Abstract
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory disorders of the gastrointestinal tract that share similar genetic risk factors. However, while fibrotic stricture of the intestine is a major characteristic of CD; it is rarely observed in UC. Deposition of collagen in the extracellular matrix contributes to the formation of fibrotic strictures in CD, but the underlying mechanisms are unknown. In the present study, we found that heat shock protein 47 (HSP47), a stress-response protein that acts as a molecular chaperone during the processing and secretion of collagen, expressed in the intestinal tissue from patients with CD. Serum HSP47 levels and anti-HSP47 antibody titers were significantly higher in patients with CD than in those with UC. Furthermore, anti-HSP47 antibody levels correlated significantly with fibrosis in CD. In addition, HSP47 inhibition significantly suppressed collagen production in fibroblasts in vitro. These findings suggest that HSP47 is a biomarker for differentiating fibrotic from non-fibrotic forms of CD. Additionally, we propose that HSP47 could be a potential target for treating fibrosis in patients with CD.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract characterized by an inappropriate inflammatory response to intestinal microbes1. IBD is typically further classified as ulcerative colitis (UC) or Crohn’s disease (CD)2. Recent investigations have identified a cluster of genetic susceptibility loci for IBD3,4. Both UC and CD often occur within the same family, suggesting that the relevant genes are common to both diseases1,5. However, the clinical manifestations of these diseases differ. UC is limited to the colon and is characterized by superficial mucosal inflammation, sometimes leading to bleeding and toxic megacolon. In contrast, CD affects any part of the gastrointestinal tract and is characterized by transmural inflammation of discontinuous lesions, often leading to fistula and abscess formation6. Fibrotic strictures are a major complication of CD but are rarely observed in UC7. Deposition of collagen in the extracellular matrix contributes to fibrotic stricture formation at wound-healing sites8. Knowledge of the molecular mechanisms underlying intestinal fibrosis has enabled the identification of several anti-fibrotic therapeutic targets9; however, further investigation into the mechanism for fibrotic stricture formation in CD is required to establish novel therapeutic targets.
Heat shock proteins (HSPs) are ubiquitous proteins that act as molecular chaperones and regulate the biosynthesis, folding, transport, and assembly of cellular proteins10. HSPs are induced in response to various stresses. Among the several subtypes of HSPs, HSP47 is a collagen-specific chaperone expressed in the endoplasmic reticulum (ER), and it plays an essential role in the processing and secretion of collagen from the ER and its subsequent transport to the Golgi apparatus11. HSP47 levels correlated positively with the degree of fibrosis and the amount of collagen secreted in rats with induced liver cirrhosis12. In addition, the number of HSP47-positive pneumocytes was significantly higher in patients with idiopathic pulmonary fibrosis than in patients with bronchiolitis obliterans13. HSP47 is involved in fibrotic stricture formation in CD, but its role in this process is unknown.
Based on the above observations, we hypothesized that high HSP47 levels are a potential indicator of CD in patients with IBD and that it may reflect disease progression of CD. In this study, we aimed to investigate the role of HSP47 in patients with IBD, particularly in those with CD.
Results
Patient characteristics
A total of 22 healthy controls, 26 patients with UC, and 32 patients with CD were included in the analysis, and their main characteristics are presented in Table 1. Age, height, weight, and body mass index (BMI) differed significantly among the three groups. There was no difference in disease evolution time between the UC and CD groups. A CAI > 4 was observed in 10 patients (38.5%) and a CDAI > 150 was observed in 9 patients (28.1%). No difference was observed in the ratio of active stage to remission stage between UC and CD groups. Eighteen patients (56.3%) with CD had been diagnosed with stricturing. Regarding the treatments being administered to patients at the time of evaluation of serum HSP47 and anti-HSP47 antibody levels, we observed that steroids were used significantly more frequently for patients in the UC group, while infliximab was used significantly more frequently for patients in the CD group (P = 0.045 and P < 0.001, respectively).
Differences in serum HSP47 and anti-HSP47 antibody levels among the control, UC, and CD groups
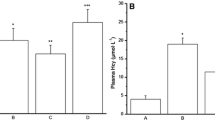
We compared serum HSP47 and anti-HSP47 antibody levels between the control, UC, and CD groups (Table 1). HSP47 levels were significantly higher in the CD group than in the UC group (P < 0.001). By contrast, neither the CD group and control group, nor the UC group and control group, differed significantly from each other (Fig. 1a). The CD group showed a significantly higher serum anti-HSP47 antibody titer than the UC and the control groups (P < 0.001, respectively), although the UC group and control group did not differ significantly from each other (Fig. 1b).
Comparison of serum HSP47 and anti-HSP47 antibody levels among control, UC, and CD groups. (a) Quantitative analysis of HSP47 levels in control (n = 22), UC (n = 26), and CD (n = 32) groups. (b) Quantitative analysis of anti-HSP47 antibody levels in control, UC, and CD groups. Each dot represents one subject, and bars indicate average ± SEM. *P < 0.001 (Dann–Bonferroni); **P < 0.001 (Scheffe test). HSP heat shock protein, UC ulcerative colitis, CD Crohn’s disease, ns not significant.
Differences in serum HSP47 and anti-HSP47 antibody levels according to disease activity in CD
To compare serum HSP47 and anti-HSP47 antibody levels according to the disease activity of CD, patients with CD were classified into two groups: a clinically active stage defined as CDAI ≥ 150 and a clinically remission stage defined as CDAI < 150 (Table 2). The CDAI ≥ 150 and CDAI < 150 groups did not differ significantly from each other in age, sex, height, weight, BMI, or current treatment. HSP47 levels did not vary significantly with CD activity (P = 0.77) (Fig. 2a). By contrast, serum anti-HSP47 antibody levels were significantly higher in the CDAI ≥ 150 group than in the CDAI < 150 group (P = 0.033) (Fig. 2b).
Comparison of serum HSP47 and anti-HSP47 antibody levels between CDAI < 150 and CDAI ≥ 150 groups. (a) Quantitative analysis of HSP47 levels in the CDAI ≥ 150 (n = 9) and CDAI (n = 23) groups. (b) Quantitative analysis of anti-HSP47 antibody levels in the CDAI < 150 and CDAI ≥ 150 groups. Each dot represents one subject, and bars indicate average ± SEM. **P < 0.001 (Student’s t test). HSP heat shock protein, CDAI Crohn’s disease activity index, ns not significant.
Differences in serum HSP47 and anti-HSP47 antibody levels between patients with CD with and without intestinal stricturing
To compare serum HSP47 and anti-HSP47 antibody levels between patients with CD with and without intestinal stricturing, the patients were classified into two sub-groups: stricturing and nonstricturing groups (Table 3). The stricturing and nonstricturing groups had similar patient characteristics, including age, sex, height, weight, clinical stage, evolution time of disease, and location of disease. Serum HSP47 levels were not significantly different between the stricturing group and nonstricturing group (P = 0.21) (Fig. 3a), but serum anti-HSP47 antibody levels were significantly higher in the stricturing group than in the nonstricturing group (P = 0.03) (Fig. 3b).
Comparison of serum HSP47 and anti-HSP47 antibody levels between stricturing and nonstricturing disease in patients with CD. Quantitative analysis of (a) HSP47 and (b) anti-HSP47 antibody levels in stricturing and nonstricturing groups in patients with CD. (b) Quantitative analysis of anti-HSP47 antibody in CD patients with and without intestinal stricturing. Each dot represents one subject, and bars indicate average ± SEM. *P = 0.033 (Student’s t test). HSP heat shock protein, CDAI Crohn’s disease activity index, ns not significant.
Optimal cut-off values for serum HSP47 and anti-HSP47 antibody levels for differentiating between CD and UC
Receiver operating characteristic (ROC) curve analyses were performed to define the cut-off values of serum HSP47 and anti-HSP47 antibody levels for differentiating between CD and UC. The area under the curve (AUC) was 0.824 for serum HSP47 levels (Fig. 4a) and 0.880 for serum anti-HSP47 antibody levels (Fig. 4b), indicating that both measurements can be used to accurately differentiate CD from UC. The ROC curve analyses determined a cut-off value of 212.9 pg/mL for serum HSP47 levels, with a sensitivity of 71.8% and a specificity of 84.6%, and a cut-off value of 0.207 (A450) for serum anti-HSP47 antibody levels, with a sensitivity of 78.1% and a specificity of 80.7%.
ROC curves of HSP47 and anti-HSP47 antibody for differentiating CD from UC. (a) ROC curve of HSP47 showing an AUC of 0.824. (b) ROC curve of anti-HSP47 antibody showing an AUC of 0.880. HSP heat shock protein, CD Crohn’s disease, UC ulcerative colitis, ROC receiver operating characteristics, AUC area under the curve.
Optimal cut-off values for serum anti-HSP47 antibody levels for differentiating CD with or without intestinal stricturing
ROC curve analyses were performed to define the cut-off values of serum anti-HSP47 antibody levels for differentiating CD with or without stricturing. The AUC was 0.714 (Fig. 5), indicating that the measurement can be used to accurately differentiate between CD with or without intestinal stricturing. The ROC curve analyses determined a cut-off value of 0.267 (A450) for serum anti-HSP47 antibody levels, with a sensitivity of 77.8% and a specificity of 64.3%.
Expression of HSP47 in the colonic mucosa of patients with CD
Immunohistochemical studies were performed on specimens biopsied from the colonic mucosa of patients with CD to confirm HSP47 localization in these tissues. The expression of collagenous tissue was confirmed by assessing α-smooth muscle actin (α-SMA) expression, which colocalized with HSP47 (Fig. 6).
Effect of HSP47 inhibitor on human fibroblasts
We investigated the effect of HSP47 inhibition on fibrosis prevention in human fibroblasts. The HSP47 inhibitor (100 μM) significantly reduced type I collagen production (Fig. 7a) without decreasing cell viability (Fig. 7b). An mTOR inhibitor, rapamycin, was used as a control and also suppressed type I collagen production; however, unlike the HSP47 inhibitor, rapamycin reduced cell viability (Fig. 6a,b). Type I collagen production normalized by cell viability decreased significantly after HSP47 inhibition in a dose-dependent manner (Fig. 7c). These results suggest that HSP47 inhibition exerts a protective effect against fibrosis without affecting cell viability.
Effect of HSP47 inhibitor on collagen production. Normal human fibroblast cells treated with control, rapamycin (10 nM), or different concentrations of HSP47 inhibitors. (a) Type I collagen production in human fibroblast cells after 24 h of treatment. (b) Cell viabilities of human fibroblasts after treatment. (c) Type I collagen production adjusted by cell viabilities. Rapa, rapamycin; HSP, heat shock protein. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way analysis of variance test with a post-hoc Dunn’s test).
Discussion
In the present study, we demonstrated that serum HSP47 and anti-HSP47 antibody levels were significantly higher in patients with CD than in patients with UC. Serum anti-HSP47 antibody levels were found to reflect CD activity. Moreover, serum anti-HSP47 antibody levels were significantly higher in patients with CD with intestinal stricturing than in patients without it. In addition, HSP47 colocalized with α-SMA, a marker for myofibroblast that plays a central role in the pathogenesis of fibrosis, suggesting its relevance in fibrosis in CD.
Intestinal stricturing is one of the major complications of CD and is often recurrent despite surgical and immunosuppressive therapies. More than one-third of patients with CD show a stenosing phenotype13. Inflammation of intestinal mucosa is associated with infiltration by immune cells, leading to epithelial damage. Deposition of collagen in the extracellular matrix during the wound-healing cycle causes fibrotic stricture8. Although the pathogenesis of inflammation in IBD, including CD, has been extensively investigated, the precise mechanisms underlying the development of fibrotic stricture in CD are not completely understood. In addition, intestinal stenosis in CD can be observed in the absence of luminal inflammatory symptoms13. Therefore, identification of a target that participates in collagen production and deposition is required.
HSP47 is a molecular chaperone exclusively expressed in the ER and is involved in the processing, assembly, folding, and secretion of collagens11,14. During fibrosis, collagen secretion is induced. HSP47 can be detected around fibrotic lesions15,16. An in vitro study using human lung fibroblast cells revealed that HSP47 is induced by a profibrotic mediator, tissue growth factor-β14. In an experimental study involving a murine model of induced liver cirrhosis, HSP47 expression was positively correlated with the degree of fibrosis12. Furthermore, serum HSP47 and anti-HSP47 levels are elevated in patients with connective tissue diseases17. The role of HSP47 in intestinal fibrosis has been investigated by Kitamura et al., who found that transcription of SERPINH1, which encodes HSP47, was elevated in the colon tissue of mice with spontaneous colitis, and that HSP47 was distributed throughout the collagenous tissues18. Increased HSP47 expression has also been observed in the intestinal tissues of patients with CD19.
In this study, we observed that serum HSP47 levels and anti-HSP47 antibody levels were significantly higher in patients with CD than in patients with UC. ROC curve analysis revealed that both HSP47 antigen and antibody levels can be used as serological markers for differentiating between CD and UC. Our observation is similar to that of a previous study, in which high HSP47 levels were detected in the sera of patients with CD20. However, this previous study did not observe a positive correlation with CD activity. We also investigated the differences in the expression of serological markers in patients with active or inactive CD and found that only anti-HSP47 levels, but not HSP47 levels, were high in patients with active CD. We compared HSP47 levels between CD patients with and without intestinal stricturing and found that HSP47 levels tended to be high in patients with intestinal stricturing. We did not observe any significant difference in serum HSP47 levels between the healthy control group and the CD group20. The heterogeneity of the patient cohort in this study could explain why this result is inconsistent with a previous study. Indeed, heterogeneity of the study cohort has been shown to diminish the diagnostic ability of HSP47 for lung fibrosis21.
Research into the pathophysiology of IBD has revealed that adipose tissue-derived free fatty acids and adipocytokines are involved in inflammation in IBD22,23. Another study suggested that HSP47 is associated with fibrosis of adipose tissue24. In this study, we observed colocalization of HSP47 with α-SMA in intestinal tissue from patients with CD. As described previously, HSP47 is considered a candidate for the anti-fibrotic treatment of CD25. Indeed, HSP47 inhibition has been shown to hinder the profibrotic mechanism of lung fibroblasts26. In this study, we observed that HSP47 inhibition significantly suppressed collagen production in fibroblasts. The mTOR inhibitor, rapamycin, also reduced collagen production, but this was accompanied by increased cell death. Since the HSP47 inhibitor inhibited collagen production without affecting cell viability, it could be an ideal agent for fibrosis prevention. Further investigation of the effect of HSP47 inhibitor on intestinal tissues from CD patients is desired to confirm the mechanistic role of HSP47 in the formation of fibrosis in CD.
Our study has certain limitations. First, this study aimed to identify noninvasive surrogate markers of CD activity—particularly fibrotic stenotic lesions—that can be isolated from blood. Further studies using intestinal biopsy samples from patients with CD are required to investigate the association between serum HSP47 levels and local HSP47 levels in the intestinal mucosa of patients with CD. Second, we demonstrated that HSP47, especially the anti-HSP47 antibody, can be used as a biomarker for CD and its activity. Although the molecular mechanism by which HSP47 contributes to collagen deposition remains to be elucidated, inhibition of HSP47 can constitute a novel antifibrotic therapy. Further research is required to elucidate the mechanism by which HSP47 contributes to intestinal fibrosis in CD. In conclusion, we show that HSP47 is a biomarker for CD and positively correlates with disease activity. Thus, HSP47 may be a potential target for the development of antifibrotic CD therapies.
Materials and methods
Study population
This observational study included 26 with UC and 32 patients with CD. All patients had already been diagnosed with IBD prior to recruitment in this study. Diagnoses of UC and CD were made based on conventional clinical, laboratory, endoscopic, histopathological, and radiological parameters27. Disease evolution time was defined as the duration from disease onset to the date of HSP47 measurement. For patients with UC, a clinical activity index (CAI) ≤ 4 was defined as being in remission and CAI > 4 was defined as being active stage28; for patients with CD, a Crohn’s disease activity index (CDAI) ≤ 150 was defined as being in remission and CDAI > 150 was defined as being active stage29. The Montreal Classification for IBD classifies CD subgroups according to factors such as age, inflammation distribution, and disease behavior30. The Montreal Classification categorizes age of onset as A1 for patients 16 years old or younger, A2 for patients 17–40 years old, and A3 for patients over 40 years old; the disease location as L1 for ileal, L2 for colonic, L3 for ileocolonic, and L4 for isolated upper disease; the disease behavior as B1 for nonstricturing and nonpenetrating, B2 for stricturing, and B3 for penetrating, further modified as p for cases involving perianal disease. The control group consisted of 22 randomly selected healthy individuals. We determined that a total number of 86 participants would provide the study with 80% power (P = 0.05; effect size: 0.46). The target number of participants was calculated using G*Power software (version 3.1.9.6; Germany)31 as in a previous report20. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Nagasaki University Hospital (approval numbers: 14052644, 13040149, and 11092637) and Tottori University Hospital (approval number: 1508A024). Written informed consent was obtained from all patients.
Cell culture and treatment
Normal human fibroblasts (ACEL, Inc., Kanagawa, Japan) were used in this study. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin–streptomycin (Nacalai Tesque). The cells were cultured in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% carbon dioxide. When required, the cells were treated with DMEM containing 5, 10, 50, or 100 μM of HSP47 inhibitor (TECHNO SUZUTA, Nagasaki, Japan). An mTOR inhibitor, rapamycin, was used as a internal control. After 24 h incubation, cell viabilities were analyzed using the WST-8 Cell Proliferation Kit (Funakoshi, Tokyo, Japan), and type I collagen production was analyzed using an enzyme-linked immunosorbent assay (ELISA) kit (ACEL, Inc., Kanagawa, Japan).
Measurement of serum HSP47 concentration and autoantibody titers
The serum concentration of HSP47 was measured using sandwich ELISA, as described previously32. The HSP47 autoantibody titers were measured using ELISA, as described previously33. In brief, recombinant HSP47 diluted to 1 μg/mL in 50 mM sodium carbonate buffer was immobilized on a 96-well microplate. After blocking with 2% bovine serum albumin and rinsing with phosphate-buffered saline, the wells were incubated with human sera (diluted 100-fold). Specific binding of serum IgG to HSP was detected by incubating with horseradish-peroxidase-conjugated antibodies specific to the γ-chain of human IgG (BioSource, Camarillo, CA, USA) and 3,3′,5,5′-tetramethylbenzidine solution. Antibody titer was determined by measuring the absorbance at 450 nm.
Immunohistochemistry
Intestinal mucosa biopsy specimens were fixed with 10% formalin and embedded in paraffin. Three-micrometer-thick sections were deparaffinized and dehydrated, followed by antigen retrieval in ethylenediaminetetraacetic acid buffer (pH 9.0) at 95 °C for 30 min. The slides were then incubated with 3% hydrogen peroxide for 10 min. After incubation with blocking solutions and rinsing with Tris-buffered saline (TBS), the slides were incubated with anti-HSP47 antibody (1:15,000; M16.10A1; Enzo Life Sciences, Inc.) or anti-α-SMA (1:1000; Cosmo Bio Co., Ltd.) at 24 °C for 60 min. The slides were then rinsed with TBS and incubated with peroxidase-labeled corresponding secondary antibody for 30 min at room temperature. Peroxidase activity was detected using diaminobenzidine. Hematoxylin was used for counterstaining.
Statistical analysis
Statistical analysis was performed using IBM SPSS 28.0.1 (IBM, Somers, NY, USA). Continuous variables were expressed as mean ± standard deviation or median with interquartile range, according to the distribution. The Kolmogorov–Smirnov test was used to assess normal distribution. Categorical variables were expressed as percentages. Differences between two groups were analyzed using Student’s t test for normally distributed variables, the Mann–Whitney U test for non-normally distributed variables, or a Chi-squared test for categorical variables. Continuous variables among the three groups were compared using one-way analysis of variance for normally distributed variables or the Kruskal–Wallis test for non-normally distributed variables. For significant differences, the Scheffe test was used for post-hoc analysis of normally distributed variables or the Dann–Bonferroni test for non-normally distributed variables. ROC curve analysis was performed to determine the optimal cut-off values of HSP47 and anti-HSP47 antibody levels for differentiating between CD and UC. Furthermore ROC curve analysis was performed to determine the optimal cut-off values of HSP47 and anti-HSP47 antibody levels for differentiating CD with or without stricturing. Optimal cut-off values were determined by minimizing the square of the distance between a point (sensitivity of 1, 1-specificity of 0) and any point on the ROC curve. Two-tailed P values < 0.05 were considered statistically significant.
Data availability
This study's datasets are available upon reasonable request.
References
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Yeshi, K. et al. Revisiting inflammatory bowel disease: Pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J. Clin. Med. 9, 1–39 (2020).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Rioux, J. D. et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 39, 596–604 (2007).
Cho, J. H. & Weaver, C. T. The genetics of inflammatory bowel disease. Gastroenterology 133, 1327–1339 (2007).
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664 (2020).
Bokemeyer, A. et al. Quantitative phase imaging using digital holographic microscopy reliably assesses morphology and reflects elastic properties of fibrotic intestinal tissue. Sci. Rep. 9, 19388 (2019).
Rieder, F., Brenmoehl, J., Leeb, S., Schölmerich, J. & Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 56, 130–139 (2007).
Santacroce, G. et al. Therapeutic targeting of intestinal fibrosis in Crohn’s disease. Cells 11, 429. https://doi.org/10.3390/cells11030429 (2022).
Bellini, S. et al. Heat shock proteins in vascular diabetic complications: Review and future perspective. Int. J. Mol. Sci. 18, 2709 (2017).
Chioran, A., Duncan, S., Catalano, A., Brown, T. J. & Ringuette, M. J. Collagen IV trafficking: The inside-out and beyond story. Dev. Biol. 431, 124–133 (2017).
Masuda, H., Fukumoto, M., Hirayoshi, K. & Nagata, K. Coexpression of the collagen-binding stress protein HSP47 gene and the alpha 1(I) and alpha 1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J. Clin. Investig. 94, 2481–2488 (1994).
Iwashita, T. et al. Involvement of collagen-binding heat shock protein 47 and procollagen type I synthesis in idiopathic pulmonary fibrosis: Contribution of type II pneumocytes to fibrosis. Hum. Pathol. 31, 1498–1505 (2000).
Nakayama, S. et al. Pirfenidone inhibits the expression of HSP47 in TGF-β1-stimulated human lung fibroblasts. Life Sci. 82, 210–217 (2008).
Abe, K. et al. Interstitial expression of heat shock protein 47 and alpha-smooth muscle actin in renal allograft failure. Nephrol. Dial. Transplant. 15, 529–535 (2000).
Shioshita, K. et al. Expression of heat shock proteins 47 and 70 in the peritoneum of patients on continuous ambulatory peritoneal dialysis. Kidney Int. 57, 619–631 (2000).
Yokota, S. I. et al. Prevalence of HSP47 antigen and autoantibodies to HSP47 in the sera of patients with mixed connective tissue disease. Biochem. Biophys. Res. Commun. 303, 413–418 (2003).
Kitamura, H. et al. Role of heat shock protein 47 in intestinal fibrosis of experimental colitis. Biochem. Biophys. Res. Commun. 404, 599–604 (2011).
Honzawa, Y. et al. Involvement of interleukin-17A-induced expression of heat shock protein 47 in intestinal fibrosis in Crohn’s disease. Gut 63, 1902–1912 (2014).
Honzawa, Y., Nakase, H., Takeda, Y., Nagata, K. & Chiba, T. Heat shock protein 47 can be a new target molecule for intestinal fibrosis related to inflammatory bowel disease. Inflamm. Bowel Dis. 16, 2004–2006 (2010).
Kakugawa, T. et al. Serum heat shock protein 47 levels are elevated in acute interstitial pneumonia. BMC Pulm. Med. 14, 48. https://doi.org/10.1186/1471-2466-14-48 (2014).
Morisaki, T. et al. High serum vaspin concentrations in patients with ulcerative colitis. Dig. Dis. Sci. 59, 315–321 (2014).
Akazawa, Y. et al. Significance of serum palmitoleic acid levels in inflammatory bowel disease. Sci. Rep. 11, 16260 (2021).
Luo, H., Liu, T., Yang, H., Ye, H. & Luo, X. Expression of collagen (types I, III, and V), HSP47, MMP-2, and TIMP-1 in retrobulbar adipose tissue of patients with thyroid-associated orbitopathy. J. Ophthalmol. 2020, 4929634 (2020).
Nakase, H., Honzawa, Y. & Chiba, T. Heat shock protein 47 is a new candidate molecule as anti-fibrotic treatment of Crohn’s disease. Aliment Pharmacol. Ther. 31, 926–927 (2010) (author reply 927).
Miyamura, T. et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem. Biophys. Res. Commun. 530, 561–565 (2020).
Nakase, H. et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 56, 489–526 (2021).
Rachmilewitz, D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: A randomised trial. BMJ 298, 82–86 (1989).
Singleton, J. W. et al. A trial of sulfasalazine as adjunctive therapy in Crohn’s disease. Gastroenterology 77, 887–897 (1979).
Satsangi, J. et al. The Montreal Classification of Inflammatory Bowel Disease: Controversies, consensus, and implications. Gut 55, 749–753 (2006).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A.-G. Statistical power analyzes using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009).
Kakugawa, T. et al. Serum heat shock protein 47 levels are elevated in acute exacerbation of idiopathic pulmonary fibrosis. Cell Stress Chaperones 18, 581–590 (2013).
Kakugawa, T. et al. High serum concentrations of autoantibodies to HSP47 in nonspecific interstitial pneumonia compared with idiopathic pulmonary fibrosis. BMC Pulm. Med. 8, 23. https://doi.org/10.1186/1471-2466-8-23 (2008).
Author information
Authors and Affiliations
Contributions
T.K., and H.I. conceptualized the study. H.K., T.T., T.K., T.S., T.M., and T.K. analyzed and interpreted the data. T.K., T.M., S.Y. and T.A. acquired the data. H.K. and T.T. drafted the manuscript. H.I. supervised the study. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurumi, H., Takata, T., Kanda, T. et al. Investigating the role of heat shock protein 47 in fibrosis in Crohn’s disease. Sci Rep 12, 10966 (2022). https://doi.org/10.1038/s41598-022-15153-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15153-2
- Springer Nature Limited
This article is cited by
-
Identification of molecular mechanisms causing skin lesions of cutaneous leishmaniasis using weighted gene coexpression network analysis (WGCNA)
Scientific Reports (2023)
-
Targeting HSP47 and HSP70: promising therapeutic approaches in liver fibrosis management
Journal of Translational Medicine (2022)