke group showed greater phase error (t|40|= 3.52, p = 0.001; Cohen’s d = 1.09, Fig. 2D) and CV of phase (t|40|= 2.85, p = 0.007; Cohen’s d = 0.91, Fig. 2E) compared with the controls.
The impact of stroke on bilateral gait coordination: the phase values from a representative walking trial of (A) a stroke participant and (B) a control participant. The dashed line represents the ideal phase of 180° during walking. The shaded region represents the variability in phase values across the strides. The individual with stroke showed greater deviation of the phase from the 180° and higher variability of phase across consecutive strides relative to the control. Compared with the control group, the stroke group showed significantly increased (C) phase coordination index, (D) phase error relative to the ideal phase of 180°, and (E) coefficient of variation (CV) of phase during overground walking. **p < 0.01.
Association between gait coordination and gait variability
Table 2 shows the relationship between gait variability and gait coordination measures in the stroke group. PCI was positively correlated to stride length variability (r = 0.49, p = 0.02) and stride time variability (r = 0.65, p < 0.001) (Table 2). The examination of correlations between stride length variability and stride time variability showed that the two variables were not highly correlated (r = 0.50). The collinearity statistics were within the accepted limits (Tolerance = 0.74, Variance Inflation Factor = 1.34), suggesting that the assumption of multicollinearity was met.
The backward multiple regression performed to examine if the gait variability in the stroke group predicted PCI, showed that stride time variability (β = 0.65, p = 0.001), but not stride length variability (β = 0.21, p = 0.30), was a significant predictor of PCI (R2 = 0.43, p = 0.001; Fig. 3). Post-hoc power analysis showed that power of 91.60% was achieved with this sample.
Association between accuracy and consistency of gait coordination and gait variability
The stride length variability and stride time variability showed a significant positive relationship with phase error and CV of phase in the stroke group (Table 2). A backward multiple regression performed to determine if gait variability predicted the accuracy and consistency of gait coordination in stroke (Variance Inflation Factor = 1.34). The regression analyses revealed that only stride time variability (β = 0.49, p = 0.02) was a significant predictor of phase error (R2 = 0.24, p = 0.02; Fig. 4A). Similarly, only stride time variability (β = 0.65, p = 0.001) was a significant predictor of CV of phase (R2 = 0.42, p = 0.001; Fig. 4B). The stride length variability did not predict phase error (β = 0.12, p = 0.59) and CV of phase (β = 0.27, p = 0.18).
Predicting phase accuracy and consistency of phase from gait variability measures. Multiple regression analysis in the stroke group showed that stride length variability significantly predicted both (A) phase error and (B) CV of phase. Stride length variability did not emerge as a significant predictor of phase error and CV of phase in any of the regression analyses. *p < 0.05; **p < 0.01.
Discussion
The current study investigated the effect of spatial and temporal gait variability on gait coordination between lower limbs during overground walking in chronic stroke survivors. The results suggest that increased stride time variability, but not stride length variability, contributes to gait incoordination in stroke survivors. Further, increased fluctuations in stride time directly impact the accuracy and consistency of gait coordination following stroke. We provide novel evidence that increased temporal variability in successive strides disrupts lower limb coordination during overground walking in stroke survivors. These findings have implications for development of targeted interventions that reduce gait variability to promote a stable walking pattern in stroke survivors.
Heightened motor variability is one of the most prominent impairments after stroke32,33,34,35,36,37. Recently, there has been considerable interest among scientists to understand the functional implications of increased motor variability on performance related outcomes in stroke survivors. In line with this motivation, here, we investigated the impact of gait variability on gait coordination to gain insights into how increased motor variability contributes to the lower limb incoordination during walking.
Rhythmic coordination between lower extremities is a fundamental element of stable walking and safe ambulation19. Commonly, gait deficits after stroke have been quantified using symmetry of limb kinematics rather than by examining the interaction between two legs. In the current study, we quantified lower limb coordination with PCI that indices the accuracy and consistency of generating the anti-phase leg movements during walking13. We found that ambulatory, chronic stroke survivors showed higher PCI indicating that lower limb coordination is significantly diminished during walking. These results are in line with previous studies showing inter-limb coordination deficits during voluntary motor tasks after stroke15,21. Furthermore, our stroke cohort showed 266.66% higher phase error and 48.78% lower phase consistency relative to the control group, suggesting that the impact of stroke is larger on accuracy rather than the consistency of the lower limb coordination required for walking. Such lower limb incoordination after stroke could impact the ability to adjust the walking pattern to environmental demands and lead to loss of balance.
Phase coordination index quantifies the inter-limb coordination in temporal domain by measuring step time relative to stride time. Post-stroke individuals adapt gait pattern to compensate sensorimotor impairments. For example, stroke survivors may decrease the stride length of the non-paretic to limit weight bearing on the weaker, paretic limb. Such intentional adaptation of stride length can affect the temporal parameters as the instance of heel contact is delayed or hastened with change in stride length38. From the biomechanical standpoint, inconsistency in both spatial and temporal gait parameters can alter gait coordination. Whether distinct neural mechanisms control spatial and temporal aspects of walking is not well understood. However, studies using split-belt and body-weight support treadmill training support that spatial and temporal strategies independently contribute to change in walking outcomes after stroke39,40. Further, spatial and temporal inter-limb coordination during overground walking has not been well investigated in stroke survivors. Only one study investigated inter-limb coordination during voluntary anti-phase ankle movements21. In this study stroke survivors had reduced ankle excursion and slow ankle movements resulting in impaired anti-phase coordination, highlighting that both spatial and temporal movement parameters affect lower limb coordination21. In the following sections, we discuss how increased gait variability in spatial and temporal domains contribute to deficits in gait coordination after stroke.
The most noteworthy finding of our study is that poor coordination between lower limbs during walking is associated with exacerbated stride time variability in stroke survivors. The PCI predominantly measures the temporal coordination of lower limbs. The stroke group showed greater stride length variability and stride time variability suggesting heightened inconsistency in timing and distance between consecutive strides. These findings are in line with previous reports showing increased gait variability in spatial and temporal domains following stroke11,41. We found that both stride length variability and stride time variability were positively correlated to PCI in the stroke group (Table 2). However, the regression analysis showed that only stride time variability was a significant predictor of PCI. Thus, the variability in timing of foot movement influences the lower limb incoordination to a greater degree than the variability in foot positioning during walking.
Increased stride time gait variability is consistently linked with mobility impairments, specifically increased fall risk in older adults and clinical populations6,9,42,43,44. Recent studies in stroke survivors found that compared with stride length variability, stride time variability contributed to a larger extent to the fear of falling and the amount of ambulatory activity8,41. Although stroke impacts both spatial and temporal stride variability, stride time variability may be more robust indicator of the decline in locomotor function. Furthermore, previous studies report that stroke survivors show better ability to modulate the stride length than stride time during walking45,46. Such reduction in the modulation of stride time may explain the association between temporal stride variability and coordination of lower extremities in stroke survivors. Further, we found that stride time variability independently predicted both phase error and CV of phase during overground walking in the stroke group. Thus, increased fluctuations in stride time directly impact the accuracy and consistency of gait coordination between lower limbs. These results further highlight that increased temporal gait variability is particularly detrimental through its impacts on both accuracy and consistency of gait coordination between lower limbs in stroke survivors. Prior work in older adults has confirmed that greater fall risk is associated with poor gait coordination between lower limbs47. Considering that increased temporal variability contributed to gait incoordination in our study, whether the link between gait variability and falls is mediated through poor gait coordination in stroke survivors constitutes a logical, next question for future investigation.
One of the limitations of our study is that the overground walking was completed over a relatively short distance of 14 m resulting in 10 to 32 strides per trial. A recent study indicated more than 23 strides are required to obtain a reliable estimation of PCI, however, this study was conducted in healthy young adults using overground and treadmill walking trials48. We intentionally selected multiple (three) shorter walking trials to ensure that all of our stroke participants were able to complete the walking trials without a break or without the use of a walking aid, that could indirectly influence gait variability. Moreover, averaging gait variability across three walking trials provided us a more conservative estimation of gait parameters and avoided artificial inflation due to a single spurious trial. Our rationale for shorter walking trials was supported by previous research indicating that as few as ten strides were sufficient to obtain gait variability measures with good reliability in individuals with pathological gait26,49. Regardless, future studies are needed to determine the number of strides required for reliable estimation of gait coordination in individuals with neurological conditions such as stroke. Gait variability between the paretic and non-paretic legs could differ in stroke survivors and may have distinct contribution to gait coordination, however, in our study gait variability of each leg was unavailable due to technical limitations of the equipment. Finally, our stroke cohort included relatively high functioning individuals (mean FMA-LE score 27.38 ± 6.08) who did not use a walking aid or orthoses for the walking task. Therefore, whether our results generalize to stroke survivors with sensorimotor impairments or individuals who use walking aids remains to be determined.
Each year, stroke survivors experience greater number of falls (25–35%) than their healthy counterparts (~ 11%), in spite of achieving community ambulation50. Previous studies suggest that despite recovery in gait speed and gait symmetry, ambulatory stroke survivors continue to experience mobility limitations and are more likely to fall than their healthy counterparts12,50,51. Our stroke cohort showed marked deficits in gait coordination (127.65% higher PCI relative to controls) despite achieving gait speeds associated with independent community ambulation (stroke group gait speed = 0.94 ± 0.29 m/s). Such differential recovery in gait speed and gait coordination underscores the need to incorporate gait coordination as a critical rehabilitation target for restoring safe ambulation after stroke. Recent studies provide some evidence that force-control and dual-task walking trainings may be effective in reducing gait variability after stroke30,52. Accordingly, our findings have direct implications on the development of interventions targeting gait variability to improve coordination during walking after stroke.
In conclusion, impaired lower limb coordination during walking in ambulatory, chronic stroke survivors is associated with exacerbated stride time variability, but not stride length variability. Increased stride time variability impairs gait coordination by impacting both accuracy and consistency of anti-phase coordination of lower limbs. These findings highlight that heightened temporal gait variability negatively impacts the quality of gait coordination in stroke survivors. Targeted motor interventions that reduce temporal gait variability could improve lower limb coordination during walking and promote stable walking in individuals with stroke.
Methods
Participants
Twenty-one ambulatory, chronic stroke survivors and 21 healthy controls participated in this study. The characteristics of each participant are shown in Table 1. The inclusion criteria for the individuals with stroke were: (1) diagnosed with a stroke at least 6 months before study enrollment, (2) ability to ambulate independently with or without a walking aid, and (3) ability to follow a three-step command. Participants were excluded if they presented (1) any neurological disorder other than stroke, (2) musculoskeletal impairments, pain or injury in the lower limbs (3) uncorrected visual or hearing impairments, and (4) global aphasia. All participants read and signed an informed consent approved by the Institutional Review Board of University of Florida before participating in the study. All procedures were performed in accordance with guidelines from Institutional Review Board of University of Florida.
Clinical assessments
In the stroke group, we performed the Fugl-Meyer Assessment (FMA) to determine the degree of motor impairments in the lower extremities. Across all participants, Frenchay Activities Index (FAI) measured the level of participation in instrumental activities of daily living.
Overground walking
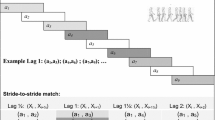
As shown in Fig. 5, the walking task involved walking for seven meters straight, turning around after the seven meter mark, and walking back seven meters straight to the starting point. Participants were instructed to walk at their preferred walking speed. Three trials were performed with a 90-s rest period between each trial. All participants walked without the use of walking aids or orthoses. The gait data were measured using six wireless inertial sensors (Opal, APDM Wearable Sensor Technologies. Inc, Portland, OR, USA) worn by the participant. The six inertial sensors were located on the two wrists, two ankles, sternum, and lumbar region. The position and acceleration data from the sensors were used to identify gait events and measure gait kinematics using the Mobility Lab software (APDM Wearable Sensor Technologies. Inc, Portland, OR, USA). The gait data were validated after each trial and stored for offline analyses. The wireless inertial sensors have been identified as a valid and reliable method for recording gait kinematics in individuals with gait disorders53,54.
Data analysis
Gait variability
The stride length variability was quantified as the coefficient of variation (CV) of stride length for each walking trial. Similarly, the stride time variability was measured as the CV of stride time. The CV of stride length and stride time were calculated over the cumulative means for each consecutive gait cycle.
Phase coordination index (PCI)
The temporal gait parameters including step time (i.e. time between heel strike of one leg to the heel strike of the other leg) and stride time (i.e. from heel strike on one leg to the next heel strike of the same leg) were measured for each gait cycle using the gait events. In a single walking trial, the phase (Φ) value for each gait cycle was calculated by dividing the step time by stride time and multiplying by 360˚ (Eq. 1). To maintain the consistency in measuring the phase for a specific leg across all participants, we first determined the average swing time for each leg within a single walking trial. Then, we used the leg with longer average swing time as the reference leg for gait cycle and measured the phase values for the opposite leg13. The absolute mean phase value was calculated across all the gait cycles. The phase error was measured as the absolute mean phase error relative to the ideal phase of 180˚ (Eq. 2). The consistency of phase across all the gait cycles was measured as the coefficient of variation (CV) of phase values (Eq. 3). The phase coordination index (PCI) for each gait trial was calculated by summating both the phase error and the CV of phase. A higher PCI value is indicative of poorer coordination.
where tLi and tSi denote the time of the ith heel strike of the legs with longer mean swing time and mean shorter swing times respectively, and tL(i + 1) > tLi > tSi.
The gait speed was computed using the time taken to walk 14 m. The number of gait cycles for each trial ranged from 10 to 32 based on participant’s walking speed. We removed the gait cycles related to turning for PCI and gait variability measurement (Fig. 5). All gait variables were measured for each trial and averaged across three trials.
Statistical analysis
The Shapiro Wilk test confirmed the normality of the data for gait variability and coordination outcome measures. We compared the spatial and temporal gait variability, PCI, phase error, and CV of phase between the groups with independent t-tests and calculated the effect size with Cohen’s d. Pearson’s bivariate correlations examined the relationship between stride length variability, stride time variability, PCI, phase error, and CV of phase for the stroke group. To determine the contribution of temporal and spatial gait variability to gait coordination in the stroke group, we performed backward stepwise multiple regression with stride length variability and stride time variability as predictor variables and PCI as the criterion variable. Given that PCI is a composite measure of phase accuracy and consistency, we performed two separate regression analyses to predict phase error and CV of phase from gait variability measures to further understand whether gait variability differentially influences one (accuracy or consistency) or both aspects of gait coordination. All the analyses were performed with SPSS 24.0 (IBM Corp) with α set at 0.05.
Ethics approval and consent to participate
All participants read and signed an informed consent form prior to participation in the study. The study procedures and the consent form were approved by the institutional review board of University of Florida.
Data availability
Data used to support study findings are included in the manuscript. Additional data can be provided up on a reasonable request to the corresponding author.
References
Hausdorff, J. M., Edelberg, H. K., Mitchell, S. L., Goldberger, A. L. & Wei, J. Y. Increased gait unsteadiness in community-dwelling elderly fallers. Arch. Phys. Med. Rehabil. 78, 278–283. https://doi.org/10.1016/s0003-9993(97)90034-4 (1997).
Christou, E. A. & Carlton, L. G. Age and contraction type influence motor output variability in rapid discrete tasks. J. Appl. Physiol. 93, 489–498. https://doi.org/10.1152/japplphysiol.00335.2001 (2002).
Cavanaugh, J. T. & Stergiou, N. Gait variability: A theoretical framework for gait analysis and biomechanics. Biomech. Gait Anal. 274, 251 (2020).
Hausdorff, J. M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2, 19. https://doi.org/10.1186/1743-0003-2-19 (2005).
Allali, G. et al. Falls, cognitive impairment, and gait performance: Results from the GOOD initiative. J. Am. Med. Dir. Assoc. 18, 335–340. https://doi.org/10.1016/j.jamda.2016.10.008 (2017).
Grimbergen, Y. A. M. et al. Falls and gait disturbances in Huntington’s disease. Mov. Disord. 23, 970–976. https://doi.org/10.1002/mds.22003 (2008).
Nakamura, T., Meguro, K. & Sasaki, H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology 42, 108–113. https://doi.org/10.1159/000213780 (1996).
Sheikh, M. & Hosseini, H. A. Investigating the relationship between spatiotemporal gait variability and falls self-efficacy in individuals with chronic stroke. Physiother. Theory Pract. https://doi.org/10.1080/09593985.2020.1771799 (2020).
Hausdorff, J. M., Rios, D. A. & Edelberg, H. K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 82, 1050–1056. https://doi.org/10.1053/apmr.2001.24893 (2001).
Herman, T., Giladi, N., Gurevich, T. & Hausdorff, J. M. Gait instability and fractal dynamics of older adults with a “cautious” gait: Why do certain older adults walk fearfully?. Gait Posture 21, 178–185. https://doi.org/10.1016/j.gaitpost.2004.01.014 (2005).
Balasubramanian, C. K., Neptune, R. R. & Kautz, S. A. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture 29, 408–414. https://doi.org/10.1016/j.gaitpost.2008.10.061 (2009).
Wang, Y. et al. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Res. 43, 69–75. https://doi.org/10.1097/mrr.0000000000000391 (2020).
Plotnik, M., Giladi, N. & Hausdorff, J. M. A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Exp. Brain Res. 181, 561–570. https://doi.org/10.1007/s00221-007-0955-7 (2007).
Plotnik, M., Giladi, N. & Hausdorff, J. M. Bilateral coordination of gait and Parkinson’s disease: The effects of dual tasking. J. Neurol. Neurosurg. Psychiatry 80, 347–350. https://doi.org/10.1136/jnnp.2008.157362 (2009).
Meijer, R. et al. Markedly impaired bilateral coordination of gait in post-stroke patients: Is this deficit distinct from asymmetry? A cohort study. J. Neuroeng. Rehabil. 8, 23. https://doi.org/10.1186/1743-0003-8-23 (2011).
Krasovsky, T. et al. Stability of gait and interlimb coordination in older adults. J. Neurophysiol. 107, 2560–2569. https://doi.org/10.1152/jn.00950.2011 (2012).
James, E. G. et al. Rhythmic interlimb coordination impairments are associated with mobility limitations among older adults. Exp. Aging Res. 43, 337–345. https://doi.org/10.1080/0361073x.2017.1333819 (2017).
James, E. G. et al. Rhythmic interlimb coordination impairments and the risk for developing mobility limitations. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1143–1148. https://doi.org/10.1093/gerona/glw236 (2017).
Pozaic, T., Grebe, A.-K., Grollmuss, M., Haeberlen, N. & Stork, W. International Conference on Biomedical and Health Informatics. 1–5. (Springer, 2015).
Roerdink, M., Lamoth, C. J., Kwakkel, G., van Wieringen, P. C. & Beek, P. J. Gait coordination after stroke: benefits of acoustically paced treadmill walking. Phys. Ther. 87, 1009–1022. https://doi.org/10.2522/ptj.20050394 (2007).
Tseng, S. C. & Morton, S. M. Impaired interlimb coordination of voluntary leg movements in poststroke hemiparesis. J. Neurophysiol. 104, 248–257. https://doi.org/10.1152/jn.00906.2009 (2010).
Schmidt, R. A. Motor schema theory after 27 years: Reflections and implications for a new theory. Res. Q. Exerc. Sport 74, 366–375 (2003).
Plotnik, M., Wagner, J. M., Adusumilli, G., Gottlieb, A. & Naismith, R. T. Gait asymmetry, and bilateral coordination of gait during a six-minute walk test in persons with multiple sclerosis. Sci. Rep. 10, 12382. https://doi.org/10.1038/s41598-020-68263-0 (2020).
Heredia-Jimenez, J., Orantes-Gonzalez, E. & Soto-Hermoso, V. M. Variability of gait, bilateral coordination, and asymmetry in women with fibromyalgia. Gait Posture 45, 41–44. https://doi.org/10.1016/j.gaitpost.2016.01.008 (2016).
Patterson, K. K. et al. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 89, 304–310 (2008).
Perera, S., Smith, C., Coffman, L. & Brach, J. Number of steps needed for reliable gait variability measurement. Gerontologist 56, 335–336 (2016).
Hyndman, D., Ashburn, A. & Stack, E. Fall events among people with stroke living in the community: Circumstances of falls and characteristics of fallers. Arch. Phys. Med. Rehabil. 83, 165–170 (2002).
Mackintosh, S. F., Hill, K., Dodd, K. J., Goldie, P. & Culham, E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin. Rehabil. 19, 441–451 (2005).
Mansfield, A. et al. Do measures of reactive balance control predict falls in people with stroke returning to the community?. Physiotherapy 101, 373–380 (2015).
Patel, P., Casamento-Moran, A., Christou, E. A. & Lodha, N. Force-control vs. strength training: The effect on gait variability in stroke survivors. Front. Neurol. 12, 667340. https://doi.org/10.3389/fneur.2021.667340 (2021).
Vergara-Diaz, G. et al. Tai Chi for reducing dual-task gait variability, a potential mediator of fall risk in Parkinson’s disease: A pilot randomized controlled trial. Glob. Adv. Health Med. 7, 2164956118775385. https://doi.org/10.1177/2164956118775385 (2018).
Cirstea, M. C. & Levin, M. F. Compensatory strategies for reaching in stroke. Brain 123, 940–953. https://doi.org/10.1093/brain/123.5.940 (2000).
Chang, S.-H., Francisco, G. E., Zhou, P., Rymer, W. Z. & Li, S. Spasticity, weakness, force variability, and sustained spontaneous motor unit discharges of resting spastic–paretic biceps brachii muscles in chronic stroke. Muscle Nerve 48, 85–92. https://doi.org/10.1002/mus.23699 (2013).
Lodha, N., Coombes, S. A. & Cauraugh, J. H. Bimanual isometric force control: Asymmetry and coordination evidence post stroke. Clin. Neurophysiol. 123, 787–795. https://doi.org/10.1016/j.clinph.2011.08.014 (2012).
Chow, J. W. & Stokic, D. S. Force control of quadriceps muscle is bilaterally impaired in subacute stroke. J. Appl. Physiol. 1985(111), 1290–1295. https://doi.org/10.1152/japplphysiol.00462.2011 (2011).
Lodha, N., Naik, S. K., Coombes, S. A. & Cauraugh, J. H. Force control and degree of motor impairments in chronic stroke. Clin. Neurophysiol. 121, 1952–1961 (2010).
Patel, P. & Lodha, N. Dynamic bimanual force control in chronic stroke: Contribution of non-paretic and paretic hands. Exp. Brain Res. 237, 2123–2133 (2019).
Varraine, E., Bonnard, M. & Pailhous, J. Intentional on-line adaptation of stride length in human walking. Exp. Brain Res. 130, 248–257. https://doi.org/10.1007/s002219900234 (2000).
Finley, J. M., Long, A., Bastian, A. J. & Torres-Oviedo, G. Spatial and temporal control contribute to step length asymmetry during split-belt adaptation and hemiparetic gait. Neurorehabil. Neural Repair 29, 786–795. https://doi.org/10.1177/1545968314567149 (2015).
Peurala, S. H. et al. Gait characteristics after gait-oriented rehabilitation in chronic stroke. Restor. Neurol. Neurosci. 23, 57–65 (2005).
Zukowski, L. A., Feld, J. A., Giuliani, C. A. & Plummer, P. Relationships between gait variability and ambulatory activity post stroke. Top Stroke Rehabil. 26, 255–260. https://doi.org/10.1080/10749357.2019.1591038 (2019).
DeMott, T. K., Richardson, J. K., Thies, S. B. & Ashton-Miller, J. A. Falls and gait characteristics among older persons with peripheral neuropathy. Am. J. Phys. Med. Rehabil. 86, 125–132. https://doi.org/10.1097/PHM.0b013e31802ee1d1 (2007).
Moon, Y., Wajda, D. A., Motl, R. W. & Sosnoff, J. J. Stride-time variability and fall risk in persons with multiple sclerosis. Mult. Scler. Int. 2015, 964790. https://doi.org/10.1155/2015/964790 (2015).
Schaafsma, J. D. et al. Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J. Neurol. Sci. 212, 47–53. https://doi.org/10.1016/s0022-510x(03)00104-7 (2003).
Bayat, R., Barbeau, H. & Lamontagne, A. Speed and temporal-distance adaptations during treadmill and overground walking following stroke. Neurorehabil. Neural Repair 19, 115–124. https://doi.org/10.1177/1545968305275286 (2005).
Nakamura, R., Handa, T., Watanabe, S. & Morohashi, I. Walking cycle after stroke. Tohoku J. Exp. Med. 154, 241–244. https://doi.org/10.1620/tjem.154.241 (1988).
James, E. G. et al. Coordination impairments are associated with falling among older adults. Exp. Aging Res. 43, 430–439. https://doi.org/10.1080/0361073x.2017.1369634 (2017).
Kribus-Shmiel, L., Zeilig, G., Sokolovski, B. & Plotnik, M. How many strides are required for a reliable estimation of temporal gait parameters? Implementation of a new algorithm on the phase coordination index. PLoS ONE https://doi.org/10.1371/journal.pone.0192049 (2018).
Kroneberg, D. et al. How many steps are enough? Assessment of gait variability in realistically confined clinical settings. Basal Ganglia 100, 3–4 (2017).
Jørgensen, L., Engstad, T. & Jacobsen, B. K. Higher incidence of falls in long-term stroke survivors than in population controls: Depressive symptoms predict falls after stroke. Stroke 33, 542–547. https://doi.org/10.1161/hs0202.102375 (2002).
Punt, M., Bruijn, S. M., Wittink, H., van de Port, I. G. & van Dieën, J. H. Do clinical assessments, steady-state or daily-life gait characteristics predict falls in ambulatory chronic stroke survivors?. J. Rehabil. Med. 49, 402–409. https://doi.org/10.2340/16501977-2234 (2017).
Baek, C. Y. et al. Effects of dual-task gait treadmill training on gait ability, dual-task interference, and fall efficacy in people with stroke: A randomized controlled trial. Phys. Ther. https://doi.org/10.1093/ptj/pzab067 (2021).
Morris, R. et al. Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 40, 095003 (2019).
Washabaugh, E. P., Kalyanaraman, T., Adamczyk, P. G., Claflin, E. S. & Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 55, 87–93. https://doi.org/10.1016/j.gaitpost.2017.04.013 (2017).
Acknowledgements
The American Heart Association (Scientist Development Award 14SDG20450151 to NL and Postdoctoral Fellowship 827097 to PP) and National Institutes of Health (K01AG070327 to NL) provided the funding support for this work.
Author information
Authors and Affiliations
Contributions
P.P.: Conceptualization, Formal analyses and data interpretation, Writing-Original Draft, Writing-Review & Editing; D.E.: Data processing, Data analysis, Writing-Sections of the manuscript, A.C.M.: Data acquisition, Writing-Review & Editing; E.A.C.: Conceptualization and Study Design, Writing-Review & Editing, Resources, Funding Acquisition N.L.: Supervision, Conceptualization and Study Design, Data interpretation, Writing-Review & Editing, Resources, Funding Acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patel, P., Enzastiga, D., Casamento-Moran, A. et al. Increased temporal stride variability contributes to impaired gait coordination after stroke. Sci Rep 12, 12679 (2022). https://doi.org/10.1038/s41598-022-17017-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17017-1
- Springer Nature Limited