Abstract
IκBζ is a transcriptional regulator that augments inflammatory responses from the Toll-like receptor or interleukin signaling. These innate immune responses contribute to the progression of nonalcoholic fatty liver disease (NAFLD); however, the role of IκBζ in the pathogenesis of NAFLD remains elusive. We investigated whether IκBζ was involved in the progression of NAFLD in mice. We generated hepatocyte-specific IκBζ-deficient mice (Alb-Cre; Nfkbizfl/fl) by crossing Nfkbizfl/fl mice with Alb-Cre transgenic mice. NAFLD was induced by feeding the mice a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD). CDAHFD-induced IκBζ expression in the liver was observed in Nfkbizfl/fl mice, but not in Alb-Cre; Nfkbizfl/fl mice. Contrary to our initial expectation, IκBζ deletion in hepatocytes accelerated the progression of NAFLD after CDAHFD treatment. Although the increased expression of inflammatory cytokines and apoptosis-related proteins by CDAHFD remained unchanged between Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice, early-stage steatosis of the liver was significantly augmented in Alb-Cre; Nfkbizfl/fl mice. Overexpression of IκBζ in hepatocytes via the adeno-associated virus vector attenuated liver steatosis caused by the CDAHFD in wild-type C57BL/6 mice. This preventive effect of IκBζ overexpression on steatosis was not observed without transcriptional activity. Microarray analysis revealed a correlation between IκBζ expression and the changes of factors related to triglyceride biosynthesis and lipoprotein uptake. Our data suggest that hepatic IκBζ attenuates the progression of NAFLD possibly through the regulation of the factors related to triglyceride metabolism.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD), which is a hepatic manifestation of metabolic syndrome or insulin resistance, has become a major healthcare issue worldwide1,2,3. The clinical range of NAFLD is broadly classified into two types4,5,6. The first is the simple nonalcoholic fatty liver (NAFL), in which lipids are deposited in hepatocytes; the second is nonalcoholic steatohepatitis (NASH), which involves the progression from NAFL to inflammation and fibrosis of the liver, and then to more severe manifestations of the disease, cirrhosis and hepatocellular carcinoma2,7. NASH is also associated with a high risk of acute complications and mortality, including cardiovascular events and extrahepatic malignancies8,9,10,11. Despite an increasing trend in NAFLD cases relative to the expansion of modern industrialized economies and the resulting global prevalence of obesity, there is no effective pharmacological therapy or cure to control the progression of NAFLD12,13. The elucidation of the pathogenesis and underlying mechanisms of NAFLD, therefore, remains an important challenge for the development of disease treatment and prevention.
Although the molecular pathogenesis of NASH has not been fully elucidated, several potential mechanisms have been indicated. The accumulation of triglycerides in hepatocytes results in an inflammatory response that is attributed to innate immunity, lipotoxicity, oxidative stress, endoplasmic reticulum stress, and autophagy, which are common mechanisms in the pathogenesis of NAFLD progression14,15. These subcellular responses activate intracellular pathways that lead to proinflammatory signals. Of these, NF-κB activation is a major intracellular event16,17. NF-κB regulates the expression of numerous proinflammatory proteins, including cytokines, chemokines, and adhesion molecules. NF-κB activation has been observed in several different animal models of NASH, as well as in humans, and is essential for the development of steatohepatitis18,19. The translocation of activated NF-κB into the nucleus has been demonstrated to activate the transcription of numerous proteins20.
IκBζ, which is encoded by the Nfkbiz gene, is a member of the nuclear IκB family, which acts as a transcriptional regulator of NF-κB signaling21. IκBζ is rapidly and robustly induced by NF-κB activation and amplifies the expression of secondary response genes, which enhance cytokine and chemokine production to regulate innate immune systems21,22. We have previously demonstrated that IκBζ augments interleukin (IL)-33-dependent cytokine and chemokine production in mast cells23. IκBζ also enhances Toll-like receptor (TLR), IL-1, IL-17, and IL-36 signaling22,24,25. Despite immune response augmentation, the disruption of IκBζ signaling increases cellular apoptosis in epithelial cells, which leads to the accumulation of lymphocytes and Sjögren syndrome-like autoimmune disease26. In addition, mutations in NFKBIZ are highly prevalent in the epithelium of ulcerative colitis patients and may prevent the progression of colorectal cancer27. Collectively, IκBζ is shown to have pleiotropic cellular functions, including immune response, apoptosis, and tumor progression. However, the specific role of IκBζ in the progression of NAFLD has not been determined. In this study, we have investigated the involvement of IκBζ in the progression of NAFLD in hepatocyte-specific Nfkbiz-deleted mice.
Results
Induction of IκBζ in the liver of mice during NAFLD development
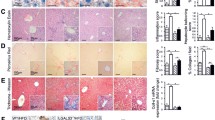
To examine the role of IκBζ in the progression of NAFLD, we first examined the expression of IκBζ after a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) feeding. The mRNA expression of Nfkbiz in the liver significantly augmented in wild-type C57BL/6 mice after CDAHFD (Fig. 1A). We further assessed the expression of NFKBIZ in the human liver and found a significant increase in NFKBIZ in moderate steatosis and a decrease in bridging fibrosis (Fig. 1B). To examine the role of IκBζ on the progression of NAFLD, we generated hepatocyte-specific IκBζ-deficient mice. Nfkbizfl/fl mice were crossed with Alb-Cre transgenic mice (Alb-Cre;Nfkbizfl/fl). Male Alb-Cre;Nfkbizfl/fl mice and littermate male control Nfkbizfl/fl mice were fed with CDAHFD for 1–8 weeks to induce NAFLD. Robust mRNA expression of Nfkbiz in the liver was observed in Nfkbizfl/fl (Fig. 1C). An increase of Nfkbiz mRNA in the liver was not observed in Alb-Cre;Nfkbizfl/fl after CDAHFD challenge (Fig. 1C), which suggests that the induction of Nfkbiz in the liver during NAFLD development was mainly derived from albumin-producing hepatocytes; thus, other cells, including Kupffer cells, endothelial cells, and hepatic stellate cells, were not associated with the increase in IκBζ in this challenge.
Alb-Cre; Nfkbizfl/fl mice accelerate the development of NAFLD. (A) C57BL/6 mice were fed an NCD or CDAHFD for 4 weeks. mRNA induction of Nfkbiz in the liver was assessed using real-time RT-PCR and was expressed as the fold increase in the Nfkbiz/Hprt1 ratio. Values are expressed as the mean ± SD (n = 3). (B) mRNA expressions of NFKBIZ in the human normal liver, moderate steatosis, and steatohepatitis with bridging fibrosis were assessed using real-time RT-PCR. Values are expressed as the mean ± SD (triplicate experiment from one sample). (C) Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice were fed a CDAHFD or NCD for 8 weeks. mRNA induction of Nfkbiz in the liver was assessed using real-time RT-PCR. Values are expressed as the mean ± SD (n = 3–12 in each point). (D) Representative macroimages of the liver obtained from Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice fed an NCD or CDAHFD for 8 weeks. (E) Representative image of hematoxylin–eosin-stained liver sections obtained from Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice fed an NCD or CDAHFD for 8 weeks. Bar = 100 µm. (F) NAFLD activity score (NAS) was performed by a blinded researcher. The scores of hepatocellular steatosis (Steatosis), lobular inflammation (Inflammation), and ballooning (Ballooning), as well as total NAS calculated by summing up the scores (NAS), are expressed as the mean ± SD (10 independent fields from per mouse, three mice per experiment). *P < 0.05, **P < 0.01, ***P < 0.001. CDAHFD, choline-deficient, L-amino acid-defined, high-fat diet; NCD, normal chow diet.
Alb-Cre; Nfkbiz fl/fl mice accelerate the progression of NAFLD
We next examined liver morphological and histological analyses after CDAHFD feeding. An obvious white color change in the liver surface was observed in Nfkbizfl/fl mice at 8 weeks after CDAHFD (Fig. 1D). On the other hand, the progression of NAFLD demonstrated by the irregular changes on the liver surface was faster in Alb-Cre;Nfkbizfl/fl mice fed with CDAHFD (Fig. 1D). Approximately half of Alb-Cre;Nfkbizfl/fl mice developed an irregular surface at 8 weeks after CDAHFD feeding (data not shown). After the CDAHFD challenge, Alb-Cre;Nfkbizfl/fl mice had lighter livers than Nfkbizfl/fl mice (Supplemental Fig. 1). Furthermore, a significant increase in the plasma aspartate aminotransferase (AST), AST/alanine aminotransferase ratio, total bilirubin, and total bile acids were observed in Alb-Cre;Nfkbizfl/fl mice (Supplemental Fig. 1). In addition, the total protein was significantly decreased in Alb-Cre; Nfkbizfl/fl mice (Supplemental Fig. 1). We further compared histological changes in the liver after CDAHFD feeding by the nonalcoholic steatohepatitis score (NAS). Alb-Cre; Nfkbizfl/fl mice tended to have accelerated steatosis, inflammation, and ballooning after the CDAHFD diet (Fig. 1E,F, Supplemental Fig. 2). The mere expression of Cre in hepatocytes did not seem to accelerate the progression of NAFLD, because early lipid deposition was similar in the liver of Alb-Cre mice and C57BL/6 mice (Supplemental Fig. 3).
Role of hepatocyte IκBζ for apoptosis, inflammation, and fibrosis during NAFLD development
Since the loss of IκBζ increased cell apoptosis in epithelial cells, which led to lymphocyte accumulation26, we next examined the signaling related to apoptosis. TUNEL staining at 4 and 8 weeks after CDAHFD feeding indicated an increase in apoptotic cells in the liver of Alb-Cre;Nfkbizfl/fl mice (Fig. 2A,B, Supplemental Fig. 4). Statistical significance between Alb-Cre;Nfkbizfl/fl mice and Nfkbizfl/fl mice was not observed at 2 weeks (Fig. 2B). We next examined the mRNA expression of the representative molecules to assess the state of apoptosis in the liver. The mRNA expressions of Bax and Bak, which are two nuclear-encoded mediators for apoptosis, were elevated in liver obtained from Alb-Cre;Nfkbizfl/fl mice fed with a commercial normal chow diet (NCD) (Fig. 2C,D). However, we could not detect significant changes in the Bax and Bak expressions between Alb-Cre;Nfkbizfl/fl mice and Nfkbizfl/fl mice fed with CDAHFD (Fig. 2C,D). There was no difference in the expressions of Bcl-xL and Bcl-2, which are negative regulators of Bax and Bak (Fig. 2E,F). We further investigated the expressions of apoptosis-related proteins by protein array analysis. We did not observe significant differences in the protein expressions of the liver at 4 weeks after the CDAHFD diet between Alb-Cre;Nfkbizfl/fl mice and Nfkbizfl/fl mice (Supplemental Fig. 5). We next examined ductular reaction after CDAHFD challenge because of the potential role of IκBζ in epithelial cell apoptosis. We assessed ductular reaction by Cytokeratin 19 (CK19) staining in liver specimens obtained from Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice and found no significant difference between groups (Supplemental Fig. 6).
Deficiency of IκBζ in hepatocytes increases liver apoptotic cells in mouse model of NAFLD. Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice were fed an NCD or CDAHFD. (A) TUNEL staining of the liver section was observed using a confocal microscope (Leica TCS SP8; Leica Microsystems). Representative image of TUNEL staining of liver sections from mice fed an NCD or CDAHFD for 8 weeks. Green, apoptotic cells; Blue, DAPI. Bar = 50 µm. (B) The mean number of apoptotic cells in one section at high magnification from mice fed CDAHFD for 2, 4, or 8 weeks or NCD (white bar, Nfkbizfl/fl; black bar, Alb-Cre; Nfkbizfl/fl). Values are expressed as the mean ± SD (four independent fields per mouse, three mice per experiment). (C, D, E, F) mRNA expression of Bax (C), Bak (D), Bcl-xl (E), and Bcl-2 (F) in the liver was assessed using real-time RT-PCR and was expressed as the fold increase in the Nfkbiz/Hprt1 ratio (white bar, Nfkbizfl/fl; gray bar, Alb-Cre; Nfkbizfl/fl). Values are expressed as the mean ± SD (n = 3–12). CDAHFD, choline-deficient, L-amino acid-defined, high-fat diet; NCD, normal chow diet.*P < 0.05, **P < 0.01, ***P < 0.001.
The development of NAFLD is characterized by hepatocyte apoptosis and the resultant inflammation and fibrosis. Since IκBζ is essential for the induction of secondary response genes via various IL and TLR responses, we analyzed mRNA expressions related to immune responses during NAFLD development with a TaqMan™ Array Mouse Immune Panel. Increased mRNA expressions of Ccl2, Tnf, and Nfkb2 in the liver were observed with the progression of NAFLD (Fig. 3A–I) (Ccl2, P = 0.028 in Nfkbizfl/fl; Tnf, P = 0.017 in Nfkbizfl/fl; Nfkb2, P = 0.025 in Nfkbizfl/fl). However, we did not detect significant differences in these mRNA expressions of the liver between Alb-Cre;Nfkbizfl/fl mice and Nfkbizfl/fl mice after being fed the CDAHFD diet (Fig. 3A–I). These data indicate that the deterioration of NAFLD in Alb-Cre;Nfkbizfl/fl mice is not attributable to transcriptional modification in apoptosis and the immune response.
The deficiency of IκBζ in hepatocytes fails to modify mRNA expression of the proteins related to immune responses in mice model of NAFLD. Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice were fed an NCD or CDAHFD for 8 weeks. Liver RNA was isolated from mice at 2 and 8 weeks after the CDAHFD challenge or at 8 weeks after NCD. mRNA expressions of Ikbkb (A), Nfkb1 (B), Nfkb2 (C), Ccl2 (D), Ccl3 (E), Il18 (F), Il1b (G), Il6 (H), and Tnf (I) were analyzed by TaqMan Array Mouse Immune Panel, according to the manufacturer’s instructions (white bar, Nfkbizfl/fl; gray bar, Alb-Cre; Nfkbizfl/fl). Values are expressed as the mean ± SD (n = 3–4). *P < 0.05. CDAHFD, choline-deficient, L-amino acid-defined, high-fat diet; NCD, normal chow diet.
We next assessed fibrosis and mRNA expression of the related proteins after CDAHFD challenge. Histological analysis to observe fibrosis by Sirius Red staining revealed an increase in the fibrosis area after CDAHFD feeding in Alb-Cre;Nfkbizfl/fl mice (Fig. 4A,B). Increased mRNA expression of Tgfb1, Acta1, Pdgfb, Timp1, and Col1a1 in the liver were observed with the progression of NAFLD (Fig. 4C–G) (P < 0.001 in Nfkbizfl/fl). Although the expression of Acta1 in Alb-Cre; Nfkbizfl/fl mice increased compared with Nfkbizfl/fl in the NCD-fed mice, we did not detect significant differences in the mRNA expression in the liver between the groups after being fed the CDAHFD diet (Fig. 4C–G).
Fibrotic changes in the liver in the mice model of NAFLD. Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice were fed an NCD or CDAHFD for 8 weeks. (A) Representative image of Sirius Red staining of the liver obtained from Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice fed an NCD or CDAHFD for 8 weeks. Bar = 100 µm. (B) Sirius Red-positive area quantified using BZ-X 700 imaging software (Keyence). The fibrosis area was expressed as the percentage of the Sirius Red-positive area. Values are expressed as the mean ± SD (four independent fields per mouse, three mice per experiment). (C–G) mRNA expressions of Tgfb1 (C), Acta1 (D), Pdgfb (E), Timp1 (F), and Col1a1 (G) were analyzed by real-time RT-PCR and expressed as the fold increase in the Nfkbiz/Hprt1 ratio (white bar, Nfkbizfl/fl; gray bar, Alb-Cre; Nfkbizfl/fl). Values are expressed as the mean ± SD (n = 3–12). *P < 0.05. CDAHFD, choline-deficient, L-amino acid-defined, high-fat diet; NCD, normal chow diet.
Role of hepatocyte IκBζ for hepatic steatosis in NAFLD
Since Alb-Cre;Nfkbizfl/fl accelerated the progression of NAFLD at an early stage, we hypothesized that lipid accumulation in the hepatocytes may have enhanced after CDAHFD challenge in Alb-Cre;Nfkbizfl/fl mice. We used histological analysis to evaluate lipid accumulation in the liver of Alb-Cre;Nfkbizfl/fl mice and Nfkbizfl/fl mice fed with NCD or CDAHFD for 2 weeks. The steatosis in the liver assessed by Oil Red O staining significantly increased in Alb-Cre;Nfkbizfl/fl mice (Fig. 5A,B), which suggests that hepatocyte IκBζ may contribute to NAFLD progression via aberrant lipid metabolism. Choline-deficient diets exacerbated steatosis by inhibiting very low-density lipoprotein (VLDL) release from the liver28. Indeed, the plasma VLDL concentration was significantly decreased by the CDAHFD diet (Fig. 5C,D). Deletion of IκBζ in hepatocytes further reduced plasma VLDL-derived triacylglycerol after the CDAHFD challenge (Fig. 5C,D). Although the levels of high-density lipoprotein (HDL)-derived cholesterol tend to be reduced by deletion of IκBζ in hepatocytes, it was not significant after the CDAHFD challenge (Supplemental Fig. 7). Changes in LDL cholesterol were not observed (Supplemental Fig. 7).
Deficiency of IκBζ in hepatocytes increases liver steatosis cells in the mouse model of NAFLD. Nfkbizfl/fl and Alb-Cre; Nfkbizfl/fl mice were fed an NCD or CDAHFD for 2 weeks. (A) Representative image of Oil Red O staining of liver sections from mice fed an NCD or CDAHFD for 2 weeks. Bar = 100 µm. (B) Oil Red O staining area of liver specimens quantified using BZ-X 700 imaging software (Keyence). The Oil red O staining area was expressed as the percentage of the Oil Red O area. Values are expressed as the mean ± SD (n = 4). (C, D) Plasma lipoprotein fractions were analyzed by high-performance liquid chromatography. (C) Representative image of the chromatogram (violet line, cholesterol; blue line, triacylglycerol). CM, chylomicron; VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein. (D) VLDL-cholesterol and VLDL-triacylglycerol in plasma were quantified (white bar, Nfkbizfl/fl; gray bar, Alb-Cre; Nfkbizfl/fl). Values are expressed as the mean ± SD (n = 3–5). *P < 0.05, **P < 0.01. CDAHFD, choline-deficient, L-amino acid-defined, high-fat diet; NCD, normal chow diet.
Overexpression of IκBζ in hepatocytes by the AAV8 vector attenuates liver steatosis
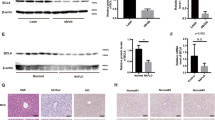
We next examined whether overexpression of IκBζ attenuates hepatic steatosis in vivo. We constructed three types of AAV8 vectors to express target proteins to liver hepatocytes; (1) firefly Luciferase (control); (2) Nfkbiz (L), a full-length mouse IκBζ; (3) Nfkbiz (D), truncated form of IκBζ without transcriptional activity26 (Fig. 6A). AAV8 vectors harboring Luciferase, Nfkbiz (L), and Nfkbiz (D) were intravenously injected into C57BL/6 wild-type mice. We confirmed overexpression of Nfkbiz in the liver at 4 weeks after the vector injection (Fig. 6B). The treated mice were further fed with CDAHFD for 4 weeks. There was no significant difference in the liver weight (data not shown) among the three groups, but the white color change of the liver due to the CDAHFD diet was significantly abolished by the hepatocyte expression of Nfkbiz (L), but not Nfkbiz (D) (Fig. 6C). Histological examination confirmed the inhibition of liver steatosis by the expression of Nfkbiz (L) (Fig. 6D,E). In contrast, hepatic apoptosis and fibrosis were not ameliorated by the expression of Nfkbiz (L) (Supplemental Fig. 8).
Overexpression of Nfkbiz in liver hepatocytes by AAV8 vector ameliorates hepatic steatosis in vivo. (A) Schematic representation of the AAV8 vector used in this study. Nfkbiz (D), a deletion mutant of IκBζ lacking trans-activating domain; Nfkbiz (L), full-length IκBζ; ITR, internal terminal repeat; ANK, ankyrin repeat. (B) C57BL/6 mice (7 weeks old) were intravenously treated with 3 × 1011 vg of AAV8 vector harboring Luciferase, Nfkbiz (D), or Nfkbiz (L). The expression of Nfkbiz in the liver obtained at 4 weeks after vector injection was assessed to measure the mRNA of Nfkbiz using real-time PCR and was expressed as the fold increase in the Nfkbiz/Hprt1 ratio. Values are expressed as the mean ± SD (n = 4–5). (C–E) At 4 weeks after AAV injection, the mice were then fed an NCD or CDAHFD for 4 weeks. (C) Representative morphology of the liver. (D) Representative image of hematoxylin–eosin staining of the liver section. Bar = 100 µm. (E) Steatosis area in the section quantified with BZ-X 700 imaging software (Keyence). The steatosis area was expressed as a percentage of the fat droplet area. Values are expressed as the mean ± SD (four independent fields per mouse, three mice per experiment). **P < 0.01, ***P < 0.001. AAV, adeno-associated virus; CDAHFD, choline-deficient, L-amino acid-defined, high-fat diet; NCD, normal chow diet.
Changes in the transcript expressions in hepatocytes due to overexpression of IκBζ
To identify the mechanisms by which the expression of IκBζ attenuates liver steatosis, we compared mRNA expressions in liver obtained from mice treated with the AAV vector harboring Nfkbiz with those treated with the AAV vector expressing Luciferase by microarray analysis. We analyzed a set of 45,037 probes to detect the mRNA expression and selected genes whose expression changed more than fivefold (P < 0.01) in liver obtained from mice treated with AAV8 vector harboring Nfkbiz (Fig. 7A,B). We identified 77 upregulated genes and 57 downregulated genes (Fig. 7B, Supplemental Fig. 9, Supplemental Tables 2 and 3). Since we noticed the downregulation of Lipin (Lpin), which is a catalyzing enzyme for the synthesis of glycolipids, we extracted genes related to triacylglycerol metabolism to synthesis VLDL in the liver (Fig. 7C, Supplemental Fig. 9).
Microarray analysis in the liver of mice treated with the AAV8 vector harboring Nfkbiz. C57BL/6 mice (7 weeks old) were intravenously treated with 3 × 1011 vg of the AAV8 vector harboring Luciferase or Nfkbiz (L). The CDAHFD challenge started at 4 weeks after vector injection. Liver RNA was isolated from mice at 4 weeks after the challenge. The expressions of transcripts in the liver were analyzed by Mouse Genome 430 2.0 Array (n = 3 in each group). The gene expressions in the liver of mice treated with the AAV vector expressing Nfkbiz (L) were compared with those treated with the AAV vector expressing Luciferase. (A) Volcano plots showing the global transcriptional changes among the groups. All genes on the Mouse Genome 430 2.0 Array were plotted. The log-fold changes in Nfkbiz (L) versus Luciferase group are represented on the X-axis. Statistically upregulated and downregulated genes are expressed in red and green, respectively (> fivefold, P < 0.01). (B) The proportion of upregulated and downregulated genes. (C) Overview of triacylglycerol metabolism in the liver. Transcripts selected for the analysis are shown in red italics. (D) Transcripts related to triacylglycerol metabolism exhibit statistically significant changes (P < 0.05). The fold changes and P values are shown on the right.
Of mRNAs related to fatty acid uptake and de novo synthesis (Fig. 7C), the expressions of fatty acid synthetase (Fasn) and transcriptional factors that regulate fatty acid synthesis including sterol regulatory element-binding protein-1 (SREBP-1, Srebf 1), SREBP-2 (Srebf 2), peroxisome proliferator-activated receptor (PPARγ) coactivator 1α (Prargc1a) were attenuated by the expression of IκBζ (Fig. 7D). The glycerol phosphate pathway is the major pathway by which triacylglycerol is generated in the liver (Fig. 7C). The expressions of some acyltransferases (Agpat1 and Agpat3), as well as the phosphatases Lipin1 (Lpin1) and Lipin2 (Lpin2), were significantly downregulated (Fig. 7D). PPARγ coactivator 1α (Ppargc1a), which is a transcriptional coactivator controlling several key hepatic metabolic pathways, was also downregulated. Triacylglycerol was further assembled with Apoprotein B (ApoB) by the microsomal triglyceride transfer protein (Mttp) and secreted into circulation as a VLDL by the vesicular transport (Fig. 7C). We also observed increases in the syntaxin family members involved in vesicular transport, including Stx1b, Stx1a, and Stx11, and the downregulation of Sec23b and Sec 31a (Fig. 7D). Furthermore, the expression of the VLDL receptor (Vldlr) was downregulated by the expression of IκBζ. Among the changes observed in the microarray analysis, we confirmed the significant decrease of Lipin1 mRNA expression by performing RT-PCR in mice treated with AAV vector harboring Nfkbiz. (Supplemental Fig. 10). Next, we assessed the changes in the transcripts related to β-oxidation by overexpression of IκBζ. As shown in Supplemental Table 4, no significant change was observed in the transcripts.
Discussion
Inflammation is important for the process of NAFLD, from hepatic lipid accumulation to liver fibrosis28,29. TLRs and ILs, which are involved in innate immunity, have been reported to promote the progression of NAFLD30. TLRs and ILs induce inflammation through transcriptional regulation via NF-κB17. IκBζ is a transcription factor that amplifies signals downstream of NF-κB, thus leading to a secondary inflammatory response31,32,33. In this study, we generated hepatocyte-specific IκBζ-deficient mice to clarify the role of IκBζ in NAFLD progression. Contrary to our initial expectation, the deletion of IκBζ in hepatocytes accelerated the progression of NAFLD. These results suggest the existence of a novel function of IκBζ in regulating the progression of NAFLD in hepatocytes.
Our data suggest that IκBζ regulates the progression of NAFLD by controlling intracellular triglyceride accumulation in hepatocytes. IκBζ-deficient mice showed enhanced hepatic fibrosis in CDAHFD-induced NAFLD. We analyzed the processes leading to fibrosis, including inflammation, apoptosis, and fat accumulation, to evaluate the influence of IκBζ deficiency. Since the expression of inflammatory cytokines and chemokines were not different in IκBζ-deficient mice after the CDAHFD challenge, it is unlikely that IκBζ directly modulates the inflammatory processes in hepatocytes. Our experiments employed the hepatocyte-specific deletion of IκBζ; however, the role of IκBζ in blood cells for inflammation may be negligible. The deletion of IκBζ was previously reported to be associated with the induction of apoptosis in epithelial cells26. Although apoptosis-inducing factors, such as Bax and Bak, were predominantly expressed in the liver in IκBζ-deficient mice fed with a normal diet, the enhancement of apoptosis induction is unlikely, because there was no significant difference in apoptosis-related protein or gene expressions during the CDAHFD challenge. On the other hand, lipid accumulation in hepatocytes, with the earliest change appearing in NAFLD, was significantly enhanced in IκBζ-deficient mice, and overexpression of IκBζ suppressed fat accumulation in NAFLD in wild-type mice. Furthermore, moderate, but not bridging fibrosis enhanced the expression of NFKBIZ, suggesting that NFKBIZ plays a role in the early stage of NAFLD. This data supports our hypothesis that IκBζ regulates lipid metabolism in hepatocytes.
IκBζ regulation of fat accumulation in hepatocytes may be partly explained by the involvement in VLDL release from the liver. CDAHFD promotes lipid accumulation by inhibiting the release of VLDL from the liver due to choline deficiency. In fact, CDAHFD reduced the concentration of VLDL-triacylglycerols in the blood. The concentration of plasma VLDL-triacylglycerol was more suppressed in IκBζ-deficient mice, and the expressions of some syntaxin members related to vesicular transport were upregulated by the expression of IκBζ. However, this activity alone does not clearly explain the apparent fat accumulation in the liver, because the reduction of plasma VLDL-triacylglycerol was only 18.8% (Nfkbizfl/fl 57.6% v.s. Alb-Cre;Nfkbizfl/fl 76.4%). The expression of IκBζ lacking a transcription factor from the AAV vector showed no inhibitory effect on lipid accumulation, which indicates that IκBζ plays a role in the regulation of lipid metabolism through its transcriptional activity.
We found that IκBζ may affect mRNA expression of genes associated with the triglyceride synthesis pathway. The synthesis of triglycerides involves three fatty acid-derived acyl-CoA esterified to a glycerol molecule34. Fatty acids are synthesized de novo from acetyl CoA and are taken up from extracellular sources35. Microarray analysis showed the downregulation of the molecules related to this process. Notably, we confirmed the downregulation of the Lipin1 by IκBζ expression. Lipin1 plays a key role in orchestrating liver metabolism, for example by generating diacylglycerol through its phosphatidate phosphatase activity36. Lipin1 also acts as a transcriptional coactivator for PPARα and PGC1α, inducing the expression of genes related to fatty acid oxidation36. Besides, liver-specific deletion in mice aggravates lipid accumulation in the liver by inhibiting of VLDL excretion and fatty acid oxidation in alcoholic liver injury37. In addition, hypoxia-inducible factor-1 prevents hepatic lipid accumulation, probably by regulating Lipin1 and PGC1α 38. In this study, the overexpression of IκBζ ameliorated fat accumulation in the liver despite the decrease in Lipin1. This discrepancy may involve the induction of a negative regulator or a compensatory mechanism for the expression of some factors to ameliorate steatosis through the expression of IκBζ. In the future, it will be necessary to study in more detail to identify a direct target that improves fat accumulation in the liver through the transcriptional activation of IκBζ.
It would be of clinical importance to understand the relationship between IκBζ regulation of lipid metabolism in the liver and the function of other organs, as well as the role of IκBζ in the metabolism of other tissues. NAFLD is closely related to the development of type 2 diabetes mellitus39,40. Insulin resistance observed in type 2 diabetes exacerbates NAFLD by increasing fatty acid influx and fatty acid synthesis in the liver41. Conversely, NAFLD promotes insulin resistance, which leads to exhaustion of pancreatic β cells and exacerbates diabetes42. Interestingly, overexpression of IκBζ in wild-type mouse hepatocytes with the AAV vector not only suppressed the development of NAFLD, but also caused weight gain (data not shown). Although the mechanism for this is not clear at present, it is possible that the regulation of lipid metabolism by IκBζ in hepatocytes is linked to a systemic metabolic process to regulate body weight. Furthermore, it would be interesting to investigate the function of IκBζ in other organs involved in lipid metabolisms, such as adipose tissue and muscle. Detailed analyses about the role of IκBζ in systemic lipid metabolism may provide insights into whether IκBζ is a new target for hyperlipidemia drugs.
This study has several limitations that should be addressed. First, the precise mechanism of hepatic lipid metabolism regulated by IκBζ has not been fully elucidated. IκBζ reportedly facilitated the transcription of a set of inflammatory genes, but IκBζ overexpression without transcriptional activity failed to ameliorate steatosis. Although it is certain that the transcriptional regulation controls fat accumulation in the liver, we could not identify key molecules regulated by IκBζ. Second, we observed tiny but significant differences between NCD-fed Alb-Cre; Nfkbizfl/fl and Nfkbizfl/fl mice. For example, NCD-fed Alb-Cre; Nfkbizfl/fl mice had higher body and liver weight (Supplemental Fig. 1). We noted similar differences in serum AST, total bilirubin, and mRNA expressions of Bax and Bak (Fig. 2). A deficiency of Nfkbiz in liver hepatocytes may harm liver function and the metabolic system, even in mice on a long-term NCD. Furthermore, the present study was mainly conducted in a mouse NAFLD model using CDAHFD. Although it is histologically similar to human steatohepatitis, it does not cause weight gain or insulin resistance observed in human43. We also used only male mice in this study, and thus sex difference in the role of IκBζ in the development of NAFLD are unknown. Finally, our results should be interpreted with caution because the study was conducted only on mice. Therefore, it is unknown whether IκBζ has a similar function in the development and progression of human NAFLD, albeit we observed the increase in NFKBIZ in moderate steatosis. Further analysis is required to determine whether the induction of IκBζ in the liver of human NAFLD patients regulates the progression of NAFLD.
In conclusion, we demonstrated that IκBζ contributes to the development of NAFLD through alterations in lipid metabolism in the liver. With the resolution of viral hepatitis by effective antihepatitis virus drugs and increases in obesity worldwide, the importance and prevalence of NAFLD is expected to further increase in the future. Further elucidation of the regulatory mechanism of steatosis by IκBζ may represent a novel and attractive therapeutic target for NAFLD, diabetes, and obesity. Further studies are required to elucidate the precise mechanisms by which IκBζ controls lipid metabolism so that an effective treatment that targets IκBζ can be developed for various diseases.
Methods
Animal experimentation
All animal experimental procedures were approved by The Institutional Animal Care and Concern Committee of Jichi Medical University (permission number: 17118-04), and animal care was conducted in accordance with the committee’s guidelines and ARRIVE guidelines. C57BL/6J mice were purchased from Japan SLC (Shizuoka, Japan). Mice containing loxP-flanked Nfkbizfl/fl were obtained from RIKEN BRC (#RBRC06410, Ibaraki, Japan)26. The deletion of both Nfkbiz alleles in hepatocytes was achieved by breeding Nfkbizfl/fl animals with Alb-Cre transgenic mice (B6.Cg-Tg(Alb-Cre)21Mgn/J)44. The PCR primer used for genotyping is shown in Supplemental Table 1. Hepatocyte-specific Nfkbiz-deleted male mice (Alb-Cre:Nfkbizfl/fl) and a littermate control male mice (Nfkbizfl/fl) were used for the experiments. Animals were maintained in isolators in the specific pathogen‐free facility of Jichi Medical University at 23 °C ± 3 °C with 12:12 h light/dark cycle and fed with a commercial normal chow diet (NCD) (CLEA Japan, Inc., Shizuoka, Japan) after weaning. We genotyped mice at 4 weeks of age, and littermates with the same genotype were maintained at a maximum of five mice per cage. To induce steatohepatitis, 7–8-week-old male mice were fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) (60 kcal% lard-based fat, choline deficient, and 0.1% methionine) (A06071302, Research Diets Inc., New Brunswick, NJ)45. When indicated, whole blood was obtained from anesthetized mice through the jugular vein using a 30 G syringe containing 1/10 sodium citrate, which was then centrifuged to obtain plasma samples. Clinical chemistry parameters were measured at Oriental Yeast Co. (Tokyo, Japan). The lipoprotein fraction was analyzed using high-performance liquid chromatography at Skylight Biotech Inc. (Akita, Japan).
Quantitative reverse transcription polymerase chain reaction
Mice anesthetized with isoflurane were intracardially perfused with PBS to remove blood, and then liver tissues were sterilely isolated. The tissues were incubated with RNAlater®Stabilizaion Reagent (QIAGEN, Venlo, Netherlands) at 4 °C overnight, and then stored at − 80 °C in a deep freezer until extraction. Total RNA was isolated from cells using an RNeasy Plus Mini Kit (Qiagen). We purchased RNAs of human liver from healthy, moderate steatosis, and steatohepatitis with bridging fibrosis (SEKISUI Medical Co., Tokyo, Japan; normal liver: #Lot. H1314, macro fat: 0%, 43 years old Caucasian male, body mass index (BMI): 30, alcohol use: none, diabetes: none; steatosis: moderate: #Lot. H0878, macro fat: 40%, 43 years old Caucasian male, BMI: 35.4, alcohol use: none, diabetes: none; steatohepatitis: bridging fibrosis: #Lot. H0613, macro fat: 50%, 65 years old Caucasian male, BMI: 32.9, alcohol use: none, diabetes: none). The RNA samples were reverse-transcribed using a PrimeScript RT Reagent kit (Takara Bio, Shiga, Japan) or SuperScript™ IV VILO™ Master Mix with ezDNase™ Enzyme (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative real-time PCR was performed using THUNDERBIRD™ Probe qPCR Mix or THUNDERBIRD™ SYBR qPCR Mix (TOYOBO, Osaka, Japan) in QuantStudio™ 12 K Flex Real-Time PCR system (Thermo Fisher Scientific). Reactions were analyzed in duplicate, and expression levels were normalized to Gapdh or Hprt1 mRNA levels (ΔCT). We then calculated ΔΔCT as the ratio of calibrator sample (an NCD-fed mouse). The calculation was automatically performed in QuantStudio™ 12 K Flex Real-Time PCR system (Thermo Fisher Scientific). The primers used in this study are shown in Supplemental Table 1. Quantitative gene expressions of targets associated with immune responses were analyzed by TaqMan™ Array Mouse Immune Panel (Thermo Fisher Scientific), according to the manufacturer’s instructions.
Histological analysis for liver steatosis and fibrosis
Mice were anesthetized with isoflurane and then intracardially perfused with PBS to remove the blood. Then, the liver tissues were dissected and fixed in 10% formalin and embedded in paraffin or were fixed with 4% paraformaldehyde, incubated with PBS containing sucrose (10%–30%), and then frozen in the presence of optimal cutting temperature compound (Sakura Fintek Japan, Tokyo, Japan). The paraffin-embedded tissue sections were dewaxed in xylene, rehydrated with ethanol, and washed with water. The sections were processed for hematoxylin and eosin staining, Sirius Red staining (Sirius Red/Fast Green Collagen Staining Kit, Chondrex Inc., Woodinville, WA, USA), or immunostaining. The frozen sections were subjected to Oil Red O staining (FUJIFILM Wako Pure Chemical, Osaka, Japan). The sections were observed using an all-in-one microscope (BZ-X700, Keyence, Tokyo, Japan). Quantitative evaluation of the target area was measured with BZ-X 700 imaging software (Keyence). When indicated, the NAFLD activity score (NAS) in hematoxylin and eosin staining was assessed by a blinded researcher (Dr. Miura, Jichi Medical University), as previously described4. Hepatocellular steatosis was scored as 0 (< 5% of liver parenchyma), 1 (5%–33%), 2 (33%–66%), and 3 (> 66%). Lobular inflammation was also scored with hematoxylin and eosin staining as 0 (no inflammatory foci), 1 (< 2 foci per 20 × field), 2 (2–3 foci per 20 × field), and 3 (> 4 foci per 20 × field).
Immunostaining
The sections were pretreated with 5% donkey serum and then treated with anti-Cytokeratin 19 (CK19) rabbit polyclonal antibody (GENETEX Inc., Irvine, CA). Immunoreactivity was detected with Simple Stain Mouse MAX-PO (Nichirei Bioscience, Tokyo, Japan), and DAB (Agilent Technologies, Santa Clara, CA, USA), followed by counterstaining with Mayer’s hematoxylin. Tissue sections were observed using an all-in-one microscope (BZ-X700, Keyence, Tokyo, Japan). Quantitative evaluation of the CK19-positive area was performed with BZ-X 700 imaging software (Keyence).
TUNEL staining of apoptotic cells in the liver section
TUNEL staining of apoptotic cells in the liver sections was performed with Mebstain Apoptosis Tunel Kit III (Medical & Biological Laboratories Co, Aichi, Japan). Briefly, paraffin-embedded tissue sections were dewaxed in xylene and then rehydrated through immersion in ethanol followed by washing with water. After proteinase K treatment, the DNA nick ends were labeled with biotin-conjugated dUTP by terminal deoxynucleotidyl transferase and then stained with streptavidin conjugated with dichlorotriazinyl aminofluorescein. The sections were mounted in Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Images were obtained using a fluorescence microscope (BZ-X700, Keyence) or a confocal microscope (Leica TCS SP8, Leica Microsystems, Wetzlar, Germany). Quantitative evaluation was determined as the apoptotic cell number of one section recorded at high magnification with BZ-X700 imaging software (Keyence).
Expression of apoptosis-related proteins
Liver tissues were harvested in 500 µL PBS containing cOmplete® protease inhibitor cocktail (Sigma Aldrich, Saint Louis, MO, USA), sonicated, and then lysed with the addition of Triton X-100 (final concentration 1%). After the freeze–thaw cycle, samples were centrifuged at 10,000 × g for 5 min to remove cell debris. The relative levels of apoptosis-related protein in the supernatants (400 µg) were determined by a Proteome Profiler Mouse Apoptosis Array Kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. The image data were quantified using the ImageQuant LAS4000 system (GE Healthcare). Band intensities were normalized to that of the internal control.
Construction of an adeno-associated virus vector
cDNAs of Nfkbiz [Nfkbiz (L) and (D)] were kindly provided by Dr. T. Maruyama (National Institutes of Health). A DNA fragment consisting of a chimeric promoter (HCRhAAT; an enhancer element of the hepatic control region of the Apo E/C1 gene and the human anti-trypsin promoter), full-length of Nfkbiz, truncate type of Nfkbiz without transcriptional activity, or Luciferase cDNA, and the SV40 polyadenylation signal were introduced between inverted terminal repeats into pAAV2. The AAV vectors were produced by triple plasmid transfection of human embryonic kidney 293 cells to generate the AAV8 vector (helper-free system), as described previously46. Titration of recombinant AAV vectors was carried out by quantitative PCR to measure the copy number of the SV40 polyadenylation signal.
Microarray analysis
The total RNAs were isolated by RNeasy Mini Kit (QIAGEN). mRNA expressions were analyzed by GeneChip Mouse Genome 430 2.0 Array (Thermo Fisher Scientific) and detected by GeneChip Scanner 3000 7G (Thermo Fisher Scientific) at Takara Bio Co. The data were analyzed with Transcriptome Analysis Console software (Thermo Fisher Scientific).
Statistical analysis
Data were analyzed using GraphPad Prism® version 8 (GraphPad Software, San Diego, CA, USA). All data are presented as the means ± standard deviation (SD). Statistical significance was determined using two-tailed Student’s t-tests. A value of P < 0.05 was considered to be statistically significant.
Data availability
The original data in this study are available upon request from the corresponding author. The datasets of gene expression data analyzed during the current study are available in the GEO repository, GSE205390.
References
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Younossi, Z. M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 70, 531–544 (2019).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Rinella, M. E. Nonalcoholic fatty liver disease: A systematic review. JAMA 313, 2263 (2015).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019).
Hagström, H. et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273 (2017).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153 (2017).
Ding, W., Fan, J. & Qin, J. Association between nonalcoholic fatty liver disease and colorectal adenoma: A systematic review and meta-analysis. Int. J. Clin. Med. 8, 323–333 (2015).
Kim, G.-A. et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 68, 140–146 (2018).
Sumida, Y. & Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 53, 362–376 (2018).
Esler, W. P. & Bence, K. K. Metabolic targets in nonalcoholic fatty liver disease. Cell Mol. Gastroenterol. Hepatol. 8, 247–267 (2019).
Parthasarathy, G., Revelo, X. & Malhi, H. Pathogenesis of nonalcoholic steatohepatitis: An overview. Hepatol. Commun. 4, 478–492 (2020).
Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 52, 1836–1846 (2010).
Luedde, T. & Schwabe, R. F. NF-κB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 8, 108–118 (2011).
Liu, T., Zhang, L., Joo, D. & Sun, S.-C. NF-κB signaling in inflammation. Sig. Transduct. Target. Ther. 2, 17023 (2017).
Sunami, Y. et al. Hepatic activation of IKK/NFκB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology 56, 1117–1128 (2012).
dele Peña, A. et al. NF-κB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 129, 1663–1674 (2005).
Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Annemann, M. et al. Atypical IκB proteins in immune cell differentiation and function. Immunol. Lett. 171, 26–35 (2016).
Yamamoto, M. et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature 430, 5 (2004).
Ohto-Ozaki, H. et al. Induction of IκBζ augments cytokine and chemokine production by IL-33 in mast cells. J. Immunol. 204, 2033–2042 (2020).
Muromoto, R. et al. IL-17A plays a central role in the expression of psoriasis signature genes through the induction of IκB-ζ in keratinocytes. Int. Immunol. 28, 443–452 (2016).
Müller, A. et al. IκBζ is a key transcriptional regulator of IL-36-driven psoriasis-related gene expression in keratinocytes. Proc. Natl. Acad. Sci. U. S. A. 115, 10088–10093 (2018).
Okuma, A. et al. Enhanced apoptosis by disruption of the STAT3-IκB-ζ signaling pathway in epithelial cells induces Sjögren’s syndrome-like autoimmune disease. Immunity 38, 450–460 (2013).
Kakiuchi, N. et al. Frequent mutations that converge on the NFKBIZ pathway in ulcerative colitis. Nature 577, 260–265 (2020).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Farrell, G. C., van Rooyen, D., Gan, L. & Chitturi, S. NASH is an Infl ammatory disorder: Pathogenic, prognostic and therapeutic implications. Gut. Liver 6, 149–171 (2012).
Miura, K. et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 139, 323-334.e7 (2010).
Motoyama, M., Yamazaki, S., Eto-Kimura, A., Takeshige, K. & Muta, T. Positive and negative regulation of nuclear factor-κB-mediated transcription by IκB-ζ, an inducible nuclear protein. J Biol Chem 280, 7444–7451 (2005).
Kitamura, H., Kanehira, K., Okita, K., Morimatsu, M. & Saito, M. MAIL, a novel nuclear IκB protein that potentiates LPS-induced IL-6 production. FEBS Lett. 485, 53–56 (2000).
Yamazaki, S., Muta, T. & Takeshige, K. A novel IκB protein, IκB-ζ, induced by proinflammatory stimuli, negatively regulates nuclear factor-κB in the nuclei. J. Biol. Chem. 276, 27657–27662 (2001).
Takeuchi, K. & Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physio Endocrinol. Metab. 296, E1195–E1209 (2009).
Cohen, J. C., Horton, J. D. & Hobbs, H. H. Human fatty liver disease: Old questions and new insights. Science 332, 1519–1523 (2011).
Bou Khalil, M., Blais, A., Figeys, D. & Yao, Z. Lipin—The bridge between hepatic glycerolipid biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta 1801, 1249–1259 (2010).
Hu, M. et al. Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology 58, 1953–1963 (2013).
Arai, T., Tanaka, M. & Goda, N. HIF-1-dependent lipin1 induction prevents excessive lipid accumulation in choline-deficient diet-induced fatty liver. Sci. Rep. 8, 14230 (2018).
Byrne, C. D. & Targher, G. NAFLD: A multisystem disease. J. Hepatol. 62, S47–S64 (2015).
El-serag, H. B., Tran, T. & Everhart, J. E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 126, 460–468 (2004).
Gluchowski, N. L., Becuwe, M., Walther, T. C. & Farese, R. V. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 14, 343–355 (2017).
Tilg, H., Moschen, A. R. & Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 14, 32–42 (2017).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018).
Postic, C. & Magnuson, M. A. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 (2000).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Path. 94, 93–103 (2013).
Ohmori, T. et al. CRISPR/Cas9-mediated genome editing via postnatal administration of AAV vector cures haemophilia B mice. Sci. Rep. 7, 4159 (2017).
Acknowledgements
This study was partly supported by research from Bristol Myers Squibb (M.H.) and Otsuka Pharmaceutical (T.O.). We thank Mai Hayashi, Tamaki Aoki, Mika Kishimoto, Yaeko Suto, Sachiyo Kamimura, Yuiko Ogiwara, Nagako Sekiya, Tomoko Noguchi, Hiroko Hayakawa, Yuji Kashiwakura, Takafumi Hiramoto, and Nobuhiko Kamoshita (Jichi Medical University) for their technical assistance and advice. ImageQuant LAS4000 digital imaging system and Optima XE-90 were subsidized by JKA through its promotion funds from KEIRIN RACE. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
H.I. designed the study, performed the experiments, analyzed data, and wrote the manuscript. M.H. designed the study, analyzed data, and revised the manuscript. N.B. and N.K. performed the experiments and analyzed data. H.O.-O. designed the study and performed the experiments. T.M. provided critical reagents and revised the manuscript. K.M., K.S., and T.R. supervised the study and revised the manuscript. T.O. designed the study, analyzed the data, and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.H. received a research grant from Bristol Myers Squibb for this study. T.O. received grant support from Otsuka Pharmaceutical for the study. All other authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishikawa, H., Hayakawa, M., Baatartsogt, N. et al. IκBζ regulates the development of nonalcoholic fatty liver disease through the attenuation of hepatic steatosis in mice. Sci Rep 12, 11634 (2022). https://doi.org/10.1038/s41598-022-15840-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15840-0
- Springer Nature Limited
This article is cited by
-
NFKBIZ regulates NFκB signaling pathway to mediate tumorigenesis and metastasis of hepatocellular carcinoma by direct interaction with TRIM16
Cellular and Molecular Life Sciences (2024)