Abstract
Identifying high-yield genotypes under low water availability is essential for soybean climate-smart breeding. However, a major bottleneck lies in phenotyping, particularly in selecting cost-efficient markers associated with stress tolerance and yield stabilization. Here, we conducted in-depth phenotyping experiments in two soybean genotypes with contrasting drought tolerance, MUNASQA (tolerant) and TJ2049 (susceptible), to better understand soybean stress physiology and identify/statistically validate drought-tolerance and yield-stabilization traits as potential breeding markers. Firstly, at the critical reproductive stage (R5), the molecular differences between the genotype’s responses to mild water deficit were explored through massive analysis of cDNA ends (MACE)-transcriptomic and gene ontology. MUNASQA transcriptional profile, compared to TJ2049, revealed significant differences when responding to drought. Next, both genotypes were phenotyped under mild water deficit, imposed in vegetative (V3) and R5 stages, by evaluating 22 stress-response, growth, and water-use markers, which were subsequently correlated between phenological stages and with yield. Several markers showed high consistency, independent of the phenological stage, demonstrating the effectiveness of the phenotyping methodology and its possible use for early selection. Finally, these markers were classified and selected according to their cost-feasibility, statistical weight, and correlation with yield. Here, pubescence, stomatal density, and canopy temperature depression emerged as promising breeding markers for the early selection of drought-tolerant soybeans.
Similar content being viewed by others
Introduction
Soybean [Glycine max (L.) Merrill] represents one of the most important sources of vegetable oil and protein in the world1. Calculation models based on the growing global population and current agricultural production suggest that crop yields, including soybean, must be doubled to provide enough food in 20502. Yield, the principal breeding target for most crop plants, is massively affected by suboptimal growth conditions primarily due to climate factors such as drought and extreme temperatures. In addition, the progressive climate change will reduce water availability for many rainfed crops like soybean, affecting their growth and productivity3. Hence, breeding for yield stabilization and drought tolerance in soybean and other crops is essential for sustainable agriculture and food production4.
The development of cultivars with improved yield under water deficit has had relatively limited success for several reasons. First, the direct selection for yield improvement under drought is expensive, time-consuming, laborious, and complex due to intrinsic genotype by environmental interactions5. Moreover, when determining plant performance under drought conditions, inherent stress susceptibility is often masked by the spillover effects of high yield potential. Consequently, a high-yield variety will often give significant yields during drought, even though the relative yield reduction can be substantial6. Instead, analytical approaches that emphasize breeding for yield stabilization through an indirect selection strategy, using morphophysiological or biochemical markers, have gained increasing attention7,8. However, the challenge for effectively using targeted breeding approaches lies in developing reliable and reproducible markers. These markers should be (i) strongly related with yield and stress-tolerance traits, where possible, (ii) be non-destructive, (iii) be easily measurable in early phenological stages, and (iv) have a high narrow-sense heritability to facilitate the selection in breeding populations9. Therefore, identifying and validating drought-tolerance traits are essential steps to obtaining valuable markers for breeding programs and selecting superior genotypes. Soybean drought tolerance has been evaluated through markers such as water use efficiency (WUE), root morphology and penetrability of hardpan, leaf wilting, excised leaf water loss, and relative water content (RWC) with varying degrees of success10.
Advances in next-generation sequencing (NGS) and subsequent evolution in multi-omics technologies have contributed to understanding some underlying mechanisms of response to water deficit in soybean and other crops11. Omics studies, however, must be complemented with morphophysiological and biochemical approaches to ensure an integrative perspective of plant adaptation to water scarcity and accurately assess the role of individual traits regarding stress tolerance and yield. Usually, the main issue in such studies is the lack of a well-defined and reliable phenotyping methodology that validates the trait's accuracy12. It is safe to say that, currently, phenotyping systems are the major operational bottleneck in plants breeding, limiting the translation of genetic and genomic analysis into stress-tolerant phenotypes.
In previous research13, our group phenotyped several soybean genotypes submitted to drought treatments during the reproductive stage in greenhouse and field conditions. Comparative studies were performed in commercial cultivars and the widely studied PI 416937, a reference slow-wilting genotype. Overall, MUNASQA exhibited the lowest yield loss and yield-based Drought Susceptibility Index (DSI) under water scarcity conditions, while TJ2049 showed the opposite behavior. Based on these results, MUNASQA (tolerant) and TJ2049 (susceptible) were selected as the two more contrasting genotypes and incorporated into the EEAOC breeding program to develop a segregating population for genetic mapping of drought tolerance.
Meanwhile, in-depth phenotyping experiments were conducted to better understand the molecular and morphophysiological mechanisms involved in these genotypes' responses to drought. Moreover, we identify and statistically validate traits associated with drought-tolerance and yield-stabilization, aiming at their future use in drought-resilience breeding strategies such as genomic selection, especially in early developmental stages.
Results and discussion
Here, we show differences in the molecular, morphophysiological, and biochemical responses of two contrasting soybean genotypes, MUNASQA (drought tolerant) and TJ2049 (drought susceptible), subjected to mild water deficit treatments in V3 (second open trefoil) and R5 (beans beginning to develop at one of the four uppermost nodes with a wholly unrolled leaf) phenological stages.
Molecular insights
Transcriptional changes were assessed in MUNASQA and TJ2049 after 72 h of exposure to mild water deficit. From 38.658 transcripts analyzed, drought-stressed MUNASQA and TJ2049 plants exhibited 2952 and 1126 transcripts with significant changes (P < 0.05) in their expression levels (Suppl. Data 1), respectively. After an FDR = 0.1, 399 and 15 transcripts were assigned as MUNASQA and TJ2049 DEGs (Suppl. Data 2). The transcript loss detected in TJ2049 might be explained by the larger variation among replicates observed in the water deficit samples and measured as SD/mean ratio for each gene (Suppl. Table 1, Fig. S2). However, in a previous exploratory 454 sequencing experiment, TJ2049 showed significantly fewer DEGs than MUNASQA (data not shown).
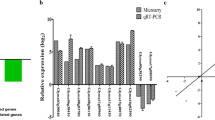
Large-scale transcriptional reprogramming has long been recognized as the first response to drought, initiating stress mitigation pathways and metabolic changes14. Moreover, the quickness to sense and respond to stresses could be essential for differentiating tolerant and susceptible genotypes. Here, after a mild drought, far from normal field levels, the genotypes exhibited dramatic changes on their transcriptional profiles and no observable phenotypic alteration. In the heat map (Fig. 1a), DEGs expression profiles were classified in 8 clusters (Suppl. Data 3), and almost 50% of MUNASQA DEGs showed repression under stress conditions, a difference reinforced by the Venn diagram (Fig. 1c).
Transcriptomic analysis of MUNASQA and TJ2049 genotypes under drought. Heat-map of all DEGs for MUNASQA and TJ2049 in drought conditions. Scale color indicates green for up-regulation and red for downregulation (a). GO enrichment in MUNASQA and TJ2049 comprises biological processes (BP, in red), molecular function (MF, in blue), and cellular component (CC in green). Relevant categories showing enrichment of DEGs for both genotypes are depicted. GO terms were plotted after applying an FDR = 0.1 Bubble size correlates with enrichment factor values; for each bubble size, the P-value is indicated (b). Venn diagram for all DEGs in MUNASQA and TJ2049 under drought conditions. DEGs were plotted after applying an FDR = 0.1 (c). Validation by qRT-PCR of ten genes selected from RNA-Seq. Log2 fold change (log2FC) was calculated based on the comparison of drought vs control for each genotype (d). Three biological replicates were used, and the experiment was performed twice with similar results.
Generally, in response to drought, plants initially trigger transcriptional control and hormone signaling, leading to metabolic adjustment for coping with low water availability. Cellular mechanisms such as water/ion uptake and transport, redox homeostasis, ROS scavenging, osmoregulation, and membrane protection are accompanied by physiological responses such as stomata regulation, root development, and protection of the photosynthesis machinery15. MUNASQA up-regulated several DEGs involved in these physiological responses (Suppl. Table 2). DEGs from "chlorophyll-binding" and "antioxidant activity" (MF category), "thylakoid", "thylakoid membranes" and "chloroplast" (CC category), and "photosynthesis", "response to water" and "response to desiccation" (BP category) (Fig. 1b), were identified. Overall, these results indicate a tightly regulated stress-response, growth, and water use mechanisms in drought-stressed MUNASQAs.
The expression of ten randomly selected DEGs by qRT-PCR (Fig. 1d) agreed with the MACE profile data for MUNASQA and TJ2049, showing a high similarity between both methodologies. Moreover, genes encoding detoxifying proteins like SOD, CAT, and APX were differentially regulated in both genotypes (Suppl. Table 3). Enzymes like these are stress-response indicators, frequently regulated at both transcriptional and post-transcriptional levels under drought16. We detected two up-regulated SOD genes under stress conditions in MUNASQA and, contrary to TJ2049, three down-regulated genes for APX and CAT. Accordingly, SOD activity measurements in R5 corroborated these differences in transcriptional activity between the two genotypes (Suppl. Table 4).
Morphophysiological and biochemical phenotyping
MACE assay, by itself, does not fully explain the extent of MUNASQA and TJ2049 responses to the applied stress. Thus, extensive morphophysiological and biochemical measurements were performed to minimize the gap between the transcriptional regulation and phenotypical alterations observed. Here, 22 markers associated with stress-response, growth, and water-use were evaluated in both genotypes exposed to mild water deficit in V3 and R5 stages.
Stress-response markers
An efficient antioxidant activity is crucial for withstanding low cellular water content, and its importance in plant drought tolerance has been extensively reported17. Here, substantial differences were found in MUNASQA and TJ2049 SOD, APX, POX, and CAT enzymes regulation over time, under water deficit, and independently of the phenological stage (Table 1). MUNASQA reached maximum activity for all enzymes after 4 d of water deficit, while the highest activity for TJ2049 occurred to 8 d after the stress onset, except for CAT. Noticeably, under non-stressed conditions, all enzymatic activities, excluding CAT, showed higher activity in the tolerant genotype than in the susceptible one, strengthening the hypothesis that TJ2049, compared to MUNASQA, presents delayed stress perception and response mechanisms. In fact, and according to Carvalho et al.16, a successful response to water deficit may depend not only on which enzymes are activated but also on the activation timing. Moreover, the general gene expression profile regarding these enzymes regulation was consistent with the biochemical results.
Regarding PRO, one of the most common osmoprotectant in plants18, MUNASQA and TJ2049 accumulated the osmolyte in response to water deficit over time and in both V3 and R5 stages (Table 1). Several studies have demonstrated a direct correlation between high osmoprotectant accumulation and drought tolerance in many crops19. Here, the tolerant genotype MUNASQA exhibited a higher and more rapid accumulation of PRO after 4 d of water deficit at both phenological stages. In agreement with our results, a recent study in soybean reported higher PRO accumulation in the drought-tolerant genotype A5009 RG, compared with the drought-susceptible ADM5004820.
When analyzing MDA production, an indicator of lipid peroxidation and stress severity21, a higher and more rapid accumulation was detected in TJ2049 plants in response to water deficit, compared to MUNASQA (Table 1).
Drought also affects leaf pigments content21. Changes in photosynthetic pigments can alter various light-harvesting processes, while the accumulation of photoprotective compounds plays an essential role in preventing photo-oxidative damage22. Here, MUNASQA and TJ2049 showed alterations in pigment content under water deficit (Table 1). The CHL was significantly reduced over time due to stress in both genotypes, but this reduction was significantly lower in the tolerant one. Similar results were found in drought-tolerant maize that showed lower CHL reductions under stress than susceptible genotypes23. Regarding CAR levels, TJ2049 and MUNASQA showed an increased synthesis in response to drought, although the last one exhibited greater and faster accumulation.
According to our results, MUNASQA drought tolerance is strongly related to a rapid stress-sensing and response capacity and an efficient ROS (reactive oxygen species) scavenging system, both essential mechanisms for stress tolerance in numerous species24.
Growth and yield markers
The ability to produce high seed yield or biomass under limited water access is considered the optimal indicator of drought tolerance in crops25,26,27. We evaluated the effect of mild water deficit in MUNASQA and TJ2049 growth and yield by monitoring changes in various markers related to biomass and seed production, including leaf area index (LAI), leaf area ratio (LAR), the net assimilation rate (NAR), relative growth rate (RGR), crop growth rate (CGR), relative yield and DSI (Table 2).
Total leaf surface area and LAI are strongly associated with canopy interception, evapotranspiration, and photosynthesis28. Here, independently of the phenological stage or water availability, MUNASQA plants exhibited a higher LAI compared to TJ2049, indicating a larger assimilatory capacity and, as a consequence, photosynthetic potential. In vegetative stages, a higher LAI denotes a more rapid canopy development, favoring greater and faster soil coverage and thus less water loss from direct evaporation. In general, drought suppresses leaf initiation and growth and consequently affects LAI29. Therefore, a decrease in LAI is expected in plants exposed to water scarcity. As expected, MUNASQA and TJ2049 plants showed a reduction in LAI in response to drought, more noticeable after 8 d of stress and in the R5 stage.
Drought also alters the LAR: the leaf area development in relation to the total biomass produced30. Here, water deficit affected the LAR in both genotypes, but only during the vegetative stage. The highest ratio between plant leaf area and biomass is reached at the beginning of the plant life cycle31, which explains the highest LAR values at the first sampling day during the vegetative stage.
In MUNASQA and TJ2049, the relationship between leaf area expansion and the biomass produced over time (NAR) was also reduced due to water deficit at both phenological stages. Changes in photosynthetic efficiency were more significant at V3, where both genotypes exhibited greater NAR values. Noticeably, well-watered MUNASQA plants showed a significantly higher NAR than TJ2049 ones, while, in response to stress, MUNASQA’s NAR increased in contrast to TJ2049 values, which were reduced. Although not as accentuated, a similar pattern was observed in plants exposed to water deficit in R5. Here, LAI, LAR, and NAR results indicated that, in response to drought, MUNASQA plants regulated photosynthates allocation to leaves and the maintenance of photosynthetic efficiency more efficiently than TJ2049 ones.
In addition, the relative growth rate (RGR) or biomass produced over time was significantly reduced by water deficit in the vegetative stage (Table 2). Interestingly, the reduction in RGR was more pronounced in TJ2049 plants. Regarding CGR, significant differences were observed in the V3 stage after 8 d of drought, while in R5 were detected after 4 d.
These results agree with the differences in yield and yield-DSI exhibited by MUNASQA and TJ2049 after water-deficit treatments in V3 and R5 (Fig. 2). According to these findings, when drought was applied in the V3 stage, TJ2049 showed a distinct but not significant yield penalty and a yield-DSI considerably higher than MUNASQA. Moreover, after a mild water deficit in R5, a highly moisture-sensitive phenological stage, TJ2049 exhibited the largest yield loss and a significantly higher yield-DSI than MUNASQA. Results that also agree with the ones reported by Pardo et al.13.
Effects of mild water deficit in MUNASQA and TJ2049 yield and yield-DSI. Yield in well-irrigated (Ψs = − 0.05 MPa) and drought-stressed (Ψs = − 0.65 MPa) V3 and R5 (a). Yield-DSI for each genotype phenotyped in V3 and R5 (b). Different letters indicate significant differences at P < 0.05 (two-way ANOVA). Error bars represent SE from independent experiments, n = 300 per trial.
Water use markers
Maintaining tissue/cellular water content and/or metabolic activity at low water potentials are physiological strategies to survive drought32. Traits like pubescence, leaf thickness, stomatal density, closure, slow wilting, canopy temperature, RWC, and WUE are essential to determining drought tolerance in plants. Thus, all these water-use parameters were assessed in MUNASQA and TJ2049 in response to drought.
After 21 d of water deficit, the genotypes exhibited drought-induced adaptations in leaf morphology traits33, such as stomata, trichrome density, and leaf thickness (Table 3). As expected, the stomatal density was substantially altered in response to drought. Here, TJ2049 plants exhibited a considerable decrease, while MUNASQA ones showed an 89 and 65% increase on abaxial and adaxial leaf surfaces. Moreover, no changes in pubescence were observed on TJ2049 plants, while in MUNASQA, the trichrome density was increased, especially in the abaxial leaf surface. In response to drought, plants can reduce their stomatal size, density, or aperture and develop higher pubescence34, mainly on the abaxial surface35. The increase in MUNASQA’s stomatal density could indicate the genotype’s ability to produce smaller but denser stomata and, therefore, reduce transpiration by a quicker onset of stomatal regulation. This modification, combined with denser trichomes, could enhance the boundary layer resistance, increase the air’s moisture outside the stomata and minimize water loss during drought, precluding significant growth penalties in terms of photosynthetic activity.
Regarding leaf thickness, no significant differences were detected under well-irrigated conditions. However, after 21 d of water deficit, MUNASQA leaves were considerably thinner, while TJ2049 ones were thicker. The knowledge about the links between leaf morpho-anatomy and its function under non-stressed/stressed conditions is relatively poor, especially for traits like leaf thickness36 that is strongly related to transpiration37 and reported by some authors as a drought-tolerance trait that maintains turgor pressure and enhances photosynthesis38. Yet, this feature, only apparent in TJ2049 plants under water deficit, could not be associated with transpiration adjustments, photosynthesis increase, or any other drought-tolerant feature.
Meanwhile, the regulation of stomatal aperture reinforces the drought-tolerant character of MUNASQA. Under non-stressed conditions, the susceptible TJ2049 showed more opened stomata (⁓22% more than MUNASQA). Moreover, after 3 d of water deficit in V3, this difference was increased to almost ⁓50% of stomatal aperture (Table 3). Although stomata represent a small percentage of the leaf lamina, large amounts of water evaporate through them33. Thus, the lack of stomatal control in TJ2049 might explain the fast-wilting phenotype in response to air desiccation (Fig. 3). Measuring the water loss of detached leaves is a selection method for drought tolerance39. Here, R5 leaves of both genotypes were removed and air-dried. After 48 h, MUNASQA exhibited a greener, healthier and slow-wilting phenotype, previously linked to drought tolerance in studies with soybean cultivars17.
MUNASQA water-saving behavior was also confirmed through parameters such as RWC and WUE, strongly regulated under drought (Table 4). The RWC, a time-specific measurement of the hydric status of a plant, is considered a physiological character recommended for drought-tolerance selection40. In V3 and R5 stages, well-irrigated MUNASQA plants showed higher RWC than TJ2049, even with a smaller canopy. As expected, under water deficit, the RWC values were reduced by 14% in MUNASQA and 20% in TJ2049, indirectly confirming the effectiveness of the stress imposed.
Water use efficiency (WUE), referring to the biomass produced per water unit, has been widely used as a breeding target in many rainfed crops, including soybean. Conservative water-use strategies are associated with high leaf WUE34. In agreement, and contrarily to TJ2049, V3 and R5 MUNASQA plants showed a gradual increase of WUE in response to water deficit that agrees with the tighter regulation of stomatal movements and the reduced water loss observed in the genotype (Table 4). Moreover, considering the discrete NAR reduction and the maintenance of a ~ 70% RWC under water deficit, we hypothesize that MUNASQA may display a stomatal control based on partial or total/partial closure intervals, therefore reducing transpiration and saving water through a smaller gas exchange (potential photosynthesis) penalty.
The CTD, regarding plant canopy temperature difference with the surrounding air, is considered a surrogate trait for stomatal conductance and a good indication of plant transpiration rate41. As expected, in response to drought, MUNASQA plants evidenced lower CTD values, a finding that supports the stomatal aperture results and strongly suggests the genotype water-saving behavior. Plants with higher stomatal conductance transpire more and thus maintain a cooler canopy42. Thus, in TJ2049 stressed-plants, the high and positive CTD confirmed a higher stomatal aperture and transpiration rate that agrees with a water-spender behavior. Moreover, TJ2049 also presented higher transpiration rates in unstressed conditions. Finding that could be evidence of a natural and predisposing difference between tolerant and susceptible genotypes.
Markers selection
Identifying and exploiting phenotyping markers will improve selection strategies for drought tolerance in legumes crops17. However, to successfully implement markers in a breeding program, it is imperative to validate their (i) accuracy, (ii) feasibility, and (iii) strength of association with the desired trait. To further understand the marker’s contribution to drought tolerance and yield stabilization in MUNASQA and TJ2049, Principal Component Analysis (PCA) was performed.
A first PCA was conducted for all the morphophysiological parameters evaluated together with absolute yield (Fig. 4). Here, data corresponding to MUNASQA stress was separated from the rest of the treatments and genotype in Principal Component (PC) I, showing the biggest dissimilarity (Fig. 4a). Meanwhile, TJ2049 stress data were separated in PC II. However, when comparing the proportion of variance explained by the different PC (Fig. 4b), the first two only explained 53,48%. Moreover, no clear association was observed between the absolute yield and the rest of the parameters, although PRO and LAR exhibited some positive relation.
In PCAs, an increase in the number of comparable variables will reduce the proportion of variance explained by those variables. Therefore, to discriminate which markers better explain the variability between genotypes and treatments, independent PCAs were conducted using subsets of parameters grouped by biological processes in (i) “stress response”, (ii) “growth” and (iii) “water use” categories (Fig. S3). In addition, markers evaluated at V3 and R5 stages were measured by Pearson's correlation to determine their strength of association between the phenological stages (accuracy) and yield stabilization (desired trait) (Tables 1, 2, and 4).
Biochemical parameters such as enzymatic and non-enzymatic ROS scavengers, leaf pigments, and MDA have been confirmed as adaptive responses to desiccation stress, frequently used for selecting plant genotypes under drought43. The PCA results showed clear discrimination of MUNASQA and TJ2049 drought responses (Fig. S3a). Here, the first two principal components (PC) explained 96.6% of total variation (PC1 = 75.0% and PC2 = 21.6%). Data were clustered by irrigation treatment in PC1, suggesting that these markers are indicators of phenotypic plasticity. The CHL was associated with well-irrigated plants, while all enzymes, MDA, CAR, and PRO, were related to drought stress.
All these markers showed high accuracy between phenological stages due to significant (P < 0.001), strong (r2 over 0.88), and positive correlations (Table 1). However, the correlation with yield showed inconsistent outcomes. PRO, MDA, and CAR were significant and positively correlated with yield. Meanwhile, except SOD, all enzymes exhibited significant and positive correlations that were too variable in the strength of association and could not be linked with a specific genotype or treatment. Thus, we considered these “stress-response” markers suitable for discriminating susceptible/resistant responses during early drought-tolerance screenings in soybean. Still, their use as breeding traits is limited due to the high environmental effect. Interestingly, a report20 found that PRO and CHL were suitable markers for ranking soybean genotypes in response to drought in vegetative stages (5 days after emergence). At the same time, MDA could be useful during R5 as a sensitivity trait.
In legumes, features like LAI, smaller leaf area, leaf area maintenance, and dry matter partitioning have been used to screen for drought tolerance17. Here, in the “growth” PCA (Fig. S3b), the first two PC explained 93.0% of total variation (PC1 = 73.0% and PC2 = 20.0%). In PC1, data were clustered by genotype, suggesting that these markers explained differences between MUNASQA and TJ2049 growth responses on their intrinsic genetic variability. Moreover, the markers analyzed were only associated with MUNASQA, LAR, and RGR associated with stressed plants. However, only LAI and LAR showed a weak correlation between phenological stage and yield (Table 2).
Water-saving features like denser leaf pubescence, a higher number of stomata, warmer canopies, RWC maintenance, and increased WUE have been associated with drought tolerance in legumes and applied in drought-resistance breeding17. Here, “water-use” markers contributed the most to discriminating drought-tolerant and susceptible responses. In the two PCA performed, one for physiological markers and the other for morphological ones, data were clustered by genotype in PC1; thus, these markers are indicators of genetic variability. In CTD, RWC and WUE PCA (Fig. S3c), the first two PC explained of total variation 87.7% (PC1 = 54.6% and PC2 = 33.1%). Markers RWC and WUE (associated with MUNASQA) and CTD (associated with TJ2049) showed significant (P < 0.001), strong (r2 over 0.87), and positive correlations between phenological stages (Table 4). Moreover, WUE and CTD were significantly associated with yield, showing positive and negative correlations depending on the water treatments applied (Table 4). In the PCA made with morphological “water-use” markers, the first two PC explained 96.6% of total variation (PC1 = 73.8% and PC2 = 22.8%) (Fig. S3d). Here, pubescence and stomata abaxial density was strongly related to MUNASQA. Although the data were insufficient to execute good correlation analysis, these morphological markers have been demonstrated as clear indicators of water-saving strategies in legumes34.
A good drought-tolerance marker linked to yield stabilization must also be accurate, cost-effective, if possible, non-destructive, and easily measurable. Hence, after evaluating marker accuracy (amid phenological stages) and assessing which ones better explained the phenotypic variability between genotypes and treatments, a final selection was performed by cost-feasibility (CF) and statistical weight (SW) rankings. Based on the CF values, the degree of complexity and cost to assess each indicator, nine markers were further selected, encompassing the categories 1 (easy and cheap) and 2 (easy and expensive) (Table 5). Subsequently, after SW re-selection, based on the percentage of variability among genotypes and treatments explained by each parameter, four markers remained with “High” SW in both PC.
Determine which markers are more suitable to assess drought tolerance often represents a challenge and depends on the researcher's criteria, e.g., whether tolerance is based on yield maintenance or intrinsic mechanisms that ensure plant survival at the expense of productivity. During this research, we assessed numerous parameters associated with plant performance and stress responses, aiming to identify a small group of markers related to yield stabilization, stress tolerance, or both, and if possible, non-destructive and easily measurable. Often, some markers are highly accurate (stable during the plant life cycle) but expensive, laborious, and/or time-consuming (low throughput). Thus, without disregarding the importance of accuracy, we also consider markers that are cost-efficient and can be assessed in a high throughput manner as strong candidates for drought-tolerance phenotyping.
During this research, the selected markers were (i) stomatal density on the adaxial and (ii) abaxial leaf surface, (iii) trichrome density on the abaxial side, and (iv) CTD. These four traits were chosen as the most efficient phenotyping markers for drought tolerance due to their high accuracy, strong association to water-saving strategies under drought, high cost-efficiency (affordable and easily measured), and non-destructive assessment, therefore ideal for high throughput screening using high-resolution imaging.
Furthermore, beyond the contributions to our soybean breeding program, we must highlight the practical applications of the in-depth phenotyping results. Overall, these findings (i) confirmed the effectiveness of the methodologies used during the research (e.g., drought imposition, sampling times), (ii) corroborate its successful application in early phenological stages, and (iii) strength the usefulness of MUNASQA and TJ2049 as model genotypes for genetic mapping studies. In this context, our group developed a segregating population of 280 F6 RIL (recombinant inbred lines) families that will be mapped for drought tolerance with SSR (simple sequence repeats or microsatellites) and SNPs (single nucleotide polymorphism) selected based on the four markers chosen in this research (stomatal and trichome densities and CTD).
Methods
Experimental approach
The response of MUNASQA and TJ2049 to mild water deficit applied in the R5 stage (beans beginning to develop at one of the four uppermost nodes with a wholly unrolled leaf) was assessed through transcriptional and leaf morphology analysis. Subsequently, comparative studies were performed to determine the genotype's response to water deficit imposed in V3 and R5 stages. Next, all markers assessed were analyzed according to their strength of association between phenological stages and yield, then were ranked by statistical weight and cost-feasibility (Fig. S1).
Plant material and growth conditions
All experiments were conducted in greenhouse conditions at the Estación Experimental Agroindustrial Obispo Colombres (EEAOC), Las Talitas, Tucumán, Argentina (S26° 50′, W65° 12′). Seeds of MUNASQA and TJ2049 were inoculated with Bradyrhizobium japonicum E109 strain (9 × 109 viable cells kg−1 of seeds) and sown in 4 L plastic pots (diameter: 18 cm, height: 21 cm) filled with GrowMix® Multipro commercial substrate (Terrafertil S.A., Argentina). Topsoil was covered with perlite to minimize water evaporation. Pots were weekly rearranged to minimize environmental effects. At the V1 stage, open leaf at the unifoliate node44, two homogeneous plants per pot were left. Comparative trials were performed at two phenological stages according to Fehr et al.44: V3 (second open trefoil) and R5 (beans beginning to develop at one of the four uppermost nodes with a wholly unrolled leaf). During all the experiments, environmental variables were assessed with sensors every 15 min, then recorded and averaged in data loggers (Cavadevices.com, Buenos Aires, Argentina) (Suppl. Table 5).
Irrigation treatments
The volumetric water content (VWC) of each pot was estimated according to Pereyra-Irujo et al.45, and the relationship between VWC and water potential (Ψs) was determined46. Pots were maintained at 22% of VWC (Ψs = − 0.05 MPa) through daily watering until stress onset. According to Pardo et al.13, the water deficit was applied by maintaining the pots at 14% of VWC (Ψs = − 0.65 MPa) for ten days. The desired Ψs was reached in 2–3 days. At the end of stress, plants were fully watered until harvest. The Ψs was daily monitored and recorded. Corrections for soil water status were made by weighing two plants per genotype and treatment every 3 days. The plant water status was monitored through the RWC47 to ensure stress occurrence.
All drought experiments were carried out for three consecutive years, always applying the previously described irrigation treatments. Water deficit in V3 and R5 phenological stages was imposed in independent plant sets; the sections below detailed the sampling process.
Experiments
MACE-transcriptomic analysis and validation
Three biological replicates per treatment (Control and Stress) and genotype (MUNASQA and TJ2049) were collected from R5 plants after 72 h of water deficit (n = 12), and RNA from fully mature expanded leaf between nodes 5 to 7 node was isolated for transcriptional analysis.
MACE-Seq libraries and sequencing were performed on an Illumina NextSeq500 machine (1 × 75 bp reads). The conversion was made with bcl2fastq2 software (version 2.19.1), and the cleaning of duplicate sequences was performed with "TrueQuant". In MACE-seq, the TrueQuant barcodes each DNA molecule before PCR amplification. As each barcode-template combination is statistically unique, PCR-duplicates can be identified and eliminated from the dataset to prevent PCR bias. Bases with low sequencing quality were clipped. Next, reads were mapped into genome version “Gmax_275_v2.0.fa” of soybean downloaded with standard parameters from Phytozome.net and Bowtie2 (version 2.2.4). Then, expression analysis was performed by in-house scripts and DESeq2 (R-package).
DEGs were defined at an FDR of 0.1 and listed as either up or down-regulated. A heat map plot was generated using R software (version 3.4.1). Then, hierarchical clustering was applied by considering a cut-off threshold of 8 expression profiles (clusters). Venn diagrams were depicted using the VennDiagram R package.
A GO enrichment analysis was performed using the topGO R package48, while the GO annotation file was extracted from agriGO website49. Each GO term, containing at least two DEGs, was analyzed by Fisher's exact test. The resulting P values were corrected by FDR multiple testing approach. GO terms with an FDR lower than 0.1 were considered for further analysis.
Ten DEGs were randomly selected and measured by qRT-PCR assays (Applied Biosystems) to validate MACE results. F-BOX gene was used as an internal reference to standardize the expression of target genes, and the ratio between treatments was calculated according to50. All primers used are listed in Suppl. Table 6. Data analysis and primer efficiencies were obtained using LinReg PCR software51. Relative expression ratios and statistical analysis were performed using fgStatistics software interface52. The cut-off for statistically significant differences was set as P < 0.05, indicated as *.
Antioxidant measurements
Additionally, antioxidant proteins encoded by DEGs detected in MACE were analyzed. Five biological samples were collected per treatment and genotype (n = 20). The enzymatic extraction was performed according to Singh et al.53. The activities of superoxide dismutase (SOD, EC 1.15.1.1)54, ascorbate peroxidase (APX, EC 1.11.1.11)55, phenol peroxidase (POX, EC 1.11.1.7)56 and catalase (CAT, EC 1.11.1.6)57 were measured, as well as the total soluble protein content58.
Leaf morphology measurements
Changes in leaf thickness (LT), adaxial and abaxial stomatal and trichrome densities (SD_AD, SD_AB, TD_AD and TD_AB) were assessed in leaves between nodes 4 to 7 of MUNASQA and TJ2049 R5 plants after 3, 10 and 21 d of water deficit. Five samples per genotype and treatment (n = 20) were taken and fixed in FAA (10% formalin, 5% acetic acid, 50% ethyl alcohol). Diaphanised sections of the central leaflet were used for superficial observations. Different standard colorations were applied according to D’Ambrogio de Argüeso (1986)59. Staining samples were visualized in a Leica DM500 optical microscope and photographed with an Arcano (5 Mpx) camera (10 measurements per sample, n = 200).
Stomatal aperture measurements
According to Gudesblat et al.60, stomatal apertures were measured in three independent assays, using MUNASQA and TJ2049 plants submitted to 72 h of water deficit in the V3 stage. The aperture of 40 stomata per treatment and genotype (n = 120) was measured in each experiment.
Wilting air desiccation assay
Response to air desiccation was evaluated in the R5 stage. Three whole leaves per genotype (n = 6) were collected and exposed to air desiccation at 32 °C. After 0, 6, 24, 36 and 48 h of air exposure, plants were photographed with a Canon Power Shot SX520 HS (14 Mpx), and the wilting rate was assessed.
Comparative analysis of genotypes responses to water deficit in V3 and R5
The responses of MUNASQA and TJ2049 to water deficit, applied in V3 and R5 stages, were compared by measuring morphophysiological and biochemical parameters grouped by biological processes (BP) (Table 6). Four treatments were defined: (i) Control-V3, (ii) Control-R5, (iii) Stress-V3 and (iv) Stress-R5, and three sampling times were performed (0, 4 and 8 d of water deficit). Ten plants per genotype, treatment and time were collected and used for markers evaluation (n = 240). Additionally, for treatments (i), (iii) and (iv), 50 plants per genotype were harvested at physiological maturity (n = 300) to quantify relative yield and calculate the relative yield DSI (Drought Susceptibility Index) according to Fischer and Maurer61.
The markers evaluated and their methodologies are detailed below.
Markers
As stress response markers, the activities of SOD, APX, POX and CAT proteins were assessed, together with the accumulation of free proline (PRO)62, malondialdehyde (MDA)63, total chlorophylls (CHL)64, and carotenoids (CAR)65.
As growth indicators, the plant total leaf area (TLA) and biomass (plant total dry weight) were determined. Then leaf area index (LAI) and leaf area ratio (LAR)66, the net assimilation rate (NAR)67, relative growth rate (RGR)68 and crop growth rate (CGR)69 were calculated.
Finally, as water-use parameters, plant RWC and WUE70 were calculated. Moreover, the canopy temperature was monitored and recorded using a FLIR ONE-3 thermal camera (0.3456 Mpx) to calculate canopy temperature depression (CTD)71.
Univariate analysis
Data from stomatal apertures were subjected to a two-way ANOVA (Factor 1: genotype, Factor 2: treatment). The remaining data were analyzed through ANOVA with post hoc contrast by Tukey's HSD test. Data were analyzed with InfoStat statistical package52 and presented as the arithmetic mean ± SE. Means were considered significantly different at P < 0.05.
Correlations, multivariate analysis and markers selection
The 22 markers strength of association between phenological stages, V3 and R5, was measured by Pearson's correlation analysis adjusted by Bonferroni (P < 0.05 indicated as *; P < 0.01 ** and P < 0.001 ***). Then, the markers correlation with relative yield was assessed. Correlation coefficients (r2) were classified as “Strong” (> ± 0.60) and “Weak” (below ± 0.59).
All markers were submitted to a PCA to discriminate main associations between markers, genotypes and treatments. However, in PCAs, an increase in the number of comparable variables will reduce the proportion of variance among treatments explained by those variables. Therefore, all markers were grouped by biological processes in (i) “stress response”, (ii) “growth”, and iii) “water use” sets and subjected to independent PCAs to discriminate which markers better explain the variability between genotypes/treatments.
Additionally, the markers were ranked by CF and SW. The markers CF, in terms of their complexity and evaluation cost, was assigned according to 4 categories: easy and cheap (1), easy and expensive (2), complicated and cheap (3) or complicated and expensive (4). Meanwhile, the SW was obtained from PCA variables coefficients (autovectors e1 and e2) that were ranked and classified in “Low” (Low = [− 2, 2]) and High (High = ℝ − [− 2, 2]), according to their weigh on PC1 and PC2. Markers strongly correlated between phenological stages, if possible, with yield, together with CF values of 1 or 2 and “High” SW in both PC, were selected as the most efficient phenotyping markers.
General guidelines statement
The authors declare that there is no conflict for the use of commercial soybean varieties for scientific research purposes cited in this article in accordance with Argentine law (Law of Seeds and Phytogenetic Creations No. 20,247/73).
References
Pagano, M. C. & Miransari, M. 1—The importance of soybean production worldwide. In (ed. Miransari, M. B. T.-A. and B. S. in S. P.) 1–26 (Academic Press, 2016). https://doi.org/10.1016/B978-0-12-801536-0.00001-3.
Ray, D. K., Mueller, N. D., West, P. C. & Foley, J. A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8, e66428 (2013).
Fuganti-Pagliarini, R. et al. Characterization of soybean genetically modified for drought tolerance in field conditions. Front. Plant Sci. https://doi.org/10.3389/fpls.2017.00448 (2017).
Kunert, K. & Vorster, B. J. In search for drought-tolerant soybean: Is the slow-wilting phenotype more than just a curiosity?. J. Exp. Bot. 71, 457–460 (2020).
Rauf, S., Al-Khayri, J. M., Zaharieva, M., Monneveux, P. & Khalil, F. Breeding strategies to enhance drought tolerance in crops. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits (2016). https://doi.org/10.1007/978-3-319-22518-0_11.
Ribaut, J. M., Jiang, C., Gonzalez-de-Leon, D., Edmeades, G. O. & Hoisington, D. A. Identification of quantitative trait loci under drought conditions in tropical maize. 2. Yield components and marker-assisted selection strategies. Theor. Appl. Genet. https://doi.org/10.1007/s001220050492 (1997).
Lafitte, H. R., Price, A. H. & Courtois, B. Yield response to water deficit in an upland rice mapping population: Associations among traits and genetic markers. Theor. Appl. Genet. https://doi.org/10.1007/s00122-004-1731-8 (2004).
Parmley, K., Nagasubramanian, K., Sarkar, S., Ganapathysubramanian, B. & Singh, A. K. Development of optimized phenomic predictors for efficient plant breeding decisions using phenomic-assisted selection in Soybean. Plant Phenomics https://doi.org/10.34133/2019/5809404 (2019).
Blum, A. Constitutive traits affecting plant performance under stress. In Edmeades, G.O., M. Bänziger, H.R. Mickelson C.B. Pena-Valdivia (eds.). Dev. Drought Low-N Toler. Maize Proc. a Symp. 25–29 March 1996 (1997).
Fried, H. G., Narayanan, S. & Fallen, B. Evaluation of soybean [Glycine max (L.) Merr.] genotypes for yield, water use efficiency, and root traits. PLoS ONE https://doi.org/10.1371/journal.pone.0212700 (2019).
Bala, M., Sinha, R., Mallick, M. A., Sharma, T. R. & Singh, A. K. Methods of gene expression profiling to understand abiotic stress perception and response in legume crops. Methods Mol. Biol. https://doi.org/10.1007/978-1-0716-0235-5_5 (2020).
Cobb, J. N., DeClerck, G., Greenberg, A., Clark, R. & McCouch, S. Next-generation phenotyping: Requirements and strategies for enhancing our understanding of genotype-phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. https://doi.org/10.1007/s00122-013-2066-0 (2013).
Pardo, E. M. et al. Drought tolerance screening under controlled conditions predicts ranking of water-limited yield of field-grown soybean genotypes. J. Agron. Crop Sci. 201, 95–104 (2015).
Shin, J. H. et al. Transcriptomic changes due to water deficit define a general soybean response and accession-specific pathways for drought avoidance. BMC Plant Biol. 15, 26 (2015).
Verslues, P. E., Lasky, J. R., Juenger, T. E., Liu, T. W. & Nagaraj Kumar, M. Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. https://doi.org/10.1104/pp.113.224014 (2014).
Laxa, M., Liebthal, M., Telman, W., Chibani, K. & Dietz, K. J. The role of the plant antioxidant system in drought tolerance. Antioxidants 8, 94 (2019).
Nadeem, M. et al. Molecular sciences review research progress and perspective on drought stress in legumes: A review. mdpi.com (2019). https://doi.org/10.3390/ijms20102541.
Carvalho, M. et al. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. https://doi.org/10.1016/j.jplph.2019.153001 (2019).
Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 40, 4–10 (2017).
Guzzo, M. C. et al. Morpho-physiological traits associated with drought responses in soybean. Crop Sci. 61, 672–688 (2021).
Fathi, A. & Tari, D. B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. https://doi.org/10.3126/ijls.v10i1.14509 (2016).
Mombeni, M. & Abbasi, A. Biochemical responses of some common bean (Phaseolus vulgaris L.) genotypes to drought stress. J. Agric. Sci. Tech. 21, 407–421 (2019).
Khayatnezhad, M. The effect of drought stress on leaf chlorophyll content and stress resistance in maize cultivars (Zea mays). Afr. J. Microbiol. Res. 6, 2844–2848 (2012).
Xu, C. et al. Physiological and transcriptomic responses of reproductive stage soybean to drought stress Associate View project Physiological and transcriptomic responses of reproductive stage soybean to drought stress View project Physiological and transcriptomic resp. Springer 37, 1611–1624 (2018).
Du, W., Yu, D. & Fu, S. Detection of quantitative trait loci for yield and drought tolerance traits in soybean using a recombinant inbred line population. J. Integr. Plant Biol. https://doi.org/10.1111/j.1744-7909.2009.00855.x (2009).
Fahad, S. et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 8, 1147 (2017).
Tardieu, F. Plant tolerance to water deficit: Physical limits and possibilities for progress. Comptes Rendus - Geosci. 337, 57–67 (2005).
Stockle, C. O. & Kemanian, A. R. Crop radiation capture and use efficiency: A framework for crop growth analysis. In Crop Physiology: Applications for Genetic Improvement and Agronomy (eds Sadras, V. O. & Calderini, D. F.) 145–170 (Academic Press, 2009).
Kerby, T. A., Cassman, K. G. & Keeley, M. Genotypes and plant densities for narrow-row cotton systems. I. Height, nodes, earliness, and location of yield. Crop Sci. https://doi.org/10.2135/cropsci1990.0011183x003000030034x (1990).
Fang, H., Liang, S. & Kuusk, A. Retrieving leaf area index using a genetic algorithm with a canopy radiative transfer model. Remote Sens. Environ. 85, 257–270 (2003).
Clavijo, J. Análisis de crecimiento en malezas. Rev. Comalfi 16, 12–16 (1989).
Katuwal, K. B., Schwartz, B. & Jespersen, D. Desiccation avoidance and drought tolerance strategies in bermudagrasses. Environ. Exp. Bot. https://doi.org/10.1016/j.envexpbot.2019.103947 (2020).
Petrov, P. et al. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. https://doi.org/10.1111/jac.12255 (2018).
Bacelar, E. L. V. A. et al. Water use strategies of plants under drought conditions. In Plant Responses to Drought Stress: From Morphological to Molecular Features https://doi.org/10.1007/978-3-642-32653-0_6 (2012).
Etherington, J. R. & Larcher, W. Physiological plant ecology. J. Ecol. https://doi.org/10.2307/2261487 (1996).
Afzal, A., Duiker, S. W., Watson, J. E. & Luthe, D. Leaf thickness and electrical capacitance as measures of plant water status. Trans. ASABE https://doi.org/10.13031/trans.12083 (2017).
Giuliani, R. et al. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (Genus Oryza). Plant Physiol. https://doi.org/10.1104/pp.113.217497 (2013).
Takai, T. et al. A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Sci. Rep. https://doi.org/10.1038/srep02149 (2013).
Bartletta, M. K., Klein, T., Jansen, S., Choat, B. & Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 113, 13098–13103 (2016).
Sourour, A. A review: Morphological, physiological, biochemical and molecular plant responses to water deficit stress. Int. J. Eng. Sci. 06, 01–04 (2017).
Manavalan, L. P. & Nguyen, H. T. Drought tolerance in crops: Physiology to genomics. In Plant stress physiology (ed. Shabala, S.) 1–23 (CABI, 2017).
Yousfi, S. et al. Comparative performance of remote sensing methods in assessing wheat performance under Mediterranean conditions. Agric. Water Manag. https://doi.org/10.1016/j.agwat.2015.09.016 (2016).
Al Hassan, M., Chaura, J., Donat-Torres, M. P., Boscaiu, M. & Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants https://doi.org/10.1093/aobpla/plx009 (2017).
Fehr, W. E., Caviness, C. E., Burmood, D. T. & Pennington, J. S. Stage of development description of soybean, Glycine Max (L.) Merrill. Crop Sci. 11(6), 929–931 (1971).
Pereyra-Irujo, G. A., Gasco, E. D., Peirone, L. S. & Aguirrezábal, L. A. N. GlyPh: A low-cost platform for phenotyping plant growth and water use. Funct. Plant Biol. https://doi.org/10.1071/FP12052 (2012).
Richards, L. A. Physical condition of water in soil. In Methods of Soil Analysis 128–152 (1965) https://doi.org/10.2134/agronmonogr9.1.c8.
Weatherley, P. E. Studies in the water relations of the cotton plant: I. the field measurement of water deficits in leaves. New Phytol. 49, 81–97 (1950).
Alexa, A. & Rahnenfuhrer, J. topGO. Alexa A and Rahnenfuhrer J (2016). topGO: Enrichment Analysis for Gene Ontology. R package version 2.28.0. (2016) https://doi.org/10.1136/jech-2013-202820.
Tian, T. et al. AgriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. (2017) https://doi.org/10.1093/nar/gkx382.
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29(9), e45 (2001).
Ramakers, C., Ruijter, J. M., Lekanne Deprez, R. H. & Moorman, A. F. M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. https://doi.org/10.1016/S0304-3940(02)01423-4 (2003).
Di Rienzo, J. . A. et al. InfoStat, versión 2008. Grup. InfoStat, FCA, Univ. Nac. Córdoba, Argentina (2008).
Singh, A., Lawrence, K., Pandit, S. & Lawrence, R. Oxidative stress in leaves, stems and roots of Withania somnifera on copper exposure. International Journal of Pharmaceutical Sciences Review and Research vol. 30 (2015).
Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 60, 6418–6424 (2012).
Nakano, Y. & Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. https://doi.org/10.1093/oxfordjournals.pcp.a077268 (1987).
Kar, M. & Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 57(2), 315−319 (1976).
Chance, B. & Maehly, A. C. B. T.-M. in E. [136] Assay of catalases and peroxidases: . in vol. 2 764–775 (Academic Press, 1955).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
D’ Ambrogio de Argüeso, A. Manual de técnicas en histología vegetal. Editorial Hemisferio sur (1986).
Gudesblat, G. E., Torres, P. S. & Vojnov, A. A. Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. https://doi.org/10.1104/pp.108.126870 (2009).
Fischer, R. A. & Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. https://doi.org/10.1071/AR9780897 (1978).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil https://doi.org/10.1007/BF00018060 (1973).
Hodges, D. M., DeLong, J. M., Forney, C. F. & Prange, R. K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta https://doi.org/10.1007/s004250050524 (1999).
Porra, R. J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. https://doi.org/10.1023/A:1020470224740 (2002).
Riemann, B. Carotenoid interference in the spectrophotometry determination of chlorophyll degradation products from natural populations of phytoplankton. Limnol. Oceanogr. https://doi.org/10.4319/lo.1978.23.5.1059 (1978).
Pallardy, S. G. Vegetative Growth. In Physiology of Woody Plants 39–86 (Elsevier, 2008). https://doi.org/10.1016/b978-012088765-1.50004-x.
Watson, D. J. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties, and within and between years. Ann. Bot. https://doi.org/10.1093/oxfordjournals.aob.a083148 (1947).
Hoffmann, W. A. & Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. https://doi.org/10.1093/aob/mcf140 (2002).
Hunt, R. Plant growth analysis: The rationale behind the use of the fitted mathematical function. Ann. Bot. https://doi.org/10.1093/oxfordjournals.aob.a085632 (1979).
Van Halsema, G. E. & Vincent, L. Efficiency and productivity terms for water management: A matter of contextual relativism versus general absolutism. Agric. Water Manag. https://doi.org/10.1016/j.agwat.2011.05.016 (2012).
Kumar, M. et al. Canopy temperature depression (CTD) and canopy greenness associated with variation in seed yield of soybean genotypes grown in semi-arid environment. South African J. Bot. https://doi.org/10.1016/j.sajb.2017.08.016 (2017).
Acknowledgements
B.W., A.B., D.F.D.P., A.V. and A.P.C. are Career Investigators of CONICET, E.M.P. is Career investigator of EEAOC, C.L. and A.P.M. are support assistants of CONICET, L.T. and L.S.P.B. were supported by post-doctoral and doctoral scholarships from CONICET. We are grateful to the EEAOC staff for greenhouse assays. We especially thank Dr. Christian W. Bachem (Wageningen University, Netherlands) for his critical reading of this manuscript.
Funding
Research was supported by grants from the Ministerio de Ciencia, Tecnología e Innovación Productiva (MINCYT): BiotecSojaSur I N°127119 and BiotecSojaSur II (Acta N°52), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-UE): GENBIONOA (N°31) and The Estación Experimental Agroindustrial Obispo Colombres (GRB2 project). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Contributions
L.T. and L.S.P.B. designed and performed the experiments, compiled the data and wrote the article; A.P.M. carried out the statistical analysis and art graph; C.L. performed the morphophysiological analysis; E.M.P. performed the RNA-seq sample preparation; A.B. and D.F.D.P. designed and performed the RNA-seq analysis; B.W. and A.V. supervised and designed experiments; A.P.C., B.W. and E.M.P. conceived the project; all authors contributed to the writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toum, L., Perez-Borroto, L.S., Peña-Malavera, A.N. et al. Selecting putative drought-tolerance markers in two contrasting soybeans. Sci Rep 12, 10872 (2022). https://doi.org/10.1038/s41598-022-14334-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14334-3
- Springer Nature Limited
This article is cited by
-
Is intrinsic water use efficiency independent of leaf-to-air vapor pressure deficit?
Theoretical and Experimental Plant Physiology (2023)