Abstract
Tuberculosis (TB) is a global health problem of major concern. Identification of immune biomarkers may facilitate the early diagnosis and targeted treatment of TB. We used public RNA-sequencing datasets of patients with TB and healthy controls to identify differentially expressed genes and their associated functional networks. GBP1 expression was consistently significantly upregulated in TB, and 4492 differentially expressed genes were simultaneously associated with TB and high GBP1 expression. Weighted gene correlation analysis identified 12 functional modules. Modules positively correlated with TB and high GBP1 expression were associated with the innate immune response, neutrophil activation, neutrophil-mediated immunity, and NOD receptor signaling pathway. Eleven hub genes (GBP1, HLA-B, ELF4, HLA-E, IFITM2, TNFRSF14, CD274, AIM2, CFB, RHOG, and HORMAD1) were identified. The least absolute shrinkage and selection operator model based on hub genes accurately predicted the occurrence of TB (area under the receiver operating characteristic curve = 0.97). The GBP1-module-pathway network based on the STRING database showed that GBP1 expression correlated with the expression of interferon-stimulated genes (GBP5, BATF2, EPSTI1, RSAD2, IFI44L, IFIT3, and OAS3). Our study suggests GBP1 as an optimal diagnostic biomarker for TB, further indicating an association of the AIM2 inflammasome signaling pathway in TB pathology.
Similar content being viewed by others
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB) infection, remains one of the most significant health problems worldwide. The situation is dire, especially in the 30 high-TB-burden countries, which are predominantly low- and middle-income countries. According to the World Health Organization1, an estimated 10 million people were newly infected with TB in 2017. Of these, only 6.4 million were diagnosed and officially notified. It is estimated that 1.3 million people die from TB each year. In addition, more than one million children developed active TB in 2016, with 250,000 children dying from the disease1. Bacille Calmette-Guérin (BCG), a live attenuated vaccine, was first administered to humans in 1921 and has since been administered to more people than any other vaccine in history2. However, recent research has shown that BCG had only an estimated 19% effectiveness (95% confidence interval 8–29%) in protecting against MTB infection3,4, with almost no protective effect in adults. The mortality rate of TB in the pre-treatment era was nearly 50%, which was substantially higher than that in older children5. This number represents 10% of the total global burden of incident TB and 15% of the associated total mortality1. Therefore, there is an urgent need to identify new targets for prevention and treatment, which requires further research into the pathogenic mechanisms involved in MTB infection and the host response.
MTB can invade susceptible organisms via the respiratory tract, digestive tract, and skin damage. The bacterium is then swallowed by macrophages at the site of the infected tissue, and antigens are extracted into the lymphocytes. The innate immune system is the host’s first line of defense against invading pathogens, and the inflammasome plays an essential role in this process6,7. The guanylate-binding protein (GBP) family of interferon-inducible GTPases promotes antimicrobial immunity and consists of seven members in humans8. GBPs can target intracellular pathogens to mediate the defense response of the host via inflammasomes, oxidative responses, and autophagy9,10,11,12,13.
GBP1 is a 65-kD GTPase protein in the GBP family that plays a crucial role in innate immunity14, comprising a conserved region with an N-terminal globular GTPase domain and a C-terminal helical domain. Isoprenylation of the C-terminal CaaX box of GBP1 can achieve membrane anchoring15. Previous studies have shown that GBP1 promotes activation of inflammasomes to mediate pyroptosis and has dual membrane-disruptive actions to induce the atypical apoptosis of Salmonella typhimurium or Toxoplasma gondii in infected macrophages16. However, it is unclear whether GBP1 is associated with inflammasome activation in TB. Therefore, in this study, we searched for differentially expressed genes (DEGs) in TB patients based on public databases, and further investigated the DEGs associated with variations in GBP1 expression and their functional networks. These findings can suggest GBP1 as a new biomarker or therapeutic target, and provide further insight into the pathogenic mechanism of TB.
Results
Identification of DEGs in TB
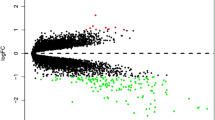
Data were extracted from the Gene Expression Omnibus (GEO) database, including the gene expression matrix, clinical characteristics, and probe set (https://www.ncbi.nlm.nih.gov/geo/). In the GSE83456 dataset (including 92 TB patients and 61 healthy controls), we found that GBP1 expression was significantly upregulated in TB samples compared to the control samples [P = 2.580e-30 and log fold change (FC) = 2.158] (Fig. 1A). This suggested that high expression of GBP1 may be associated with TB. Along with GBP1, compared to the control samples, we identified a total of 5302 DEGs in TB, 3274 of which were upregulated and 2028 of which were downregulated (Fig. 1B). Moreover, there were 5023 DEGs in the GBP1-high samples compared to the GBP1-low samples (divided according to the median GBP1 expression level), including 2129 upregulated genes and 2894 downregulated genes (Fig. 1C). A total of 4492 genes were found at the intersection of the two DEG sets, which indicated a relation to the expression of GBP1 and to TB. The 25 most strongly upregulated and 25 most strongly downregulated genes in TB are visualized using a heat map in Fig. 1D. The upregulated genes mainly included interferon-stimulated genes (ISGs) and inflammasome activation-related genes (CASP5 and CARD17).
Differential expression gene analysis. (A) GBP1 is up-regulated in TB (P = 2.580e-30, 92 TB patients and 61 normal people). (B) Volcano plot of the TB-control, red represents up-regulated genes, blue represents down-regulated genes, and black represents no significantly differentially expressed genes. (C) Volcano plot of the GBP1-low/high, red represents up-regulated genes, blue represents down-regulated genes, and black represents no significantly differentially expressed genes. (D) A heatmap of 25 most up-regulated and 25 most down-regulated genes.
Module associated with TB
Weight gene correlation network analysis (WGCNA) was performed using the expression profile of genes commonly associated with TB and GBP1 expression to identify the key module with the strongest correlation to TB. Among the 12 modules identified (Fig. 2A), the turquoise module was significantly positively correlated with TB (correlation coefficient = 0.73, P = 2e-26; Fig. 2B). According to the criteria of a gene significance (GS) value > 0.6 and module membership (MM) > 0.9, 11 genes were identified as hub genes (GBP1, HLA-B, ELF4, HLA-E, IFITM2, TNFRSF14, CD274, AIM2, CFB, RHOG, and HORMAD1). Correlation analysis showed that GBP1 was strongly correlated with the other hub genes, and the correlation coefficients between GBP1 and HLA-B, HLA-E, CD274, AIM2, and CFB were all > 0.7 (Fig. 2C). Module function enrichment analysis further showed that turquoise module genesare significantly involved in biological processes related to regulation of the innate immune response, neutrophil activation, and neutrophil-mediated immunity (Fig. 2D), and the module was significantly enriched in the NOD receptor signaling pathway (Fig. 2E).
Weighted correlation network analysis. (A) Recognition module, each module was given an individual color as identifiers, including 12 different modules. (B) Correlation heat map of gene modules and phenotypes, the red was positively correlated with the phenotype, blue was negatively correlated with the phenotype. (C) The correlation between GBP1 and hub genes,red indicated negative correlation and blue indicated positive correlation. (D) Biological processes of module genes, the significance of enrichment gradually increased from blue to red, and the size of the dots indicates the number of differential genes contained in the corresponding pathway17,18,19. (E) KEGG pathways analysis of module genes. The significance of enrichment gradually increased from blue to red, and the size of the dots indicated the number of differential genes contained in the corresponding pathway17,18,19.
Network of GBP1
We extracted the genes found to interact with GBP1 in the modules based on the STRING database that also showed differential expression (logFC) in TB (Fig. 3A). The correlation analysis of GBP1 and these genes in the GSE83456 dataset was consistent with the results of the network construction diagram (Fig. 3B). According to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, these significant genes were enriched in the RIG-I-like receptor signaling pathway. Both network and correlation analyses showed that GBP1 is strongly correlated with the interferon-stimulated genes BATF2, GBP5, EPSTI1, RSAD2, IFI44L, IFIT3, and OAS3.
Network construction of GBP1 modular genes. (A) The PPI network construction of GBP1 and each modular genes based on the STRING database, red was up-regulated genes and blue was down -regulated genes, the value was adjusted by the false discovery rate (FDR). (B). The correlation between GBP1 and modular genes, red indicates negative correlation and blue indicates positive correlation.
Combined predictive marker of TB
We extracted the expression profiles of the hub genes to construct a least absolute shrinkage and selection operator (LASSO) model (Fig. 4A). Using the LASSO method, four genes were identified with non-zero regression coefficients and a minimum lambda value of 0.09878891. The model index of these four genes was defined as GBP1 × 0.1568 + HLA − B × 0.0845 + ELF4 × 0.0965 + TNFRSF14 × 0.0279. The area under the receiver operating characteristic (ROC) curve (AUC) (Fig. 4B) of this four gene-based model was 0.97 in the training set, indicating good predictive ability; thus, the LASSO model may be used as a biomarker of TB. This was further validated in two independent GEO datasets used as validation set (GSE42834 and GSE19491) with AUC values of 0.998 and 0.96, respectively (Fig. 4C and D). Furthermore, we found that GBP1 was also highly upregulated in the blood samples of TB patients in the GSE42834 and GSE19491 datasets (Fig. 4E, F). This indicated that GBP1 and its related hub genes are strongly associated with TB and could potentially serve as diagnostic biomarkers.
A model for predicting TB and verification of expression of GBP1 in differential datasets. (A) LASSO model. (B) ROC curves analysis of train set (GSE83456). (C) ROC curves analysis of validation (GSE42834). (D) ROC curves analysis of validation (GSE19491). (E) GBP1 is up-regulated in TB (P = 2.580e-30, 35 TB patients and 113 normal samples are contained). (F) GBP1 is up-regulated in TB (P = 2.580e-30, 68 TB patients and 36 normal samples are contained).
Discussion
According to the 2018 Global Tuberculosis Report of the World Health Organization1, there were approximately 10 million new cases of TB (5.8 million men, 3.2 million women, and 1 million children) worldwide in 2017, leading to approximately 1.6 million deaths. At present, the global control of TB faces major challenges, including the lack of effective vaccines, rapid and sensitive diagnostic methods, and effective treatment strategies for drug-resistant bacteria. Studying the body’s immune regulation mechanism against MTB will help to identify new methods for the diagnosis and treatment of TB. Previous studies have focused on the mechanism of adaptive immunity in TB, specifically in T helper cells. In recent years, increasing attention has been paid to the role of the innate immune system. Innate immunity is not only the first barrier of the body’s immune system but is also an indispensable factor in initiating adaptive immunity. Innate immune components can quickly recognize foreign antigens depending on the expression of various pattern recognition receptors20.
In the present study, we found that GBP1 expression was generally upregulated in TB patients, suggesting that high expression of GBP1 may play a role in fighting MTB infection. Furthermore, ISGs and inflammasome-activation genes such as CASP5 and CARD17 were highly expressed in both the TB and GBP1-high groups, suggesting that GBP1 is associated with the interferon signaling pathway and inflammasome activation in TB. According to WGCNA, a total of 12 modules associated with TB were identified. Moreover, according to the criteria of GS > 0.6 and MM > 0.9, 11 genes were identified as hub genes in the TB module, including GBP1, HLA-B, ELF4, HLA-E, IFITM2, TNFRSF14, CD274, AIM2, CFB, RHOG, and HORMAD1. Among them, HLA-B and HLA-E belong to the major histocompatibility complex class I protein complex and are involved in the presentation of foreign antigens to CD8( +) T cells in TB21,22. ELF4 plays a role in the development and function of natural killer (NK) and NK T cells, as well as in innate immunity, and controls the proliferation and homing of CD8 + T cells via the Kruppel-like factors KLF4 and KLF223; however, its mechanism of action in TB has not yet been explored. IFITM encodes a transmembrane protein induced by interferon that can restrict the intracellular growth of MTB24. MTB can promote regulatory T-cell expansion via the induction of programmed death-1 ligand 1 (CD274) on dendritic cells25. AIM2 encodes a type of inflammasome that can recognize intracellular double-stranded DNA (dsDNA) and mediate the release of the pro-inflammatory cytokines interleukin (IL)-1B and IL-1826. Furthermore, we found that GBP1 expression was highly correlated with the expression levels of HLA-B, HLA-E, CD274, AIM2, and CFB, which further supported that GBP1 may play an important role in innate immunity such as antigen presentation and AIM2 inflammasome activation.
We further explored the TB and GBP1-related biological processes and pathways. Functional enrichment analysis showed that modules with a strong correlation to TB were significantly involved in several immune-related biological processes, including the innate immune response, neutrophil activation, neutrophil-mediated immunity, and the NOD receptor (NLR) signaling pathway. Thus, interactions between GBP1 and AIM inflammasome-related NLRs could contribute to TB infection and pathogenesis.
Multiple studies have demonstrated that GBP1 plays an important role in MTB infection; however, its mechanism of action remains controversial. Interferon-gamma was found to initiate transcription of the mouse Gbps gene, which is mobilized as part of a new inducible antimicrobial program to combat infection27. Moreover, GBP7 participates along with GBP1 in the trafficking of mono-ubiquitinated protein cargo to autolysosomes to generate ubiquitin (Ub)-derived antimicrobial peptides in TB27. Specifically, GBP1 binds to p62-Ub for delivery to LC3b + membranes and for engulfment, and is then recycled after p62-Ub delivery. GBP7 recruits ATG4B for LC3b + membrane elongation and closure around this cargo to generate lytic peptides that kill mycobacteria after fusion with phagosomes27. Zak et al.28 also showed that GBP1 plays a protective role during TB infection. Moreover, Ali et al.29 suggested that Ms_PE31 of MTB activated the NF-κB signaling pathway to mediate the expression of IL-10 and GBP1 protein in macrophages. They further suggested that IL-10 and GBP1 might inhibit the activation of caspase-3 and lead to the attenuation of macrophage apoptosis, thereby fostering the intracellular survival of mycobacteria and establishment of infection (Fig. 5). In summary, the role of GBP-1 in MTB infection remains unclear.
MTB can infect cells such as macrophages and dendritic cells, and subvert them to propagate and disseminate within the host. MTB releases components such as bacterial DNA into the cytosol via mechanisms that are still not completely understood30. AIM2 recognizes intracellular dsDNA and mediates the release of the pro-inflammatory cytokines IL-1B and IL-18, which has been associated with protection against pulmonary tuberculosis (PTB)26. However, no study has yet explored whether GBP1 is involved in activation of the AIM2 inflammasome in TB. The dual membrane-disruptive actions of GBP1 in Salmonella Typhimurium or T. gondii infection promotes the detection of dsDNA by AIM2 and induces cell pyroptosis16,31. Pyroptosis has also been found to be significantly associated with TB and to play a crucial role in the host defense against intracellular MTB30,32,33,34. Our study provides support for these previous findings, further revealing the interaction between GBP1 and the AIM2 inflammasome signaling pathway.
In addition, the expression profiles of hub genes were extracted to construct a LASSO model, in which the regression coefficients of the four genes were non-zero values. ROC curve analysis showed that the LASSO model had a high AUC value, showing strong discrimination against TB in healthy individuals, indicating that it may serve as a biomarker of TB. This was verified using an independent dataset. We further found that GBP1 expression was consistently upregulated in various datasets, which supports the involvement of high GBP1 expression in the pathogenesis of TB.
In summary, this study demonstrates that bioinformatics analysis can reveal important molecular pathways in TB. However, the potential key pathways and genes require further verification in molecular experiments.
Methods
Data
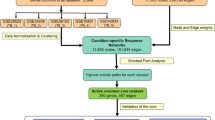
The R/Bioconductor package GEOquery was used to extract the GSE83456, GSE42834, and GSE19491 data from the GEO database. The GSE83456 dataset includes whole blood samples from 92 TB patients (47 with extrapulmonary tuberculosis and 45 with PTB) and 61 healthy individuals, which was used to explore the potential involvement of GBP1 in TB. There was no difference in sex or age distribution between the TB and control groups (Table 1). In addition, the GSE42834 and GSE19491 datasets were used for validation, including samples from 35 PTB patients and 113 controls and 68 PTB patients and 36 controls, respectively. The workflow of the study is illustrated in Fig. 6. The original studies for compiling these datasets were approved by the Central London 3 Research Ethics Committee (09/H0716/41); CPP Sud-Est IV, France, CCPPRB; and les Hôpitaux Universitaires Pitié Salpêtrière, Paris. All participants provided written informed consent.
Gene expression profiling of datasets
The samples were the ones used to generate the described GEO datasets. Three milliliters of whole blood were collected from the children by standard phlebotomy before treatment and vigorously mixed immediately. RNA was isolated from the whole blood using a MagMAX-96 Blood RNA Isolation kit (Applied Biosystems/Ambion). Total RNA was globin-reduced using the GLOBINclear 96-well format kit (Applied Biosystems/Ambion). An Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA) was used to assess the total and globin-reduced RNA integrity. RNA targets (cRNA) were amplified using an Illumina CustomPrep RNA amplification kit (Ambion, Austin, TX, USA). Labeled cRNA was hybridized to Sentrix HT12 V3 BeadChip arrays overnight, followed by washing, blocking, staining, and scanning. Signal intensity analysis (per-chip normalization) was performed using Illumina BeadStudio version 2 software35. All gene expression measurements were log2-transformed and normalized according to the manufacturer’s instructions.
Identification of DEGs between TB and control groups
The limma package in R was used for differential expression analysis. Using the median expression level of GBP1 as the cut-off point, the subjects were divided into GBP1-high and GBP1-low expression groups. We used the lmFit and eBayes functions in the limma package to identify DEGs between the TB and control groups and between the GBP1-high and GBP1-low groups. P < 0.05 adjusted by the false discovery rate was considered to indicate a significant difference in expression level.
WGCNA
WGCNA was performed for genes that were commonly upregulated or downregulated in both the TB vs. control and GBP1-low vs. GBP1-high comparisons. We identified potential functional modules for biological function characterization of each subgroup. Subsequently, the soft thresholding power value (power = 7) was screened during module construction using the soft threshold function. The distance between each gene pair was evaluated on the basis of the similarity of the topological overlap matrix. In addition, average and dynamic methods were used for hierarchical clustering analysis, and then the clustering tree and gene modules were constructed. After merging the original 12 modules based on their similarities, Spearman correlation analysis in the WGCNA package was used to calculate the correlation coefficients and associated p-values between clinical features and functional modules. The clusterProfiler package in R was used to perform functional enrichment analyses of these functional modules.
Identification of hub genes and construction of the GBP1-module-pathway network
In WGCNA, GS is defined as the correlation between a gene and a phenotype and MM is defined as the measure of importance of a gene in a module according to the formula MM(i) = cor(x i, ME). In this study, a gene with GS > 0.6 and MM > 0.9 was defined as a hub gene among the candidate gene modules. In addition, DEGs that interact with GBP1 were extracted from the STRING database (https://STRING-db.org/). The correlation between the DEGs interacting with GBP1 and hub genes was then explored.
LASSO model and ROC curve analysis
LASSO has strong predictive value and is considered suitable for selecting the best features for high-dimensional data. We extracted the expression profiles of hub genes to construct the LASSO model for distinguishing TB and healthy samples using the glmnet package. Regression coefficients from LASSO analysis were used to create a model index for each sample to weigh the expression values of selected genes using the following formula: index = ExpGene1*Coef1 + ExpGene2*Coef2 + ExpGene3*Coef3 + …., where “Coef” is the regression coefficient of a gene, derived from the LASSO Cox regression, and “Exp” represents the expression values of the gene. To evaluate the ability of the LASSO model to identify TB, we used the pROC package to perform ROC curve analysis on the test (GSE83456) and validation (GSE42834 and GSE19491) datasets.
All methods were performed in accordance with the relevant guidelines and regulations.
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request (2231365607@qq.com; zhouyulin@163.com).
References
World Health Organization. Global Tuberculosis Report 2018. https://www.who.int/tb/publications/global_report/en/.
Basu Roy, R., Whittaker, E., Seddon, J. A. & Kampmann, B. Tuberculosis susceptibility and protection in children. Lancet Infect. Dis. 19, e96–e108 (2019).
Roy, A. et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ 349, g4643 (2014).
Hawn, T. R. et al. Tuberculosis vaccines and prevention of infection. Microbiol. Mol. Biol. Rev. 78, 650–671 (2014).
Jenkins, H. E. et al. Mortality in children diagnosed with tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 17, 285–295 (2017).
Chauhan, S., Jena, K. K., Mehto, S. et al. (2021). Innate Immunity and Inflammophagy: Balancing the Defence and Immune Homeostasis. FEBS J.
Ta, A. & Vanaja, S. K. Inflammasome activation and evasion by bacterial pathogens. Curr. Opin. Immunol. 68, 125–133 (2021).
Shenoy, A. R. et al. Emerging themes in IFN-gamma-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiology 212, 771–784 (2007).
Gomes, M. T. R., Cerqueira, D. M., Guimarães, E. S., Campos, P. C. & Oliveira, S. C. Guanylate-binding proteins at the crossroad of noncanonical inflammasome activation during bacterial infections. J. Leukoc. Biol. 106, 553–562 (2019).
Kim, B. H., Shenoy, A. R., Kumar, P., Bradfield, C. J. & MacMicking, J. D. IFN-inducible GTPases in host cell defense. Cell Host Microbe 12, 432–444 (2012).
Liu, B. C. et al. Constitutive interferon maintains GBP expression required for release of bacterial components upstream of pyroptosis and anti-DNA responses. Cell Rep. 24, 155-168.e5 (2018).
Santos, J. C. et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 (2020).
Costa Franco, M. M. et al. Brucella abortus triggers a cGAS-independent STING pathway to induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J. Immunol. 200, 607–622 (2018).
Qiu, X. et al. Down-regulation of guanylate binding protein 1 causes mitochondrial dysfunction and cellular senescence in macrophages. Sci. Rep. 8, 1679 (2018).
Tripal, P. et al. Unique features of different members of the human guanylate-binding protein family. J. Interferon Cytokine Res. 27, 44–52 (2007).
Fisch, D. et al. Human GBP1 differentially targets Salmonella and Toxoplasma to license recognition of microbial ligands and Caspase-mediated death. Cell Rep. 32, 108008 (2020).
Kanehisa, M. & Goto, S.; KEGG: Kyoto Encyclopedia of Genes and Genomes.Nucleic Acids Res. 28, 27–30 (2000). [pubmed] [doi].
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019) [pubmed] [doi].
Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M., & Tanabe, M.; KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545-D551 (2021). [pubmed] [doi]
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Lewinsohn, D. A. et al. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLOS Pathog. 3, 1240–1249 (2007).
Prezzemolo, T. et al. Detailed characterization of human Mycobacterium tuberculosis specific HLA-E restricted CD8+ T cells. Eur. J. Immunol. 48, 293–305 (2018).
Szabo, A. & Rajnavolgyi, E. Finding a fairy in the forest: ELF4, a novel and critical element of type I interferon responses. Cell. Mol. Immunol. 11, 218–220 (2014).
Ranjbar, S., Haridas, V., Jasenosky, L. D., Falvo, J. V. & Goldfeld, A. E. A role for IFITM proteins in restriction of Mycobacterium tuberculosis infection. Cell Rep. 13, 874–883 (2015).
Trinath, J., Maddur, M. S., Kaveri, S. V., Balaji, K. N. & Bayry, J. Mycobacterium tuberculosis promotes regulatory T-cell expansion via induction of programmed death-1 ligand 1 (PD-L1, CD274) on dendritic cells. J. Infect. Dis. 205, 694–696 (2012).
Figueira, M. B. A. et al. Single-nucleotide variants in the AIM2 - Absent in melanoma 2 gene (rs1103577) associated With protection for tuberculosis. Front. Immunol. 12, 604975 (2021).
Kim, B. H. et al. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332, 717–721 (2011).
Zak, D. E. et al. A blood RNA signature for tuberculosis disease risk: A prospective cohort study. Lancet 387, 2312–2322 (2016).
Ali, M. K. et al. Mycobacterium tuberculosis PE31 (Rv3477) attenuates Host Cell Apoptosis and Promotes Recombinant M. smegmatis Intracellular Survival via Up-regulating GTPase Guanylate Binding Protein-1. Front. Cell. Infect. Microbiol. 10, 40 (2020).
Costa Franco, M. M. S. et al. AIM2 senses Brucella abortus DNA in dendritic cells to induce IL-1β secretion, pyroptosis and resistance to bacterial infection in mice. Microbes Infect. 21, 85–93 (2019).
Kutsch, M. et al. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 39, e104926 (2020).
Stutz, M. D. et al. Macrophage and neutrophil death programs differentially confer resistance to tuberculosis. Immunity 54, 1758-1771.e7 (2021).
Rastogi, S., Ellinwood, S., Augenstreich, J., Mayer-Barber, K. D. & Briken, V. Mycobacterium tuberculosis inhibits the NLRP3 inflammasome activation via its phosphokinase PknF. PLOS Pathog. 17, e1009712 (2021).
Qu, Z. et al. Mycobacterial EST12 activates a RACK1-NLRP3-gasdermin D pyroptosis-IL-1β immune pathway. Sci. Adv. 6 (2020).
Bloom, C. I. et al. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS ONE 8, e70630 (2013).
Acknowledgements
This study was supported by the Science and Technology Project of Suzhou, China (grant number SKJY2021106).
Author information
Authors and Affiliations
Contributions
T.S. and L.H. designed the study, analyzed the data, and wrote the main manuscript. J.T. and Y.Z. helped perform the analysis with constructive discussions. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, T., Huang, L., Zhou, Y. et al. Role of GBP1 in innate immunity and potential as a tuberculosis biomarker. Sci Rep 12, 11097 (2022). https://doi.org/10.1038/s41598-022-15482-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15482-2
- Springer Nature Limited