Abstract
Stressful situations are common in everyday life and disturb homeostasis. So, an exercise session is a strategy to mitigate blood pressure (BP) peaks in response to stress (i.e., BP reactivity), decreasing the cardiovascular risk. This is a systematic review and meta-analysis that aims to verify the effects of a single session of physical exercises on BP reactivity to stress in adults. The searches were performed in digital databases (MEDLINE, LILACS, EMBASE, SPORTDiscus, and PsycInfo) and 29 studies were included, totaling 795 individuals (quantitative analysis: k = 25, n = 659). As for exercise characteristics, 21 of the 29 studies focused on aerobic exercises, and 23 studies focused on low to moderate intensities. As for the stress tests, we have them in the following order from the most to the least frequent: stroop color and word test, cold pressor test, arithmetic test, public speaking, handgrip, trier social stress test, and study task. Favorable metanalytic results (standardized mean differences through random-effects approach) for the exercises were found, with attenuated reactivity in systolic BP (pooled effect size = − 0.38 [− 0.49; − 0.27], representing average reductions of 3.7 ± 3.8 mmHg), diastolic BP (pooled effect size = − 0.51 [− 0.70; − 0.33], representing average reductions of 2.9 ± 3.7 mmHg), and mean BP (pooled effect size = − 0.51 [− 0.72; − 0.31], representing average reductions of 4.1 ± 3.3 mmHg). So, acute physical exercise lowers systolic, diastolic, and mean blood pressure reactivity in response to stressor tasks. However, given the small magnitude of effects, the clinical relevance of this result must be interpreted with caution and be better explored.
Similar content being viewed by others
Introduction
Stressful situations are common in modern life and can cause transient alterations in autonomic, catecholaminergic, and neural networks in response to it1,2,3. Although these alterations are expected to prepare the body for the challenge, prolonged, frequent, or exaggerated responses to stress can be indicative of future cardiovascular risk2. In this way, simple laboratory stress tests that disturb the homeostasis in a controlled manner were previously associated with the development of future cardiovascular events, depression, and decreased telomere length4. These tests involve different types of stressors, such as physical (e.g., cold), mental (e.g., arithmetic task), or a mix of both5. Besides, one of the simplest and most frequent ways to assess stress reactivity responses is based on changes in blood pressure (BP) (i.e., hypertensive peaks)5.
In a broad context, high BP is one of the main preventable factors associated with premature death globally6 and is associated with the risk of cardiovascular events, strokes, and kidney disease7. In this context, one of BP's control strategies is to perform physical exercises. Evidence shows that even after a single exercise session, BP can be below baseline levels at rest8 but its influence on BP reactivity to stressful situations is still poorly understood. Despite that, it has already been suggested that cardiovascular responses to stress are better indicators of left ventricular mass9 and the development of hypertension10,11 than resting BP, reiterating the importance of studying these responses.
In 2006, a meta-analysis by Hamer and collaborators12 evaluated the acute effects of aerobic exercise on BP reactivity to several laboratorial stress tests (i.e. stroop color and word test, arithmetic test, cold pressor test, and study task) and found favorable results with attenuated hypertensive peaks in adults (effect size between 0.38 and 0.40). However, in addition to new studies being produced since then, responses to non-aerobic exercise are still unclear. Thus, the aim of the present systematic review and meta-analysis is to verify the acute effects of physical exercise on stress-related BP reactivity in adults. The hypothesis is that the exercise will be able to mitigate stress reactivity, with a similar magnitude to those demonstrated in isolated aerobic exercises12.
Methods
This systematic review and meta-analysis followed PRISMA guidelines13,14, had its protocol published (available at: https://doi.org/10.17504/protocols.io.bhw3j7gn)15, and was registered on PROSPERO (CRD42020194353).
Eligibility criteria
Studies with the following characteristics were eligible: (1) population: human, both sexes, adults (i.e. > 18 years), regardless of health or training status; (2) intervention: a session of physical exercise; (3) control: a session without exercise; (4) outcome of interest: BP reactivity under stress (peak BP during a stress test or BP variation from basal levels); (5) languages: English, Portuguese or Spanish; (6) study designs: randomized clinical trials or crossovers; (7) publication dates: no time limit; (8) other characteristics: in studies with more than two intervention arms, only comparisons with the control group were considered, dividing the control sample proportionately to avoid sample duplication in the final analysis.
Search strategy
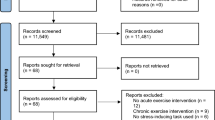
The searches were performed on April 26th/2022, in digital databases (MEDLINE, LILACS, EMBASE, SPORTDiscus, and PsycInfo). Also, in the reference lists of the included studies, and through manual search on other websites (“https://core.ac.uk/” and “https://scholar.google.com/”). The flow diagram is shown in Fig. 1, and the list of studies excluded from full-text screening are available in Supplementary Data S1. The search was organized into the following categories of terms: exercise intervention, BP, and stressors. Parentheses and intersection boolean operators (i.e. “AND”) were used to separate the categories, and union operators (i.e. “OR”) were used to separate the terms of each category. In this way, these terms were searched in title, abstract, and keywords indexed in the aforementioned databases in the following format:
(Exercise OR “Exercise Therapy” OR “Physical activity” OR “Physical training” OR Aerobic OR Cycling OR Bicycle OR Treadmill OR “Cycle ergometer” OR Cyclergometer OR “Cycle-ergometer” OR Swimming OR Swim OR Running OR Run OR “Hand grip” OR “Hand-grip” OR Walking OR Walk OR "Weight training” OR "Weight-training” OR “Weight exercise” OR “Weight-exercise” OR “Resistance exercise” OR “Resistance training” OR Strength OR Pilates OR Yoga OR Ioga OR Taichi OR “Tai chi” OR “Tai-chi” OR Isometric OR Hiit OR Hit OR Siit OR Sit OR “High intensity” OR “Moderate intensity” OR ”Low intensity” OR “Combined training” OR “Combined exercise” OR “Concurrent training” OR “Concurrent exercise”) AND (“Arterial pressure” OR "Blood pressure" OR Diastolic OR Systolic) AND ("Reactivity" OR "Cold pressor" OR "Stroop" OR "Stress test" OR Psychosocial OR “Psychosocial test” OR “Psychosocial stress” OR “Psychosocial task” OR “Stress task” OR “math task” OR “Speech task” OR Speech OR Math OR Arithmetic OR “Arithmetic test” OR “Arithmetic task”).
Screening and data extraction process
During the process of screening (title and abstract, and full-text), data extraction, and risk of bias assessment, the studies were evaluated in duplicate by independent reviewers. After checking the responses, the reviewer’s disagreements were resolved by consensus or by a third reviewer when necessary. The reviewer’s agreement was estimated from Cohen's kappa in both full-text screening (κ = 0.671; p < 0.001; 13 disagreements were resolved by a third reviewer) and risk of bias assessment (κ = 0.867; p < 0.001).
Before the data extraction phase, one of the reviewers standardized codes for all studies included in the following analyses. Thus, each reviewer independently filled an electronic datasheet detailing the characteristics of the studies and the data was compared to assess agreement and identify errors. This datasheet included: identification code, author last name, publication year, language, study design, sample sizes, health and fitness status, age, sex, hypertension status, other comorbidities, exercise intensity, exercise volume (measured in minutes), exercise mode (aerobic, resistance, combined or yoga), stressor test, BP measure device/technique, and BP reactivity measures (sample sizes, mean and standard deviation. If other types of measures were reported, the mean and standard deviation were requested from the authors, and in case of null or negative answers, the results were transformed (when possible). When there was not sufficient data for meta-analysis, the authors were contacted to request further information. Studies in which the data are presented without numerical description, it was extracted through a web-based software (https://automeris.io/WebPlotDigitizer).
Statistical analysis
Pooled estimates were calculated using standardized mean differences (SMD) with confidence intervals (95% CI), using “R” programming language through the packages "meta"16 and "metafor"17. For the pooled effect, were considered the values of BP reactivity under stress (peak BP during a stress test or BP variation from basal levels) after an exercise session and after a control session without exercise, as a comparator. In studies with multiple stressors, we used the mean and pooled dispersion between the stressors. The heterogeneity was measured by I2 and Kendall's tau using the Hunter Smith method for heterogeneity variance estimators18,19. Due to the different characteristics of interventions, population, and stress tests, we selected a random-effects approach to summarize the metanalytic results.
The sensitivity analysis was done through the search for outliers and influential points using externally standardized residuals (values farther than 1.96 standard deviations in the standardized residuals graph), difference in fits (identifying values above 1 or below − 1), covariance ratio (identifying values below 1) and Cook’s distance methods (identifying values far above the other studies). In addition, we visually evaluated the overlap of confidence intervals in the forest plot, and studies without overlap would be considered outliers. In addition, subgroup analyses by type of stressor, the number of stressors, participants' sex, exercise mode, and studies design were made. The individual study assessment of the risk of bias was conducted through “Risk of Bias 2.0” method from the Cochrane collaboration20 and its graphical visualization by the “R” package "robvis"21. Publication bias analysis was carried out through Egger’s regression22 and trim and fill funnel plots23. Quality of evidence was accessed throught Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach24.
Results
Studies characteristics
Studies included 388 women, 387 men, and 20 individuals in which sex was not disclosed. In addition, of the 29 studies25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53, only 4 (14%) included hypertensive patients31,39,44,53, 22 (76%) had a mean age of less than 30 years25,26,27,28,29,30,32,33,34,37,40,41,42,43,46,50,51,52,54,55,56, 4 (14%) were from 30 to 40 years old35,38,47,48, and only 4 (14%) were over 40 years old31,39,44,53. As for stress tests, we have as the most frequent the stroop color and word test (13 studies)29,31,32,33,36,37,38,43,44,46,47,48,49, followed by cold pressor32,34,35,37,39,41,43,50,51,52,53 (11 studies), arithmetic test25,27,29,30,39,40,42,45,48 (9 studies), public speaking (3 studies)29,32,38, handgrip (2 studies)36,48, and Trier Social Stress Test28 and Study task26 (1 study each). As for the time interval between the exercise session and the stressor task, only 2 studies (7%) performed more than 60 min later42,44, 7 studies (24%) performed between 31 and 60 min later29,32,35,40,49,53,54 and 23 studies (79%) performed in up to 30 min later25,26,27,30,31,33,34,37,38,39,40,41,43,45,46,47,48,50,51,52,57.
As for exercise characteristics, 2 studies included intervention with Yoga (7%)30,34, 4 (14%) with resistance exercises41,42,51,52 and 2 (7%) with combined exercises35,53, all the others focused on aerobic exercises (21 studies, 72%)25,26,27,28,29,31,32,33,36,37,38,39,40,43,44,45,46,47,48,49,50,53. Furthermore, the exercise sessions lasted between 10 and 120 min (average of 30–60 min). As for intensity, 1 study used self-selection34, 5 used high intensity29,42,49,50,52 and all others used low to moderate intensity (50–85% of the individual maximum; e.g. heart rate max, 1RM, VO2max)25,26,27,28,30,31,32,33,35,36,37,38,39,40,41,43,44,45,46,47,48,51,53.
Regarding experimental designs, 5 (17%) studies used a randomized clinical trial approach25,27,29,32,46, and 24 (83%) adopted a crossover design26,28,30,31,33,34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53. As the main results, 13 (45%) studies demonstrated improvements in systolic blood pressure (SBP)25,31,32,35,37,38,40,44,47,49,51,52,53, 14 (48%) in diastolic blood pressure (DBP)25,31,32,35,37,38,39,40,41,44,46,47,51,52, and 8 (out of 12; 67%) in mean blood pressure (MBP)29,31,37,38,46,47,51,52. The others (12; 41%) had null results since no study has shown harmful BP reactivity effects of exercise26,27,28,30,33,34,36,42,43,45,48,50. Besides that, four studies did not present data dispersion measures to be included in the meta-analysis25,26,27,28. The general characteristics of all studies are shown in Table 1.
Meta-analysis results
Among 25 studies included in meta-analysis29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53, 9 presented multiple possible comparisons according to the exercise mode34,52, exercise volume32,46,51, exercise intensity47,50, parents smoking habit33, or participants smoking habit43. Besides that, 23 studies demonstrate results for SBP (34 comparisons), 24 for DBP (35 comparisons) and 12 for MBP (18 comparisons), as shown in Table 1. The forest plots of SBP, DBP and MBP reactivity are present in Figs. 2, 3 and 4, respectively. We found small but favorable results to exercise in both SBP (SMD = − 0.38 [− 0.49; − 0.27], representing mean reductions of 3.7 ± 3.8 mmHg), DBP (SMD = − 0.51 [− 0.70; − 0.33], representing mean reductions of 2.9 ± 3.7 mmHg) and MBP reactivity (SMD = − 0.51 [− 0.72; − 0.31], representing mean reductions of 4.1 ± 3.3 mmHg). We also highlight that 20 (80%) of the studies were carried out in healthy non-athlete individuals aged up to 40 years 29,30,32,34,35,36,37,40,41,42,43,46,47,48,49,50,51,52,55,58. Thus, by isolating this population as an sensitivy analysis, we maintain the results for SBP (SMD = − 0.36 [− 0.48; − 0.25]), DBP (SMD = − 0.48 [− 0.67; − 0.30]), and MBP (SMD = − 0.41 [− 0.57; − 0.25]).
Other sensitivity analyses showed that 4 studies31,32,39,49 can be outliers and/or influential points in DBP and 1 study31 in MBP reactivity. New analysis disregarding these studies showed a DBP effect size of − 0.37 [− 0.50; − 0.24] and a MBP effect size of − 0.48 [− 0.65; − 0.31]. Subgroup analyses were performed in SBP and DBP, but none of these analyses reported significant differences between subgroups, either comparing: study design, participants sex, exercise mode, stress type or number of stressors. The summary of these analyses can be seen in Table 2. An additional analysis comparing the stressors showed no effects differences (SBP p = 0.81; DBP p = 0.47) between the cold pressor test (SBP SMD = − 0.42 [− 0.62; − 0.23]; DBP SMD = − 0.56 [− 0.80; − 0.32]), arithmetic test (SBP SMD = − 0.36 [− 0.54; − 0.17]; DBP SMD = − 0.36 [− 0.56; − 0.17]), stroop color and words test (SBP SMD = − 0.36 [− 0.65; − 0.06]; DBP SMD = − 0.35 [− 0.68; − 0.03]) or other tests (SBP SMD = − 0.51 [− 0.78; − 0.24]; DBP SMD = − 0.68 [− 1.24; − 0.13]).
Bias and quality of evidence assessment
In general, studies present a low to moderate risk of bias in all domains (Fig. 5). Just one study mentions the previous existence of protocols or clinical study records, making it difficult to analyse bias related to the selection of reported results. None of the studies reported intention-to-treat analysis, conflicts of interest or participants were blinded to interventions, what is expected in physical exercise interventions and does not seem to be a major problem in this type of intervention59. Tests for subgroup differences showed no differences between studies at high risk of bias in relation to others in SBP (p = 0.37) and MBP (p = 0.11). A difference was identified in DBP (p < 0.01), however the effect favors studies with lower risk of bias (SMD = − 0.58 [− 0.77; − 0.38]) compared to studies with high risk of bias (SMD = 0.03 [− 0.35; 0.41]). The publication bias tests showed no asymmetries in the funnel plot for SBP (Egger’s regression p = 0.818), DBP (Egger’s regression p = 0.398) or MBP reactivity (Egger’s regression p = 0.557). However, four omitted results are expected by trim and fill funnel plots only in SBP (Fig. 6). Quality of evidence analysis show moderate (SBP) to high (DBP) certainty of evidence (Table 3).
Discussion
Our main results were that 60% (18 out of 30) of the included studies reported attenuated BP peaks (either in SBP, DPB, and/or MBP) after acute exercise and none showed deleterious results from the exercise. The metanalytic results suggest that acute exercise attenuates BP reactivity to stress. This effect occurred mutually in SBP (SMD = − 0.38 [− 0.49; − 0.27]), DBP (SMD = − 0.51 [− 0.70; − 0.33]) and MBP (SMD = − 0.51 [− 0.72; − 0.31]) in magnitudes similar to previous meta-analyse about the effects of acute aerobic exercise (SBP Effect size = 0.38; DBP Effect size = 0.40)12. Besides that, only 30% of the studies included non-aerobic exercises which make the results for these exercise mode difficult to generalize. Lastly, there is a scarcity of studies with hypertensive individuals (10%) and with a population over 40 years old (13%). As for the quality of the evidence, the possible publication bias in SBP may be due only to a physiological response, since the expected omitted results would favor interventions with exercises. In this way, the quality of evidence of SBP would be high.
In this sense, we reaffirm the need for studies with high cardiovascular risk patients, since these responses contribute to the construction of the clinical picture of these patients and may indicate an increase in left ventricular mass9, augmented carotid atherosclerosis60, increased risk of cardiovascular mortality61, development of hypertension11, and an increased risk of developing several cardiovascular diseases2,4. We also extend this need for studies with the elderly, who, in addition to having the aforementioned advantages of having a high incidence of cardiovascular diseases62, seem to have very promising responses when compared to younger people63, so studies exploring specific age stratus are needed. We also emphasize that, in addition to expanding and confirming favorable responses to aerobic exercise12, the present study is, as far as we know, the first to demonstrate positive meta-analytic effects of resistance exercise in BP reactivity. It is worth mentioning that these results are anchored in a smaller volume of evidence, and should be interpreted with caution, but it provides an optimistic direction for future studies with this exercise mode.
Regarding intervention characteristics, studies that compare different exercise loads showed mixed results. As an example, three studies evaluated different exercise intensities, and one was favorable to higher intensities25, another obtained a very discreet advantage at greater intensities47, and the latter found no differences between groups40. Concerning exercise session duration, a study shows favorable effects of longer sessions51, and the others found no differences32,46. Finally, a study compared continuous aerobic exercise of moderate intensity with high-intensity interval exercise and also found no significant differences50. Although still scarce, the results with resistance exercises are also inconsistent, with higher training volumes (50 min compared to 30 min) seem to be more effective at moderate intensity51, but low41 or very high intensities42 show little or no favorable results when performed for 30–45 min. Thus, evidence on differences arising from the characteristics of exercise load control is still scarce, therefore a meta-analysis clustering intensity groups was not possible. However, the evidence is greater in moderate exercises for 30–60 min.
Overall, when exploring studies heterogeneity, we found that reductions in peak DBP appear to be more heterogeneous than those in SBP. In addition, the greatest effects found are usually in subgroups with fewer studies, and most of the heterogeneity seems to be driven by studies published before the year 2000. So, regarding the effect on DBP response, several results must be highlighted. The first is that, in sex comparisons, the high heterogeneity in men (I2 = 74%) draws attention and seems to be explained by Ebbesen et. al study32. This study has a very favorable effect on exercise and is not overlapping with other studies, and with its suppression, we have important reductions in heterogeneity and effect size (I2 = 25%; SMD = − 0.33[− 0.54; − 0.12]). The large volume of exercise in this study (from 60 to 120 min) may also explain this difference. Also, there is an important DBP responses heterogeneity related to studies that include both sexes (I2 = 76%), which is expected due to the lower specificity of the population. A point that still draws a lot of attention in comparisons by sex, is the large effect size related to studies without defined sex (− 1.16 [− 1.72; − 0.59]). However, this subgroup has only 2 studies, and one of them31 has an exceptionally large effect (− 2.06 [− 3.28; − 0.85]).
Besides that, there is high DBP responses heterogeneity in studies with aerobic exercise (I2 = 73%). The main characteristics of these studies that may explain their differences to the others in the subgroup are the inclusion of hypertensive patients31,39, the high volume of exercise (from 60 to 120 min)32 and the self-selected exercise intensity strategy34. Regarding the studies with resistance exercises, the heterogeneity is significantly reduced (from I2 = 60%, to I2 = 22%, with SMD = − 0.72[− 1.00; − 0.45]) with the suppression of one study42. This heterogeneity might be explained by the alternative training with an intensity much higher than that of other studies (eccentric phase training at 120% of 1 repetition maximum test). Furthermore, the high DBP responses heterogeneity (I2 = 88%) and the greater effect size (− 0.96 [− 1.69; − 0.22]) in studies with RCT design are also noteworthy. In this regard, when we remove the study from Ebbesen et al.32, drastically reduces the heterogeneity and effect size of the subgroup (I2 = 0%; SMD = − 0.28[− 0.64; 0.08]). This might be explained by the large volume of exercise in this study (from 60 to 120 min) compared to the others in the subgroup (up to 45 min).
Considering types of stressors, there are moderate effects in studies that present physical tests (isolated or both), but mental tests alone have small effects. This may indicate greater effects of exercise in situations of physical stress than in situations of mental stress. Furthermore, the high DBP responses heterogeneity of the group with associated physical and mental stressors (I2 = 86%) draws attention, but it was expected due to the heterogeneity of the stress tests. However, 2 studies stand out in this subgroup for having lower results that don’t overlap with the others32,39. The main characteristics of these studies that can explain their differences in relation to the others are the large volume of training (from 60 to 120 min) in one study32 and the inclusion of hypertensive patients in the other39. Another interesting fact is that both studies were carried out in the 1990s. However, it is difficult to be precise about the role of the stressors types, since most of the tests inflict isolated mental stress, and after the suppressions proposed in this analysis, only 4 studies remained in the subgroup with both types of stressors. The suppression of these studies reduces the associated stressor types subgroup heterogeneity (I2 = 0%) and effect size (0.02 [− 0.37; 0.41]), generating a situation in which studies with isolated effects (mental or physical) have effects weak to moderate while in tests with both associated have null effects. These studies also have no overlap with the others from the multiple stressors subgroup of DBP analysis, and their suppression generates a reduction in heterogeneity (from I2 = 85%, to I2 = 0%) and in effect size (from − 0.68 [− 1.24; − 0.13], to − 0.17 [− 0.45; 0.11]) in this subgroup. However, even if the effect on these two subgroups becomes null, we emphasize that they are small subgroups (between 4 and 5 studies after suppressions) and the overall effect remains favorable to exercise (SMD = − 0.37 [− 0.51; − 0.23]).
Another source of heterogeneity could be the fact that several stress tests were used, from classically standardized and widely used protocols such as the cold pressor test64 to less restricted but with greater ecological validity as study task26. In this sense, we believe that a convergence of these characteristics is necessary, to combine sufficient standardization of methods with greater continuity with the stress experienced in daily life5. Thus, studies with multiple stressors such as the Trier Social Stress Test (that includes public speaking with a simulated job interview and arithmetic task) and the Maastricht Acute Stress Test (that includes cold pressure stress, negative feedback, and arithmetic task) seem to be good alternatives for future studies5.
As the types of stressors, their mechanisms of action are also diverse. So, these stressor types differences may have occurred due to different mechanisms triggers, with mental stressors appearing to activate frontal lobes and limbic structures that connect to the hypothalamus, while physical stressors recruit the brainstem and hypothalamus1,5. Furthermore, in the present study, physical stressors are mainly represented by the cold pressor test, and in this sense, local exposure to cold causes a rapid vasoconstriction response as a thermoregulatory measure65. This response is primarily mediated by noradrenaline via the α2-adrenergic receptor, and subsequently by peripheric responses, such as reduction of endothelial nitric oxide synthase activity (reducing the nitric oxide-mediated vasodilation) and increase in mitochondrial reactive oxygen species (resulting in vasoconstriction via Rho-kinase signaling mechanisms)65. But we emphasize that the mechanisms responsible for the responses to different stress tests are still poorly explored in the literature and should be encouraged.
Furthermore, in a broader sense, when a stressful situation is imposed, it generates a response that includes diverse mechanisms1,2,3. So, it is an instantaneous activation of the autonomic nervous system to produce physiological arousal with parasympathetic withdrawal66,67, changing the dynamics of neural networks, with a dominance of salience network over executive control and default mode networks68,69, and stimulation of the hypothalamic–pituitary–adrenal axis1,2. These central changes generate increased release of catecholamines3,70, opioids/β endorphin71,72, and specially cortisol73,74. So, the isolated and interaction effects75 of these mechanisms may explain the BP reactivity to stress3,76. Exercise, in turn, seems to mitigate stress reactivity by reducing vascular resistance39, norepinephrine77, and hypothalamic–pituitary–adrenal axis responses78, in addition to causing increased β2-mediated vasodilation77 and levels of endorphins79. Finally, there are also psychosocial effects of exercise such as improved self-efficacy and distraction from negative feelings80.
It should be emphasized that the present review has some limitations, such as the multiplicity of stress tests and exercise prescription, which makes it difficult to generalize the results. Besides that, these results are mostly in healthy and young populations and therefore cannot be easily generalized to populations with different health conditions. Thus, in future studies, we encourage the research of stressors similar to everyday life, involving different situations, sensations, emotions, and especially extended stressors like those found in sports, social fragility, and scholar/work environment. In this sense, we highlight a study26, which despite achieving null results, has an interesting approach with great ecological validity (40 min of studying with undergraduate students). Finally, we also encourage studies that allow a better understanding of exercise load control (e.g., intensity, volume), and in older populations with different morbidities, which can help to improve individual intervention strategies. As a clinical application, physical exercise can be a strategy to reduce hypertensive peaks in individuals who present stressful situations during activities of daily living, thereby reducing cardiovascular risk.
Conclusion
In summary, acute physical exercise lowers SBP, DBP, and MBP reactivity to stressor tests. However, these results refer mainly to healthy younger adults, who represented a largest part of the analyzed sample. Moreover, given the small magnitude of effects, the clinical relevance of this result must be interpreted with caution and be better explored. Further studies would help understand the effect of different exercise modalities to apply to different clinical profiles (e.g. normotensive vs. hypertensive subjects), helping explore the clinical application of this screening tool. Also, in future studies, we encourage the researcher to use stressors similar to everyday life, making research results more applicable.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rab, S. L. & Admon, R. Parsing inter- and intra-individual variability in key nervous system mechanisms of stress responsivity and across functional domains. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2020.09.007 (2020).
Huang, C.-J., Webb, H. E., Zourdos, M. C. & Acevedo, E. O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 4, 314 (2013).
Chrousos, G. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381 (2009).
Turner, A. I. et al. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 114, 104599 (2020).
Bali, A. & Jaggi, A. S. Clinical experimental stress studies: Methods and assessment. Rev. Neurosci. 26, 555–579 (2015).
Arima, H., Barzi, F. & Chalmers, J. Mortality patterns in hypertension. J. Hypertens. 29, S3–S7 (2011).
Muntner, P. Response to letter to editor “2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults”. J. Am. Soc. Hypertens. 12, 239 (2018).
Halliwill, J. R., Buck, T. M., Lacewell, A. N. & Romero, S. A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise?. Exp. Physiol. 98, 7–18 (2013).
Georgiades, A., Lemne, C., de Faire, U., Lindvall, K. & Fredrikson, M. Stress-induced laboratory blood pressure in relation to ambulatory blood pressure and left ventricular mass among borderline hypertensive and normotensive individuals. Hypertension 28, 641–646 (1996).
Wood, D. L., Sheps, S. G., Elveback, L. R. & Schirger, A. Cold pressor test as a predictor of hypertension. Hypertension 6, 301–306 (1984).
Matthews, K. A., Woodall, K. L. & Allen, M. T. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension 22, 479–485 (1993).
Hamer, M., Taylor, A. & Steptoe, A. The effect of acute aerobic exercise on stress related blood pressure responses: A systematic review and meta-analysis. Biol. Psychol. 71, 183–190 (2006).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6, e1000097 (2009).
Page, M. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. https://doi.org/10.31222/osf.io/v7gm2 (2020).
Mariano, I. M., Amaral, A. L. & Puga, G. M. Protocol of a systematic review with meta-analysis: Acute effects of physical exercise on blood pressure responsiveness to non-cardiopulmonary stress tests https://doi.org/10.17504/protocols.io.bhw3j7gn (2020).
Balduzzi, S., Rücker, G. & Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 22, 153–160 (2019).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Raudenbush, S. W., Hunter, J. E. & Schmidt, F. L. Methods of meta-analysis: Correcting error and bias in research findings. J. Am. Stat. Assoc. 86, 242 (1991).
Petropoulou, M. & Mavridis, D. A comparison of 20 heterogeneity variance estimators in statistical synthesis of results from studies: A simulation study. Stat. Med. 36, 4266–4280 (2017).
Higgins, J. P., Savović, J., Page, M. J. & Sterne, J. A. RoB 2: A revised cochrane risk-of-bias tool for randomized trials. BMJ 1–24 (2019) (in press). https://methods.cochrane.org/
McGuinness, L. A. & Higgins, J. P. T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods https://doi.org/10.1002/jrsm.1411 (2020).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000).
Schünemann, H. J. et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 336, 1106–1110 (2008).
Roy, M. & Steptoe, A. The inhibition of cardiovascular responses to mental stress following aerobic exercise. Psychophysiology 28, 689–700 (1991).
Flory, J. D. & Holmes, D. S. Effects of an acute bout of aerobic exercise on cardiovascular and subjective responses during subsequent cognitive work. J. PsychosomReasearricch 35, 225–230 (1991).
Roth, D. L. Acute emotional psychophysiological effects of aerobic exercise. Psychophysiology 26, 593–602 (1989).
Leow, S. et al. The effect of antecedent exercise on the acute stress response and subsequent food consumption: A preliminary investigation. Physiol. Behav. 229, 113256 (2021).
Bartholomew, J. B. Stress reactivity after maximal exercise: The effect of manipulated performance feedback in endurance athletes. J. Sports Sci. 18, 893–899 (2000).
Benvenutti, M. J. et al. A single session of hatha yoga improves stress reactivity and recovery after an acute psychological stress task-A counterbalanced, randomized-crossover trial in healthy individuals. Complement. Ther. Med. 35, 120–126 (2017).
Boone, J. B., Probst, M. M., Rogers, M. W. & Berger, R. Postexercise hypotension reduces cardiovascular responses to stress. J. Hypertens. 11, 449–453 (1993).
Ebbesen, B. L., Prkachin, K. M., Mills, D. E. & Green, H. J. Effects of acute exercise on cardiovascular reactivity. J. Behav. Med. 15, 489–507 (1992).
Hamer, M., Jones, J. & Boutcher, S. H. Acute exercise reduces vascular reactivity to mental challenge in offspring of hypertensive families. J. Hypertens. 24, 315–320 (2006).
Monroe, D. C., Yin, J., McCully, K. K. & Dishman, R. K. Yoga aids blood pressure recovery after exposure of forehead to cold: A pilot study. Altern. Ther. Health Med. 24, 12–17 (2018).
Moreira, S. R., Lima, R. M., Silva, K. E. S. & Simões, H. G. Combined exercise circuit session acutely attenuates stress-induced blood pressure reactivity in healthy adults. Braz. J. Phys. Ther. 18, 38–46 (2014).
Péronnet, F. et al. Blood pressure and plasma catecholamine responses to various challenges during exercise-recovery in man. Eur. J. Appl. Physiol. Occup. Physiol. 58, 551–555 (1989).
Probst, M., Bulbulian, R. & Knapp, C. Hemodynamic responses to the stroop and cold pressor tests after submaximal cycling exercise in normotensive males. Physiol. Behav. 62, 1283–1290 (1997).
Rejeski, W. J., Thompson, A., Brubaker, P. H. & Miller, H. S. Acute exercise: Buffering psychosocial stress responses in women. Health Psychol. 11, 355–362 (1992).
West, S. G. et al. Postexercise vasodilatation reduces diastolic blood pressure responses to stress. Ann. Behav. Med. 20, 77–83 (1998).
Alderman, B. L., Arent, S. M., Landers, D. M. & Rogers, T. J. Aerobic exercise intensity and time of stressor administration influence cardiovascular responses to psychological stress. Psychophysiology 44, 759–766 (2007).
Heffernan, K. S. et al. Carotid artery reactivity during sympathetic activation following acute resistance exercise. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 27, 417–421 (2017).
Paine, N. J. et al. Eccentric-exercise induced inflammation attenuates the vascular responses to mental stress. Brain. Behav. Immun. 30, 133–142 (2013).
Rooks, C. R., McCully, K. K. & Dishman, R. K. Acute exercise improves endothelial function despite increasing vascular resistance during stress in smokers and nonsmokers. Psychophysiology 48, 1299–1308 (2011).
Santaella, D. F. et al. Aftereffects of exercise and relaxation on blood pressure. Clin. J. Sport Med Off. J. Can. Acad. Sport Med. 16, 341–347 (2006).
Someya, N., Ikemura, T. & Hayashi, N. Effect of preceding exercise on cerebral and splanchnic vascular responses to mental task. J. Physiol. Anthropol. 31, 17 (2012).
Hobson, M. L. & Rejeski, W. J. Does the dose of acute exercise mediate psychophysiological responses to mental stress?. J. Sport Exerc. Psychol. 15, 77–87 (1993).
Rejeski, W. J., Gregg, E., Thompson, A. & Berry, M. The effects of varying doses of acute aerobic exercise on psychophysiological stress responses in highly trained cyclists. J. Sport Exerc. Psychol. 13, 188–199 (1991).
Szabo, A. et al. Psychophysiological profiles in response to various challenges during recovery from acute aerobic exercise. Int. J. Psychophysiol. 14, 285–292 (1993).
Neves, F. J. et al. Hemodynamic mechanisms of the attenuated blood pressure response to mental stress after a single bout of maximal dynamic exercise in healthy subjects. Braz. J. Med. Biol. Res. 45, 610–616 (2012).
Meireles, K. et al. Acute effects of moderate-intensity and high-intensity exercise on hemodynamic and autonomic reactivity to the cold pressor test in young adults with excess body weight. Blood Press. Monit. https://doi.org/10.1097/MBP.0000000000000422 (2020).
Lima da Silva, M. F. et al. The volume of resistance exercises influences blood pressure reactivity to stress. Rev. Bras. Med. do Esporte 21, 438–441 (2015).
Farah, N. M. F., Amran, A. D. & Che Muhamed, A. M. Attenuation of stress-induced cardiovascular reactivity following high-intensity interval exercise in untrained males. J. Sports Sci. 39, 2755–2762 (2021).
de Cerqueira Wanderley, E. et al. Hemodynamic response after concurrent cross exercise in hypertensive women/Respuestas hemodinámicas post ejercicio concurrente cruzado en mujeres hipertensas. Rev. Bras. Med. do Esporte 26, 122–125 (2020).
Peronnet, F., Massicotte, D., Paquet, J. E., Brisson, G. & de Champlain, J. Blood pressure and plasma catecholamine responses to various challenges during exercise-recovery in man. Eur. J. Appl. Physiol. Occup. Physiol. 58, 551–555 (1989).
Ikemura, T., Someya, N. & Hayashi, N. Autoregulation in the ocular and cerebral arteries during the cold pressor test and handgrip exercise. Eur. J. Appl. Physiol. 112, 641–646 (2012).
de Oliveira, V. N. et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl. Physiol. Nutr. Metab. 37, 334–344 (2012).
Steptoe, A., Kearsley, N. & Walters, N. Cardiovascular activity during mental stress following vigorous exercise in sportsmen and inactive men. Psychophysiology 30, 245–252 (1993).
Throne, L. C., Bartholomew, J. B., Craig, J. & Farrar, R. P. Stress reactivity in fire fighters: An exercise intervention. Int. J. Stress Manag. 7, 235–246 (2000).
Armijo-Olivo, S. et al. Blinding in physical therapy trials and its association with treatment effects. Am. J. Phys. Med. Rehabil. 96, 34–44 (2017).
Kamarck, T. W. et al. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged finnish men. Circulation 96, 3842–3848 (1997).
Carroll, D. et al. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology 49, 1444–1448 (2012).
Yazdanyar, A. & Newman, A. B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 25, 563–577 (2009).
Uchino, B. N., Birmingham, W. & Berg, C. A. Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 65, 154–162 (2010).
Hines, E. A. & Brown, G. E. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. Am. Heart J. 11, 1–9 (1936).
Alba, B. K., Castellani, J. W. & Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 104, 1202–1214 (2019).
Smeets, T. Autonomic and hypothalamic–pituitary–adrenal stress resilience: Impact of cardiac vagal tone. Biol. Psychol. 84, 290–295 (2010).
Castaldo, R. et al. Acute mental stress assessment via short term HRV analysis in healthy adults: A systematic review with meta-analysis. Biomed. Signal Process. Control 18, 370–377 (2015).
Hermans, E. J., Henckens, M. J. A. G., Joëls, M. & Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 37, 304–314 (2014).
van Oort, J. et al. How the brain connects in response to acute stress: A review at the human brain systems level. Neurosci. Biobehav. Rev. 83, 281–297 (2017).
Brummett, B. H., Boyle, S. H., Kuhn, C. M., Siegler, I. C. & Williams, R. B. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology 46, 862–869 (2009).
McCubbin, J. A. Stress and endogenous opioids: Behavioral and circulatory interactions. Biol. Psychol. 35, 91–122 (1993).
Allen, A. J., McCubbin, J. A., Loveless, J. P. & Helfer, S. G. Effects of estrogen and opioid blockade on blood pressure reactivity to stress in postmenopausal women. J. Behav. Med. 37, 94–101 (2014).
Foley, P. & Kirschbaum, C. Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 35, 91–96 (2010).
Herman, J. P. et al. Comprehensive Physiology 603–621 (Wiley, 2016). https://doi.org/10.1002/cphy.c150015.
Gianaros, P. J. & Wager, T. D. Brain-body pathways linking psychological stress and physical health. Curr. Dir. Psychol. Sci. 24, 313–321 (2015).
Myers, B. Corticolimbic regulation of cardiovascular responses to stress. Physiol. Behav. 172, 49–59 (2017).
Brownley, K. A. et al. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med. Sci. Sports Exerc. 35, 978–986 (2003).
Nyhuis, T. J., Masini, C. V., Sasse, S. K., Day, H. E. W. & Campeau, S. Physical activity, but not environmental complexity, facilitates HPA axis response habituation to repeated audiogenic stress despite neurotrophin mRNA regulation in both conditions. Brain Res. 1362, 68–77 (2010).
Harber, V. J. & Sutton, J. R. Endorphins and exercise. Sports Med. 1, 154–171 (1984).
Mikkelsen, K., Stojanovska, L., Polenakovic, M., Bosevski, M. & Apostolopoulos, V. Exercise and mental health. Maturitas 106, 48–56 (2017).
Author information
Authors and Affiliations
Contributions
I.M.M. had the idea for the article. I.M.M., A.L.A., P.A.B.R. and G.M.P. participated in the study planning and structuring. I.M.M. and A.L.A. performed the literature search and data analysis. P.A.B.R. and G.M.P. drafted and critically revised the work. I.M.M., A.L.A., P.A.B.R., and G.M.P. approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mariano, I.M., Amaral, A.L., Ribeiro, P.A.B. et al. A single session of exercise reduces blood pressure reactivity to stress: a systematic review and meta-analysis. Sci Rep 12, 11837 (2022). https://doi.org/10.1038/s41598-022-15786-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15786-3
- Springer Nature Limited