Abstract
The spotted lanternfly (SLF), Lycorma delicatula (Hemiptera: Fulgoridae), has the potential to become a global pest and is currently expanding its range in the United States. In this study, we investigated the dispersal patterns of SLF in Ailanthus altissima during its oviposition period in South Korea using a fluorescent marking system. Oviposition patterns of SLF were then analyzed by surveying egg masses in A. altissima patches. The recapture rate of fluorescent-marked SLF rapidly decreased to 30% within the first two weeks. During the oviposition period, seven cases of among-patch dispersal of SLF adults were observed. The minimum distance that SLF could have traveled to achieve these among-patch dispersal events ranged from 10 to 1740 m, with most events spanning under 60 m. Also, the number of A. altissima trees on which fluorescent marked SLF were detected increased until September. Based on the egg mass survey, a total of 159 egg masses were detected from 38 out of 247 A. altissima trees. Furthermore, 79.2% of egg masses were located < 2.5 m above the ground. Finally, a generalized linear mixed model showed that tree height and diameter at root collar (DRC) of A. altissima trees had significant effects on the number of egg masses.
Similar content being viewed by others
Introduction
Lycorma delicatula (White) (Hemiptera: Fulgoridae), spotted lanternfly (SLF), is an invasive agricultural pest native to China1,2. This pest has invaded South Korea, Japan, and the United States, causing serious problems in agricultural and forest landscapes1,3,4,5,6. In South Korea, SLF was first reported in 2004, and the area of infestation increased over 8000-fold from 2006 to 20107,8,9,10. In the US, it was first detected in Berks County, Pennsylvania in 2014. Since then, SLF infestation has expanded to nine different states including New Jersey, Virginia, and Delaware by 20223,6,11. Furthermore, recent modeling studies predicted that SLF may become a global threat. Portions of Asia, Oceania, South America, North America, Africa and Europe might be susceptible to its invasion based on their temperature profiles1,6.
SLF has a broad range of host plants which facilitate the successful establishment of this pest in new areas. It is known to feed on more than 70 plant species including various ornamental and fruit trees such as apples (Malus spp.) (Rosaceae), grapes (Vitis vinifera) (Vitaceae), and peaches (Prunus persica) (Rosaceae), as well as other woody trees such as black locust (Robinia pseudoacacia) (Fabaceae), willow tree (Salix spp.) (Salicaceae), and Korean evodia tree (Evodia danielli) (Rutaceae)4,10. Moreover, in invaded areas including South Korea and the US, SLF is known to switch hosts during its development, with the range of host plants becoming narrower through its univoltine life cycle10. In temperate region, nymphs feed on a variety of host plants from 81 taxa, after emerging from eggs in May12,13,14,15. As they start to develop into adults from late July, SLF adults feed on a narrower range of host plants of 47 taxa. Finally, the adults oviposit egg masses on hosts belonging to 28 taxa as well as non-plant materials such as stones and metal fences between late September and November4,10,12,13.
Among various host plants of SLF, Ailanthus altissima (Simaroubaceae), the tree-of-heaven, is native to Southeast Asia and known as one of the major host plants in China, South Korea, and the US4,16. Furthermore, A. altissima has already been introduced and established in multiple continents including East Asia, Europe, and North America, making it readily available for SLF even in areas where the insects have yet to invade17. Previous studies indicate the importance of A. altissima as a major host plant of SLF. First, A. altissima is known as the most preferred and suitable feeding host of SLF along with V. vinifera. Lee et al.16 observed a high survivorship of SLF nymphs and adults on A. altissima as well as their preference for this plant compared with different ornamental and fruit trees. Second, A. altissima was reported to be one of the most preferred oviposition sites4,10. Liu et al.18 found that A. altissima was one of the four oviposition host plants favored by this insect along with black cherry, black birch, and sweet cherry in Pennsylvania, US. Finally, A. altissima contains high concentrations of cytotoxic alkaloids, which SLF may utilize for their own defence against predators4,13,19.
Therefore, it is essential to understand the abundance and distribution of SLF on A. altissima in order to develop effective management programs. Previous studies demonstrated that SLF adults shift host plants from woody and non-herbaceous plants, on which the insects feed as they emerge in July, towards A. altissima between September and November12,13,18. In addition, SLF adults are known to mate and reproduce during this period utilizing multiple substrates including A. altissima for oviposition20. However, the dispersal patterns of SLF adults within and among A. altissima patches after their arrival have not yet been investigated. Especially, given that SLF is a univoltine species in the invaded areas, investigating the dispersal patterns of this pest on A. altissima and characterizing their oviposition pattern can provide valuable information for its management.
In our study, we surveyed the abundance and distribution of SLF on A. altissima patches from September to November when SLF adults are known to shift their host plant to A. altissima and lay eggs13,18. In particular, to investigate the dispersal pattern of SLF within and among different A. altissima patches, we tracked the movement of SLF using a fluorescent marking system (FMS)21,22. Then, in December, we surveyed the location, number, and size of SLF egg masses on A. altissima trees and analyzed the oviposition patterns relative to the traits of A. altissima surveyed.
Results
Effect of fluorescent marking on SLF
Fluorescent marking did not significantly affect the survivorship and flight behavior of SLF adults. First, the survivorship of SLF was not significantly affected by fluorescent marking (χ2 = 0.81, df = 1, P = 0.37; Fig. 1a). Second, no significant effect of fluorescent marking on flight behavior of SLF was observed. Fluorescent-marked and unmarked control groups did not show significant differences in the number of pecks on the forewings required to initiate flight (t = 0.93, df = 1, P = 0.36; Fig. 1b), flight duration (t = 0.71, df = 1, P = 0.48; Fig. 1c), and flight distance (t = 1.23, df = 1, P = 0.22; Fig. 1d). Moreover, the overall flight direction was not substantially different between fluorescent-marked and unmarked groups; the flight direction of SLF generally aligned along the north and northeast axes (Fig. 1e).
Assessment of the effect of fluorescent marking on performance of Lycorma delicatula. (a) survival rate of fluorescent-marked and unmarked L. delicatula over 16 days, (b) number of pecks to initiate the flight of L. delicatula, (c) flight duration, (d) flight distance, and (e) flight direction. In (e), points’ distance from the center indicates the number of trials in which individuals flew in a given direction. NS indicates no significant difference detected between two groups.
Dispersal pattern of SLF on A. altissima
The number of fluorescent-marked SLF adults showed a large decrease during the first two weeks of survey yielding average recaptures rates of 8.7 ± 6.6% (Mean ± SE) and 10.5 ± 4.3% in Tan stream and Gyeongan stream, respectively (Fig. 2). After the first two weeks, fluorescent-marked SLF adults were occasionally detected from both streams until late October, with no fluorescent-marked individuals observed in November.
Number of fluorescent-marked Lycorma delicatula observed from Ailanthus altissima patches from September to November among patches A–D (a, c, e, and g) in Tan stream, and patches E–G (b, d and f) in Gyeongan stream. Black arrow indicates the date on which L. delicatula that had been marked from different patch at the onset of the experiment was observed.
A total of 12 events were recorded in which SLF individuals marked from different patches at the onset of the experiment were observed (Fig. 2; Table 1). Because fluorescent marking in this study did not allow us to identify different individuals, we were able to confirm seven among-patch dispersals among the 12 events recorded (Table 1). For example, it cannot be confirmed whether an SLF individual observed in patch E on October 6th was a new individual that had moved from patch F or the same individual observed on September 25th that remained in patch E. For this reason, we assigned ‘unidentified’ for among-patch dispersal with no record of minimum distance moved in Table 1. Minimum dispersal distance was estimated for the seven confirmed among-patch dispersal cases by measuring the shortest distance between two patches where SLF was marked and marked SLF was observed. Among-patch dispersal was recorded in all study patches except for patch B (Fig. 2; Table 1). In addition, we observed fluorescent-marked SLF in neighboring patches adjacent to study patches. In Tan stream, a fluorescent-marked SLF from patch B was detected two times in the neighboring patch on September 28th and October 1st (Fig. 3). In Gyeongan stream, fluorescent-marked SLF from patch E were observed three times from a neighboring patch located between patch F and G, on September 22nd, 25th, and 29th, and another fluorescent-marked individual from patch G was detected on September 29th (Fig. 3).
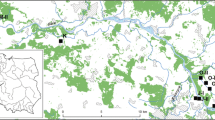
Ailanthus altissima patches selected to study dispersal and oviposition patterns of Lycorma delicatula in (a) Tan stream and (b) Gyeongan stream. Colors filled in each patch indicates the color of fluorescent paint for marking of L. delicatula adults. See “Materials and methods”for descriptions of study and neighboring patches.
Fluorescent-marked SLF also displayed within-patch movement in most of the study patches during the observation period (Fig. 4). For within-patch movement, patches A and B were not included in analysis due to low numbers of A. altissima (< 5 trees) in the patches (Table 1). Except for patch C, the cumulative proportions of A. altissima trees on which marked SLF were detected gradually increased in September. In patch D of Tan stream, the number of trees with fluorescent-marked SLF detection gradually increased from the initial 9–36% by September 28th until the end of the survey (Fig. 4c). Similar to patch D, the proportion of A. altissima trees with fluorescent-marked SLF detected at least once gradually increased until October 2nd in Gyeongan stream. The overall number of trees increased gradually from the initial 3%, 11%, and 6% to 17%, 56%, and 26% in patches E, F, and G, respectively (Fig. 4).
Oviposition pattern of SLF
In Tan stream, the tree heights of A. altissima ranged from 0.40 to 5.15 m, and in Gyeongan stream, the heights ranged from 0.20 to 5.90 m (Fig. 5a,b). Among the 116 trees surveyed in Tan stream, those < 2.5 m in their heights comprised 89%, and similarly, in Gyeongan stream, 86% of the 131 trees surveyed were < 2.5 m (Table 2; Fig. 5a,b). The DRC of A. altissima trees ranged from 0.32 to 11.15 cm and from 0.64 to 14.33 cm in Tan and Gyeongan streams, respectively (Fig. 5c,d). Trees with DRC < 5 cm were dominant comprising 85% and 72% of A. altissima trees in Tan stream and Gyeongan stream, respectively. Trees of which trunks were cut off by local administration were present only in Gyeongan stream comprising 45% of A. altissima trees in the stream.
From the two streams, we found a total of 159 egg masses on 38 out of 247 A. altissima trees (Table 2). In Tan stream, 67 out of 69 egg masses were located < 2.5 m above the ground, whereas 59 out of 90 egg masses were detected < 2.5 m above the ground in Gyeongan stream. In both streams, ca. 30% of the egg masses were located < 20 cm above the ground. However, a substantial proportion of egg masses in Gyeongan stream were detected > 2.5 m above the ground, yielding 34% of the total egg masses (Table 2). 126 egg masses located < 2.5 m above the ground contained 4257 eggs, yielding an average of 34.06 ± 1.88 (Mean ± SE) eggs per egg mass (Table 2). In addition, we found 252 scattered eggs on seven A. altissima trees.
Using a generalized linear mixed model, the effects of tree height, DRC, and their interaction, and cut status on the number of egg masses on A. altissima trees in study patches were evaluated. Significant effects of tree height (F1,241 = 12.78, P < 0.001) and DRC (F1,241 = 12.54, P < 0.001) were detected (Supplementary information SI 1).
Discussion and conclusion
To reliably track SLF adults, we tested the effect of fluorescent marking on survivorship and flight ability and found no significant adverse effects of fluorescent-marking on SLF adults. Furthermore, the durability of the method was confirmed in the field survey of this study, in which fluorescent-marked individuals were recaptured after a maximum of 47 days. Previously, Nixon et al.23 reported that fluorescent marking did not significantly affect horizontal and vertical dispersal capacity of SLF nymphs and adults. Moreover, Keller et al.24 observed dispersal of SLF nymphs in a deciduous forest, where fluorescent-marked individuals were detected a maximum of 65 m away from the release point. Therefore, the fluorescent marking system can be an effective tool to study the movement of SLF for both nymphs and adults. In addition to the conventional use of fluorescent marking for nighttime tracking21,22,25,26, fluorescent marking was effective in this study for the detection of SLF under the shadow created by tree canopy even during daytime.
As previously reported from many mark-recapture studies, the recapture rate of fluorescent-marked SLF also showed a consistent decrease during the study period with a rapid decrease during the early survey period24,27,28. Indeed, the recapture rates dropped on average to 10% during the first two weeks. Nevertheless, our results provide empirical evidence for the first time demonstrating active dispersal of natural SLF population among A. altissima patches. During the survey, we recorded 12 cases between September and October in which SLF individuals that had been marked from different patches at the onset of the experiment, were found in study patches. Based on these observations, we confirmed that at least seven SLF individuals displayed among-patch dispersal. Marked SLF were recaptured up to 27 days after the onset of the survey. In general, the marked individuals were found in A. altissima patches located close to the original patches from which SLF were marked at the onset of the survey. The minimum dispersal distance of marked SLF ranged between 10 and 60 m in most cases. Indeed, SLF has been reported to show descending flight behavior and short-range dispersals with each flight-bout ranging from 20 to 40 m in field conditions29,30. Interestingly, one marked SLF in this study was found to have dispersed at least 1.7 km within 22 days suggesting strong dispersal capacity during its oviposition period. Although it has not been evaluated in field conditions, Wolfin et al.30 suggested that the potential flight distance of SLF could be > 3 km based on laboratory observation.
Based on the survey of egg masses, we found that the tree height and DRC of A. altissima had significant effects on the number of egg masses on A. altissima trees. In addition, we observed significant positive correlations between these tree characteristics and the number of egg masses. These finding can serve as important information to prioritize sampling and management efforts based on tree characteristics especially when targeting the removal of egg masses from A. altissima. Indeed, a large-scale management practice was conducted in South Korea to identify and remove SLF egg masses during the early stages and outbreak of this invasive species31. With regard to vertical locations of egg masses on A. altissima, 79.2% of the masses were found within 2.5 m above the ground. In the surveyed areas, A. altissima trees were < 6 m in their height due to their age and local management practices such as tree trunk cutting. This would make it easier for investigators to locate and remove SLF egg masses for management practice. However, caution is needed when surveying substantially larger A. altissima as reported in previous studies in the US. Keller et al.32 found that 75% of egg masses were located above 6 m on A. altissima trees when the tree heights ranged from 5.5 to 23.8 m, and the lowest number of egg masses was located zero to 3 m above the ground.
In this study, we found that SLF actively dispersed in A. altissima patches during oviposition period and oviposited only on a small number of A. altissima trees within the patch. That is, although SLF adults were frequently observed on A. altissima trees while feeding or resting during the study, only a small portion of those trees were used for oviposition sites by SLF. This oviposition pattern of SLF may be attributed to the differential importance of A. altissima as feeding host versus oviposition substrate. SLF adults display higher preference towards A. altissima compared with other host plants from late August in South Korea and the US13,18. Nevertheless, SLF are known to deposit eggs on surfaces of various substrates including 28 taxa of host plants and inorganic materials such as stones and metal fences12,20. Indeed, a large number of SLF egg masses were detected on E. danielli and cement walls in the vicinity of A. altissima patches inspected in our survey (personal observation). Therefore, further studies are warranted to examine how SLF adults utilize different oviposition substrates following feeding on preferred host plants such as A. altissima and how this alternation would affect the likelihood of successful overwintering of egg masses or their survival against natural predators.
As one of the major host plants of SLF, A. altissima is already present in many countries expanding its geographical range, and the invasion of SLF is expected to be facilitated by the distribution of A. altissima. Especially in South Korea and the US, this invasive insect displays preference toward A. altissima in September through November when adults mate and oviposit, highlighting the importance of A. altissima in the life history of SLF4,13,18. In this study, we report for the first time dispersal patterns of natural SLF populations on A. altissima with a maximum of 1.7 km dispersal distance between A. altissima patches. We identified significant traits of A. altissima affecting the number of SLF egg masses on the trees. Our findings provide baseline information for developing proactive and efficient management strategies against SLF based on their dispersal and oviposition patterns on a major host plant.
Materials and methods
Fluorescent marking
Dispersal of SLF adults was tracked using a fluorescent marking system (FMS), which has been demonstrated to be applicable for multiple insect species including SLF nymphs21,22,24. To mark the SLF, either red, yellow, or blue fluorescent paint (#1166R, #1166Y, #1166B, BioQuip Products, USA) was diluted with distilled water (1:4). The mixture was then gently sprayed three times (ca. 20 mg each time) on each SLF individual using a mist sprayer from a distance of 30–50 cm (SI 2). Throughout the field survey, a handheld ultraviolet (UV) laser (PX 600 mW, class IIIB purple laser, 405 nm, Big Lasers, USA) was used to detect fluorescent-marked SLF individuals25.
Effect of fluorescent marking on SLF
Prior to field survey, the potential effects of fluorescent marking on the survivorship and flight behavior of SLF adults (sex ratio 1:1) were evaluated. SLF adults were collected using sweeping nets (BioQuip Products, USA) from Gyeonggi-do, South Korea (37°47′85.95″ N, 127°11′64.58″E) in September 2020. Two hours after fluorescent marking of SLF, both fluorescent-marked and unmarked SLF were subjected to survivorship and flight behavior assessment.
Survivorship of insects was measured on two A. altissima trees (ca. 2 m in height) located in Gachon University, South Korea (37°45′38.50″N, 127°13′37.75″E). Two fluorescent-marked and two unmarked insects were placed in a cylindrical mesh cage [25 × 30 cm (radius × height)] enclosing a tree branch; a total of 20 groups were tested (n = 40). Then, survivorship of SLF was determined once every two days until no individuals were alive. Survivorship was compared between fluorescent-marked and unmarked SLF using Kaplan-Meir survivorship analysis (JMP 12, SAS Institute Inc., USA).
The effects of fluorescent marking on flight behavior were evaluated in an open space (986 m2) in Gachon University, South Korea (37°45′08.37″N, 127°12′79.69″E) at 26 ± 1 °C and a relative humidity of 30 ± 5%. To induce flight of SLF adults, a wooden square rod [3 × 3 × 100 cm (width × length × height)] was established upright at the center of the arena. The SLF adult was placed individually 10 cm away from the top on the wooden square rod. To minimize any unnecessary stimuli from experimenter, SLF flight was induced by following the same sequence: once the insect climbed up the rod and oriented itself staying still to a random direction, then an experimenter carefully positioned at the back of the insect and gently pecked the forewings using tweezers to initiate its flight33,34. Pecking was intended to mimic predatory behavior of birds. Once the insect jumped away, an operator followed the individual until it landed on the ground (n = 30). The experiment was conducted for 2 h between 13:00–15:00 and marked and unmarked SLF were randomly tested during the evaluation. The number of pecks to initiate the flight, flight duration, and flight distance of SLF were compared using t-test (JMP 12, SAS Institute Inc., USA).
Field study sites
Dispersal patterns of SLF adults in A. altissima patches and their oviposition patterns were investigated in multiple A. altissima patches located along two streams in Gyeonggi-do, South Korea: Tan stream in Seongnam-si (37°48′01.80″N, 127°11′56.03″E) and Gyeongan stream in Gwangju-si (37°41′54.21″N, 127°27′12.37″E). Both Tan and Gyeongan streams run along suburban residential areas in their respective cities, with pedestrian lanes built along the streams. We selected seven A. altissima patches as study patches when more than 10 SLF adults were found per patch (Fig. 3). In the study patch, all SLF individuals or ca. up to 30 adults were florescent-marked. In addition, when the number of SLF adults was less than 10 from an A. altissima patch, those patches were designated as neighboring patches (Fig. 3). Dispersal and oviposition of SLF adults were monitored from both study and neighboring patches during the study.
In Tan stream, four study patches (patches A–D) and one neighboring patch, which were distributed over ca. 1760 m, were selected (Fig. 3a). Areas around the patches were generally covered with grass and shrubs, and the areas were occasionally managed by local administration. Deciduous trees were regularly planted along the pedestrian lanes. There were a total of four, four, 61, and 47 A. altissima trees in patches A to D, respectively (Table 2). Compared with Tan stream, A. altissima patches were located closely to each other in Gyeongan stream: three study patches (patches E–G) and three neighboring patches were spread over only ca. 90 m (Fig. 3b). Vegetation surrounding A. altissima patches consisted of grasses and small shrubs as well as deciduous trees planted along the border of residential area nearby. There were a total of 69, nine, and 53 A. altissima trees in patches E to G, respectively (Table 2). Unlike Tan stream, 45% of A. altissima trees had trunks having cut off by local administration in Gyeongan stream (Table 2; Fig. 5).
Dispersal pattern of SLF on A. altissima
Three fluorescent paint colors were used to mark SLF individuals in the study patches (Fig. 3; SI 2). Insects that took off during marking were captured and excluded from the experiment. Among the selected study patches, SLF adults were generally distributed throughout each patch, while SLF adults were observed only from one out of 61 A. altissima trees in patch C. As a result, in Tan stream, 15 (color of paint used to fluorescent-marking; red), 31 (yellow), 11 (blue), and 32 (red) adults were marked from patches A to D, respectively, whereas in Gyeongan stream, 30 (red), 30 (blue), and 33 (yellow) adults were marked from patches E to G, respectively. Starting from September 14th, 2020 in Tan stream and September 18th in Gyeongan stream, fluorescent-marked SLF adults on A. altissima trees in both study and neighboring patches were counted with a UV laser twice a week (Fig. 3). Survey continued until no individuals were observed from the study patches.
Oviposition pattern of SLF on A. altissima
Oviposition pattern of SLF was surveyed on all A. altissima trees in the study patches in December in both streams (Table 2). For the survey, SLF egg masses were categorized into three types as follows: egg mass with waxy layer, egg mass without waxy layer, and scattered eggs (SI 3). Eggs that were not covered with waxy layer and did not form aggregates were categorized as scattered (SI 3). In the field, A. altissima trees were visually inspected to identify SLF egg mass, and the number of egg masses and their distances from the ground were recorded. In addition, the number of eggs per egg mass was recorded for egg masses located < 2.5 m above the ground. When egg masses were covered with waxy layer, brushes were used to gently remove the powder-like waxy layer.
To characterize the A. altissima trees surveyed for SLF egg mass, we recorded the following morphological characteristics: tree height, DRC, and cutting status. Heights of A. altissima trees < 2 m were measured using an iron steel measuring tape, and those > 2 m were estimated with reference to the investigator’s height. DRC of A. altissima was determined by measuring the circumference of the stem at the root collar using a tape measure. In case of multi-stemmed A. altissima trees, the diameter of each stem was first determined individually, and the DRC of the tree was calculated using the following equation:
where n is the number of stems and di denotes the stem diameter of the A. altissima tree.
The effects of A. altissima height, DRC, their interactions, and cutting status of the trees on the number of egg masses were analyzed using a generalized linear mixed model (GLMM) assuming a Poisson distribution with a log link function (JMP 12, SAS Institute Inc., USA). Patch was included as random effect in the model. Due to concerns over collinearity between tree height and DRC, variance inflation factor (VIF) was calculated: VIF > 5 generally indicates collinearity35,36. VIF between height and DRC was 1.56, and therefore the two variables were included together in the GLMM model.
Policy statement
Experiments involving Ailanthus altissima were conducted in compliance with relevant institutional, national, and international guidelines and legislation.
References
Jung, J. M., Jung, S., Byeon, D. H. & Lee, W. H. Model-based prediction of potential distribution of the invasive insect pest, spotted lanternfly Lycorma delicatula (Hemiptera: Fulgoridae), by using CLIMEX. J. Asia. Pac. Biodivers. 10, 532–538 (2017).
Liu, G. Some extracts from the history of entomology in China. Psyche 46, 23–28 (1939).
Barringer, L. E., Donovall, L. R., Spichiger, S. E., Lynch, D. & Henry, D. The first new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 125, 20–23 (2015).
Dara, S. K., Barringer, L. E. & Arthurs, S. P. Lycorma delicatula (Hemiptera: Fulgoridae): A new invasive pest in the United States. J. Integr. Pest. Manag. 6, 20 (2015).
Hong, K. J., Lee, J. H., Lee, G. S. & Lee, S. The status quo of invasive alien insect species in plant quarantine in Korea. J. Asia Pac. Entomol. 15, 521–532 (2012).
Wakie, T. T., Neven, L. G., Yee, W. L. & Lu, Z. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 113, 306–314 (2020).
Han, J. M. et al. Lycorma delicatula (Hemiptera: Auchenorrhyncha: Fulgoridae: Aphaeninae) finally, but suddenly arrived in Korea. Entomol. Res. 38, 281–286 (2008).
Kim, S. S. & Kim, T. W. Lycorma delicatula (White) (Hemiptera: Fulgoridae) in Korea. Lucanus 5, 9–10 (2005).
Lee, K. Y., Kim, S. K., Kim, I. H. & Kim, K. S. Seasonal occurrence of spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae) and its control efficacy using EFAM at the vineyards. Korean J. Pestic. Sci. 15, 303–309 (2011).
Lee, D.-H., Park, Y.-L. & Leskey, T. C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia Pac. Entomol. 22, 589–596 (2019).
Eshenaur, B. Spotted Lanternfly range in the U. S. https://nysipm.cornell.edu/environment/invasive-species-exotic-pests/spotted-lanternfly/spotted-lanternfly-range-us/ (2022).
Barringer, L. E. & Ciafré, C. M. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 49, 999–1011 (2020).
Kim, J. G., Lee, E. H., Seo, Y. M. & Kim, N. Y. Cyclic behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on host plants. J. Insect. Behav. 24, 423–435 (2011).
Lee, Y. S., Jang, M. J., Kim, J. Y. & Kim, J. R. The effect of winter temperature on the survival of lantern fly, Lycorma delicatula (Hemiptera: Fulgoridae) eggs. Korean J. Appl. Entomol. 53, 311–315 (2014).
Park, J. D. et al. Biological characteristics of Lycorma delicatula and the control effects of some insecticides. Korean. J. Appl. Entomol. 48, 53–57 (2009).
Lee, J. E. et al. Feeding behavior of Lycorma delicatula (Hemiptera: Fulgoridae) and response on feeding stimulants of some plants. Korean. J. Appl. Entomol. 48, 467–477 (2009).
Sladonja, B., Sušek, M. & Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 56, 1009–1034 (2015).
Liu, H. Oviposition substrate selection, egg mass characteristics, host preference, and life history of the spotted lanternfly (Hemiptera: Fulgoridae) in North America. Environ. Entomol. 48, 1452–1468 (2019).
Ohmoto, T., Koike, K. & Sakamoto, Y. Studies on the constituents of Ailanthus altissima Swingle II. Alkaloidal constituents. Chem. Pharm. Bull. 29, 390–395 (1981).
Liu, H. Seasonal development, cumulative growing degree-days, and population density of spotted lanternfly (Hemiptera: Fulgoridae) on selected hosts and substrates. Environ. Entomol. 49, 1171–1184 (2020).
Kho, J. W., Jung, M. & Lee, D.-H. Evaluating the efficacy of two insect detection methods with Riptortus pedestris (Hemiptera: Alydidae): Portable harmonic radar system and fluorescent marking system. Pest. Manag. Sci. 75, 224–233 (2019).
Kim, J. Y., Kho, J. W., Jung, M. & Lee, D.-H. Assessment of potential effects and detection efficacy of a fluorescent marking system on a medically important hard tick, Haemaphysalis longicornis (Acari: Ixodidae). Pest. Manag. Sci. 75, 2735–2743 (2019).
Nixon, L. J., Ludwick, D. C. & Leskey, T. C. Horizontal and vertical dispersal capacity and effects of fluorescent marking on Lycorma delicatula nymphs and adults. Entomol. Exp. Appl. 169, 219–226 (2021).
Keller, J. A. et al. Dispersal of Lycorma delicatula (Hemiptera: Fulgoridae) nymphs through contiguous, deciduous forest. Environ. Entomol. 49, 1012–1018 (2020).
Rice, K. B. et al. Handheld lasers allow efficient detection of fluorescent marked organisms in the field. PLoS ONE 10, e0129175 (2015).
Longland, W. S. & Clements, C. Use of fluorescent pigments in studies of seed caching by rodents. J. Mammal. 76, 1260–1266 (1995).
Silva, P. G. D. & Hernández, M. I. M. Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS ONE 10, e0126112 (2015).
Kirkpatrick, D. M. et al. Estimating monitoring trap plume reach and trapping area for nymphal and adult Halyomorpha halys (Hemiptera: Pentatomidae) in crop and non-crop habitats. Environ. Entomol. 48, 1104–1112 (2019).
Baker, T. C. et al. Progression of seasonal activities of adults of the spotted lanternfly, Lycorma delicatula, during the 2017 season of mass flight dispersal behavior in eastern Pennsylvania. J. Asia Pac. Entomol. 22, 705–713 (2019).
Wolfin, M. S., Myrick, A. J. & Baker, T. C. Flight duration capabilities of dispersing adult spotted lanternflies, Lycorma delicatula. J. Insect. Behav. 33, 125–137 (2020).
Rural Development Administration. Press. https://www.korea.kr/news/pressReleaseView.do?newsId=155455345 (2010).
Keller, J. A. et al. Dispersion patterns and sample size estimates for egg masses of spotted lanternfly (Hemiptera: Fulgoridae). Environ. Entomol. 49, 1462–1472 (2020).
Kang, C. K., Lee, S. I. & Jablonski, P. G. Effect of sex and bright coloration on survival and predator-induced wing damage in an aposematic lantern fly with startle display. Ecol. Entomol. 36, 709–716 (2011).
Kang, C. K., Moon, H., Sherratt, T. N., Lee, S. I. & Jablonski, P. G. Multiple lines of anti-predator defence in the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae). Biol. J. Linn. Soc. 120, 115–124 (2017).
O’brien, R. M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 41, 673–690 (2007).
Thompson, D. J., Hassall, C., Lowe, C. D. & Watts, P. C. Field estimates of reproductive success in a model insect: behavioural surrogates are poor predictors of fitness. Ecol. Lett. 14, 905–913 (2011).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT). (No. 2021R1A2C1010679).
Author information
Authors and Affiliations
Contributions
D-H. L., M. J., Y.S. L and D-H. G. conceptualized and designed the study; D-H. G, M. J. and J-W. K. performed the field experiments; M. J. and J-W. K. performed statistical analysis; M. J. and J-W. K. wrote the manuscript; D-H. L. and Y.S. L. critically reviewed and amended the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jung, M., Kho, JW., Gook, DH. et al. Dispersal and oviposition patterns of Lycorma delicatula (Hemiptera: Fulgoridae) during the oviposition period in Ailanthus altissima (Simaroubaceae). Sci Rep 12, 9972 (2022). https://doi.org/10.1038/s41598-022-14264-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14264-0
- Springer Nature Limited