Abstract
New representatives of the Cretaceous cranefly genus Antodicranomyia (Diptera: Limoniidae) are reported from Albian-Cenomanian Charentese (French) amber. The newly reported specimens allow for an emended diagnosis of the type species A. azari, as well as the description of a new species, Antodicranomyia rubra sp. nov., which is mostly distinguished from the type species by features of its wing venation, antennae, and genitalia. As a rare, extinct genus known only from French amber, Antodicranomyia is compared with its closest relative genera Antocha, Dicranomyia and Antohelia. The evolutionary implications and paleohabitat of Antodicranomyia are discussed. The new discovery adds to the knowledge of the crane flies’ diversity and evolution in the mid-Cretaceous.
Similar content being viewed by others
Introduction
Limoniidae, or craneflies, are an old lineage of Diptera dating back to the Late Triassic, and currently known by ca. 11.000 extant species1,2,3,4. The family is frequently encountered in both modern and fossil entomofauna4,5. The oldest Limoniidae—Architipula youngi Krzemiński, 19921 (representative of Architipulinae, considered as the oldest group of Limoniidae6), is known from Late Triassic of North America1. The family is abundantly documented as soon as in the Early Jurassic (Toarcian) of Europe 7,8,9,10 and Asia11,12. In the Cretaceous, the Limoniidae comprise lineages known since the Triassic and Jurassic periods, as well as the earliest representatives of genera that are still extant today, such as Helius Lepeletier et Serville13, Dicranoptycha Osten Sacken14 or Trichoneura Loew15,16,17,18,19,20,21,22; but also some genera that are documented exclusively from the Cretaceous period, such as Antodicranomyia Perrichot, Nel and Krzemiński23. This genus was hitherto known only by its type species, Antodicranomyia azari Perrichot, Nel and Krzemiński23, which was described based on two males in Albian-Cenomanian amber from the Archingeay deposit in Charentes, south-western France23. Two new specimens of Antodicranomyia are reported herein: a further male of the type species, also from the Archingeay deposit; and a male assignable to a new species from another, slightly younger Charentese amber deposit (Fig. 1). It is the second evidence of the extinct genus Antodicranomyia, still apparently endemic to the mid-Cretaceous Charentese (French) amber.
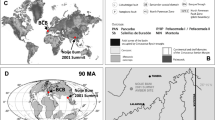
Geographical and geological setting of the Cretaceous Charentese amber deposits (modified from Perrichot et al.27). (A) simplified geological map with indication of amber localities; (B) synthetic regional stratigraphic section with indication of amber outcrops. Red dots and stars denote the outcrops yielding Antodicranomyia species considered in the present study.
Results
Systematic palaeontology
Order Diptera Linnaeus24.
Infraorder Tipulomorpha Rohdendorf12.
Family Limoniidae Speiser25.
Subfamily Limoniinae Speiser25.
Genus Antodicranomyia Perrichot, Nel and Krzemiński23.
Type species: Antodicranomyia azari Perrichot, Nel and Krzemiński23: 76, Figs. 1 and 2.
Antodicranomyia azari Perrichot, Nel and Krzemiński23. (Fig. 2).
Emended diagnosis. Wing 3 × as long as wide; tip of Sc distad sc-r 3 × as long as sc-r; r-m nearly oblique to the basal deflection of M1+2; tip of R3+4 straight to weakly upcurved; basal part of R5 slightly arched, twice to three times as long as crossvein r-m; crossvein m-cu aligned with basalmost point of d-cell; vein M3 as long as or slightly longer than d-cell; distance between tips of R5 and R3+4 approximately 2 × to 2.5 × distance between M1 and R5; distance between tips of R1 and R3+4 approximately 1.3 × to 2 × the distance between R3+4 and R5; male genitalia with lobe (branch I = ventral gonostylus) and clasper (branch II = dorsal gonostylus) of gonostyli approximately 2 × as long as wide, shorter than half of gonocoxite, narrowed, slightly broadened distally, bearing apical setae; lobe longer than clasper, armed with one small apical spine, bearing long setae distally on the inner surface and apically.
Remarks. The following features allowed specimen no. MNHN.F.A30189 (formerly ARC 182.6) to be designated as a paratype of A. azari: vein sc-r is one third the tip of Sc, r-m is nearly oblique to the basal deflection of M1+2; crossvein m-cu is aligned with fork of Mb.
Type material. Holotype MNHN.F.A30188 (formerly ARC 186.2), male, paratype MNHN.F.A30189 (formerly ARC 182.6), partial specimen preserved by head and one wing; both in amber from Font-de-Benon quarry (Arc 1 in Fig. 1B), between Archingeay and Les Nouillers, Charente-Maritime department, France. Early-Late Cretaceous, latest Albian-earliest Cenomanian, lithological subunit A1, level A1sl2 sensu Néraudeau et al.26, A1sl-A sensu Perrichot et al.27.
New material examined. Specimen IGR.ARC-259, wing of male.
Type locality & Type horizon. Amber from the same deposit and stratum as the type material.
Additional description. Wing (Fig. 2A, B) with d-cell 0.55 mm long; M3 0.58 mm long.
Remark. The specimen displays a poorly preserved base of right wing and head, and the left wing is the only well visible part.
Antodicranomyia rubra sp. nov. (Figs. 3, 4 and 5).
Antodicranomyia rubra sp. nov. No. IGR.CDL-23.1 (male), holotype. (A) antenna; (B) part of hind leg; (C) genitalia; (D) wing. Abbreviations: fm–femur; tb–tibia; scp–scape; ped–pedicel; oug–outer branch (clasper) of gonostylus; ing–inner branch (lobe) of gonostylus; gx–gonocoxite; C–costal vein; Sc–subcostal vein; h–humeral vein; sc-r, r-r–cross-veins; Rs, R1, R2, R3+4, R5radial veins; Mb, M1+2, M3, M4–medial veins; Cu–cubital vein; A1, A2–anal veins; d–d-cell.
Antodicranomyia rubra sp. nov. No. IGR.CDL-23.1 (male), holotype. (A) wing; (B) enlarged view of distal half of wing; (C) genitalia. Abbreviations as given in Fig. 3.
Zoobank ID. urn:lsid:zoobank.org:pub:B52B8DC1-002E-4996-A172-81054794B98F (for this publication), urn:lsid:zoobank.org:act:A5469291-0EDF-4EF3-8A85-01125E5C4628 (for the new species).
Diagnosis. Wing 3.5 × as long as wide; tip of Sc distad sc-r subequal to sc-r in length; r-m nearly aligned to the basal deflection of M1+2; tip of R3+4 upcurved; basal part of R5 curved, 3 × as long as crossvein r-m; crossvein m-cu before fork of Mb; vein M3 longer than d-cell; distance between tips of R5 and R3+4 approximately twice the distance between M1 and R5; distance between tips of R1 and R3+4 approximately 1.5 × the distance between R3+4 and R5; male genitalia with lobe (branch I = ventral gonostylus) and clasper (branch II = dorsal gonostylus) of gonostyli elongate, approximately 7 × as long as wide, narrowed, longer than half the length of gonocoxite; gonocoxite 3 × as long as wide; clasper strongly sclerotized, slightly broadened distally, bearing one thick apical seta-like spine; lobe longer than clasper, armed with two apical setae, pale, not very wide.
Remark. Such features like the rostrum distinctly shorter than head, the male antenna with 13 flagellomeres, the wing with Sc ending beyond the origin of Rs and veins r-r and r-m nearly aligned, the male genitalia with narrow lobe and clasper of gonostyli, additionally with the clasper strongly sclerotized, warrant placement of the new species in the genus Antodicranomyia as defined by Perrichot et al.23.
Etymology. The specific name is from the Latin rubra meaning red, and refers to ‘La Montée Rouge’ (the red slope), name of the access road to the type locality in Cadeuil.
Type material. Holotype IGR.CDL-23.1, male.
Type locality & Type horizon. Amber from Cadeuil quarry along ‘la Montée Rouge’(Cdl 2 in Fig. 1B) at Sainte-Gemme, Charente-Maritime department, France. Late Cretaceous, Early Cenomanian, lithological subunit A2, level A2a sensu Perrichot et al.27.
Description (male). Body (Fig. 4A) pale brown, 4.30 mm long, with dark brown head.
Head (Fig. 4A, B) 0.42 mm long, with moderately large, oval, almost holoptic, glabrous eyes; many strong, thick and comparatively short setae on occiput and vertex; rostrum distinctly shorter than head; antenna (Figs. 3A, 4A, B, E) 1.10 mm long, 15-segmented, shorter than head and thorax combined; scape elongate, cylindrical, massive, narrower than other segments of antenna; pedicel short, shorter and wider than scape, approximately as long as wide, widened in midlength; flagellomeres rather short and wide, becoming progressively slender toward antennal tip; flagellomeres 1–7 approximately as long as wide, 8–15 approximately twice as long as wide; last flagellomere longer than penultimate one. Antenna with few moderately elongate setae, approximately as long as their respective bearing-segments; palpus (Fig. 4B) four-segmented, rather elongate, palpomeres cylindrical, last palpomere short.
Thorax (Fig. 4A, B): wing (Fig. 3D) 4.13 mm long, 1.10 mm wide; vein Sc surpassing midlength of wing and midlength of vein Rs; vein R1 rather short, ending opposite approximately 0.3 × length of vein R3+4; d-cell 0.22 mm long, twice as long as wide; crossvein r-m rather short, as long as a one third of basal deflection of R5; R1 1.5 × as long as r-r (R2); crossvein m-cu before fork of vein Mb on M1+2 and M3+4; crossvein m-m half the length of basal deflection of M3; M3 0.50 mm long; A1 and A2 elongate, almost straight.
Legs (Fig. 4A, C, D) with strong, numerous short setae.
Abdomen (Figs. 3C, 4A, 5C): genitalia (Fig. 5C) 0.66 mm long; gonocoxite 0.43 mm long; lobe 0.22 mm long, clasper 0.20 mm long.
Remark. The specimen is well preserved, although missing apical portions of some legs.
Comparison. The new species differs from A. azari mainly by the male genitalia with more elongate, narrowed clasper and lobe. In A. rubra sp. nov., the clasper is approximately 7 × as long as wide and is longer than half the length of gonocoxite, while in A. azari the clasper is at most 3 × as long as wide, shorter than half the length of gonocoxite. Both species also differ by the wing venation: vein sc-r is subequal to the tip of Sc in A. rubra sp. nov., one third the tip of Sc in A. azari; r-m is nearly aligned to the basal deflection of M1+2 in A. rubra sp. nov., but nearly oblique to the basal deflection of M1+2 in A. azari; the tip of R3+4 and basal part of R5 are curved, with basal part of R5 3 × as long as r-m in A. rubra sp. nov., vs. tip of R3+4 straight or at most weakly upcurved and basal part of R5 just slightly arched and twice to three times as long as r-m in A. azari. A feature of the wing vein which makes it easy to distinguish between both species is the position of crossvein m-cu: in A. rubra sp. nov. this vein is anteriad fork of Mb, while it is aligned with fork of Mb in A. azari. Moreover, M3 is distinctly longer than d-cell in the new species, but as long as or only slightly longer than d-cell in A. azari.
Discussion
As reflected by Perrichot et al.23 in their selection of the genus name, Antodicranomyia shares features with the extant genera Antocha Osten Sacken14 and Dicranomyia Stephens28. Antocha currently comprises 161 extant species within three subgenera Antocha, Orimargula Mik29, and Proantocha Alexander4,30, while only two extinct species left unclassified in subgenus are known31,32. Dicranomyia is represented by over 1150 extant species within 25 subgenera (Table 1) including Alexandriaria Garrett33, Caenoglochina Alexander34, or Caenolimonia Alexander35, and the genus has a rather extensive fossil record of almost 30 species. The earliest representatives of Antocha date back to the Cenomanian Burmese amber31, while Dicranomyia first occurs in the Paleocene36. Subgenera such as Dicranomyia, Melanolimonia, or Sivalimnobia are known from the earliest Eocene Mo Clay Formation of Denmark or from Eocene Baltic amber36,37,38.
As suggested by Perrichot et al.23, Antodicranomyia and Antocha are very similar in their male gonostyli, but Antodicranomyia differs by its long, sclerotized aedeagus and (from almost all subgenera of Antocha) by its narrow lobe of gonostylus. The wide anal area on the wing of Antodicranomyia distinguishes this genus from Caenoglochina. Some species of this subgenus similarly have narrow lobes of gonostyli, however these are broader and apically truncate, in most of Dicranomyia lobe (outer gonostylus) of gonostyli is ballon-like with rostral prolongation, clasper (inner gonostylus) is strongly sclerotized, hooked39. The male genitalia with two elongate gonostyli are characteristic for Antochini, but Antodicranomyia differs from this tribe by its wing venation which is characteristic of the Limoniini. The wing of Antocha is characteristicaly wide with almost square-shaped anal lobe, in Antodicranomyia anal lobe is well developed, but not so expressive as in Antocha40. The 15-segmented antennae of Antodicranomyia are intermediate between the 14-segmented ones of Limoniini and the 16-segmented ones of Antochini and could suggest a tendency to the reduction of antennal segments within this tribes23.
Based on the reconstructed paleohabitat of the Archingeay and Cadeuil deposits26,27,40,41,42,43 Antodicranomyia apparently lived in coastal (estuarine) to back-swamp, warm and moist forests dominated by a variety of conifers (Cheirolepidiaceae, Cupressaceae (taxodioids), Araucariaceae and Podocarpaceae trees) and ferns (mainly Gleicheniaceae, Schizaeaceae), with few Ginkgoales, Bennettitales, herbaceous angiosperms, and more or less influenced by marine conditions.
The history of Antodicranomyia somewhat parallels that of Antohelia Kania39. Antodicranomyia shares some features with Antocha and Dicranomyia, two extant genera with a long temporal range (since the mid-Cretaceous for Antocha, the Paleocene for Dicranomyia), while Antodicranomyia is restricted to the mid-Cretaceous French amber (Fig. 6).
Chronostratigraphic distribution of Antocha, Antodicranomyia, Antohelia, Dicranomyia, Helius fossil species with chosen fossil sites localization. Stratigraphic chart according to International Stratigraphic Chart, International Commission of Stratigraphy (v. 2021/05) https://stratigraphy.org/chart (accessed on 16 September 2021).
Antohelia similarly shares some features with Antocha and Helius, two genera that are known since the Cretaceous16,17,19,20,21,44,45 and are still living today, while Antohelia is apparently restricted to the Eocene Baltic amber18. Such extinct lineages as Antodicranomyia and Antohelia uniquely enlighten our knowledge on the past diversity, paleohabitat, and evolutionary history of craneflies. Especially, when it is possible to indicate such features as differentiation of number of antennomeres within Antodicranomyia (15), in relation to 14-segmented antennae of Limoniini and the 16-segmented ones of Antochini, thus showing the tendency to the reduction of antennal segments23.
Material and methods
The Charente-Maritime and Charente departments of south-western France comprise several Early-Late Cretaceous deposits of fossil resin known under the collective name Charentese amber (Fig. 1A). The present study is based on inclusions in amber from the Archingeay 1 (Arc 1) and Cadeuil 2 (Cdl 2) deposits, as defined by Perrichot et al. (2010: figs. 1, 2)27. Now filled up with water forming lakes, both sites are former quarries that each exposed two lignitic, amber-bearing layers within sand and clay beds (Fig. 1B). The geological setting of the deposits have been detailed elsewhere26,27,41. The amber bed considered herein for Arc 1 was assigned to the ‘lithological subunit A1’ and dated as latest Albian or earliest Cenomanian based on palynomorph evidence40,42,43. Cdl 2 was assigned to the lithological subunit A2 which was similarly dated as Early Cenomanian40. A new male specimen of Antodicranomyia azari is reported in amber from the Archingeay deposit (Arc 1), which already yielded the type material of this species described in 200723. And the male of a new species is reported from the Cadeuil deposit (Cdl 2). All specimens newly reported herein are deposited in the Geological Department and Museum of the University of Rennes (IGR), France.
The specimens were examined using a Nikon SMZ 1500 stereomicroscope equipped with a Nikon DS-Fi1 camera. The measurements were taken with NIS-Elements D 3.0 software. The length of head was measured as the length of the head capsule. Measurements of the discal cell were taken from the proximal to distal ends of the d-cell; measurements of the vein M3 were taken from the point of connection of vein m-m with vein M3 to the margin of wing; the length of genitalia was measured from the posterior margin of tergite IX to the apex of gonocoxite. Drawings and photographs were made by Iwona Kania-Kłosok, with line drawings traced from the photographs. Terminology follows Krzemiński1 for the wing venation nomenclature, and Ribeiro46 or Perrichot et al.23 for the male genitalic nomenclature.
Data availability
Requests for materials should be addressed to V.P.
References
Krzemiński, W. Triassic and Lower Jurassic stage of Diptera evolution. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 65, 39-59 (1992).
Shcherbakov, D. E., Lukashevich, E. D. & Blagoderov, V. A. Triassic Diptera and initial radiation of the order. Int. J. Dipterol. Res. 6, 75–115 (1995).
Krzemiński, W. & Krzemińska, E. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta Zool. Crac. 46, 153–184 (2003).
Oosterbroek, P. CCW: catalogue of the crane-flies of the World (Diptera, Tipuloidea: Pediciidae, Limoniidae, Cylindrotomidae, Tipulidae) (version May 2021). https://ccw.naturalis.nl/stats.phphttp://nlbif.eti.uva.nl/ccw/index.php. Last update 15 March 2022 (2021).
Evenhuis, N. L. Catalogue of Fossil Flies of the World (Insecta: Diptera) 600 (Backhuys Publishers, Leiden, 1994).
Kopeć, K., Soszyńska-Maj, A., Kania-Kłosok, I., Coram, R. A. & Krzemiński, W. Morphology of the oldest fossil subfamily of Limoniidae (Diptera, Architipulinae) in the light of exceptionally preserved Mesozoic material. Sci. Rep. 11, 24137 (2021).
Handlirsch, A. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch für Paläontologen und Zoologen. Wilhelm von Engelmann, Leipzig pp. 1430 (1906-1908).
Handlirsch, A. Neue Untersuchungen über die fossilen Insekten mit Ergänzungen und Nachträgen sowie Ausblicken auf phylogenetische, palaeogeographische und allgemein biologische Probleme. II. Teil. Annalen des Naturhistorischen Museums in Wien 48, 1–240 (1937).
Tillyard, R. J. The panorpoid complex in the British Rhaetic and Lias. Fossil Insects 3, 1–78 (1933).
Krzemiński, W., Zessin, W. The Lower Jurassic Limoniidae from Grimmen (GDR). Deutsche Entomologische Zeitschrift 37, 39-43 (1990).
Kalugina N.S. Infraorder Tipulomorpha. in Insecta Diptera of the Jurassic in Siberia, (eds Kalugina N.S. and Kovalev V.G.) Akademiya nauk SSSR, Paleontologicheskij Institut, Moskovskoe Obshchestvo Ispytatelej Prirody pp. 47–62 (1985). [in Russian]
Rohdendorf, B.B. [Historical development of dipterous insects]. Trudy Paleontologicheskogo Instituta AN SSSR, 100, 1–311 (1964). [In Russian, English translation in Rohdendorf, 1974]
Lepeletier, A.L.M. & Serville, J.G.A. Entomologie, ou histoire naturelle des crustacés, des arachnides et des insectes. Encyclopedie Methodique, Histoire Naturelle 10, 345-833 (1828).
Osten Sacken, C. R. New genera and species of North American Tipulidae with short palpi, with an attempt at a new classification of the tribe. Proc. Acad. Nat. Sci. Phila. 1859, 197–254 (1860).
Loew, H. Uber den Bernstein und die Bernsteinfauna. Programm Koniglichen Realschule zu Meseritz 1850, 3–44 (1850).
Krzemiński, W., Kania, I. & Azar, D. The early Cretaceous evidence of rapid evolution of the genus Helius Lepeletier & Serville 1828 (Limoniidae, Diptera). Cretac. Res. 48, 96–101 (2014).
Kania, I., Krzemiński, W. & Azar, D. The oldest representative of Helius Lepeletier & Serville 1828 (Diptera: Limoniidae) from Lebanese amber (Early Cretaceous). Insect Syst. Evol. 44, 231–238 (2013).
Kania, I., Wang, B. & Szwedo, J. Dicranoptycha Osten Sacken, 1860 (Diptera, Limoniidae) from the earliest Cenomanian Burmese amber. Cretac. Res. 52, 522–530 (2015).
Kania, I., Krzemiński, W. & Arillo, A. First representative of the genus Helius Lepeletier and Serville, 1828 (Diptera, Limoniidae) from the Lower Cretaceous Álava amber (Spain). Cretac. Res. 63, 33–38 (2016).
Kania, I., Krzemiński, W. & Arillo, A. A new peculiar species of the genus Helius Lepeletier & Serville, 1828 (Diptera, Limoniidae) from Cretaceous Álava amber (Spain). Earth Environ. Sci. Trans. R. Soc. Edinb. 107, 1–7 (2017).
Kania-Kłosok, I., Krzemiński, W. & Arillo, A. Two new long-rostrum cranefly species from the Cretaceous Iberian amber (Diptera, Limoniidae, Helius). Sci. Rep. 11, 12851 (2021).
Kania-Kłosok, I., Krzemiński, W., Kopeć, K. & Arillo, A. The oldest evolutionary lineage of Trichoneura Loew, 1850 (Diptera, Limoniidae) and the first evidence of this genus in Cretaceous Spanish amber. Insects 12, 411 (2021).
Perrichot, V., Nel, A. & Krzemiński, W. A new crane fly (Diptera: Limoniidae) from the Cretaceous amber of France. Alavesia 1, 75–80 (2007).
Linnaeus, C. Systema nature per regna tria naturae, secundum classes, ordines, genera, species, cum caracteribus, differentiis, synonymi, locis. Tomus I. Editio decima, reformata. L. Salvii, Holmiae [= Stockholm] pp. 824 (1758).
Speiser, P. 4 Orthoptera. Orthoptera Nematocera. Wissenschaftliche Ergebnisse der Schwedischen Zoologische Expededition nach Kilimandjaro, Meru Diptera 10, 31–65 (1909).
Néraudeau, D. et al. A new amber deposit from the Cretaceous (Uppermost Albian-Lowermost Cenomanian) of southwestern France. Cretac. Res. 29, 925–929 (2008).
Perrichot, V., Néraudeau, D. & Tafforeau, P. Charentese Amber. In Biodiversity of fossils in amber from the major world deposits (ed. Penney, D.) 192–207 (Siri Scientific Press, 2010).
Stephens, J.F. A systematic catalogue of British insects, being an attempt to arrange all the hitherto discovered indigenous insects in accordance with their natural affinities. Containing also the references to every English writer on entomology, and on the principal foreign authors. With all the published British genera to the present time. 2 (Insecta Haustellata), London, pp. 388 (1829).
Mik, J. Zur Kenntnis der Limnobia anomala O. S. Wiener Entomologische Zeitung 2, 198–202 (1883).
Alexander, C. P. Undescribed species of Japanese crane-flies (Tipulidae, Diptera). Ann. Entomol. Soc. Am. 12, 327–348 (1919).
Podenas, S. & Poinar, G. O. New crane flies (Diptera:Limoniidae) from Burmese amber. Proc. Entomol. Soc. Wash. 111(2), 470–492 (2009).
Scudder, S. H. Tertiary Tipulidae, with special reference to those of Florissant, Colorado. Proc. Am. Philos. Soc. 32, 163–245 (1894).
Garrett, C. B. D. New Tipulidae from British Columbia (Diptera). Proc. Entomol. Soc. Wash. 24, 58–64 (1922).
Alexander, C. P. The crane-flies of Jamaica (Diptera, Tipulidae). Bull. Inst. Jam. Sci. Ser. 14, 1–86 (1964).
Alexander, C. P. Notes on the tropical American species of Tipulidae (Diptera). VII. The tribe Limoniini, genus Limonia, concluded; Helius, Orimarga, and others; tribe Pediciini; subfamily Cylindrotominae. Stud. Entomol. (N.S.) 10, 277–352 (1967).
Krzemiński, W. New fossil Tipuloidea (Diptera) from the Fur formation of Denmark in the collection of the Natural History Museum in London. Pol. J. Entomol. 70, 333–339 (2001).
Henriksen, K. L. Eocene insects from Denmark. Commun. Paleontol. Mus. Min. Geol. Univ. Copenhague 18, 1–36 (1922).
Krzemiński, W., Kania, I. & Durak, R. A new species of Dicranomyia Stephens, 1829 (Diptera: Limoniidae) from Baltic amber (Eocene). Neues Jahrbuch für Geologie und Palaöntologie Abhandlungen 277/2, 167–74 (2015).
Kania, I. The taxonomy of selected genera of the subfamily Limoniinae (Diptera: Limoniidae) from Baltic amber (Eocene), with notes on their phylogeny. Ann. Zool. 65(1), 71–100 (2015).
Peyrot, D., Barrón, E., Polette, F. & Néraudeau, D. Early Cenomanian palynofloras and inferred resiniferous forests and vegetation types in Charentes (southwestern France). Cretac. Res. 94, 168–189 (2019).
Néraudeau, D. et al. Un nouveau gisement à ambre insectifère et à végétaux (Albien terminal probable): archingeay (Charente-Maritime, France). Geobios 35(2), 233–240 (2002).
Dejax, J. & Masure, E. Analyse palynologique de l’argile lignitifère à ambre de l’Albien terminal d’Archingeay (Charente-Maritime, France). Comptes Rendus Palevol. 4, 53–65 (2005).
Peyrot, D., Jolly, D. & Barrón, E. Apport de données palynologiques à la reconstruction paléoenvironnementale de l’Albo-Cénomanien des Charentes (Sud-Ouest de la France). Comptes Rendus Palevol 4, 151–165 (2005).
Rayner, R. J. & Waters, S. B. A Cretaceous crane-fly (Diptera: Tipulidae): 93 million years of stasis. Zool. J. Linn. Soc. 99, 309–318 (1990).
Ribeiro, G. C. A new fossil Helius (Diptera, Limoniidae) from Burmese amber. Stud. Dipterol. 9(2), 403–408 (2002).
Ribeiro, G. C. Homology of the gonostylus parts in crane flies, with emphasis on the families Tipulidae and Limoniidae (Diptera, Tipulomorpha). Zootaxa 1110, 47–57 (2006).
Theischinger, G. The Limoniinae (Diptera: Tipulidae) of Australia V. The genera Helius Le Peletier and Serville, Toxorhina Loew, Limonia Meigen (part), Tonnoiromyia Alexander and Collessophila gen. nov. (all tribe Limoniini) and Atarba Osten-Sacken, Amphineurus Skuse, Erioptera Meigen, Cheilotrichia Rossi, Gonomyia Meigen and Idiocera Dale (all tribe Eriopterini). Stapfia 36, 37–276 (1994).
Alexander, C. P. New species of crane-flies from North Queensland (Tipulidae, Diptera). Can. Entomol. 53, 205–211 (1921).
Savchenko, E.N. & Krivolutskaya, G.O. Limoniidae of the south Kuril Islands and south Sakhalin. Akad. Nauk. Ukr. SSR, Kiev pp. 1–160 (1976).
Meigen, J.W. Systematische Beschreibung der bekannten europaischen zweiflugeligen Insekten. Schulz, Hamm 6, i-xiv, 1–401 (1830).
Alexander, C. P. New subgenera and species of crane-flies from California (Ptychopteridae and Tipulidae: Diptera). Trans. Am. Entomol. Soc. 92, 103–132 (1966).
Savchenko, E. N. Idiopyga Savtshenko nom. Nov. (Diptera, Limoniidae). Vestn. Zool. 6, 81 (1987) ([in Russian]).
Alexander, C. P. New species of craneflies from tropical America (Diptera: Tipulidae) I. J. Kansas Entomol. Soc. 38, 401–407 (1965).
Alexander, C. P. The crane-flies of Jamaica. Entomol. Bull. 4(3), 19–29 (1928).
Bigot, J.M.F. Essai d une classification generale et synoptique de l ordre des insectes dipteres (3e memoire). Tribu de Tipulidii (mihi). Annales de la Societe Entomologique de France (3)2, 447–482 (1854).
Alexander, C.P. New or little-known Tipulidae from Venezuela (Diptera). Part V. Boletin Entomologia Venezolana 3, 171–192 (1944).
Schiner, J.R. Diptera. Reise der osterreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859, unter den Befehlen des Commodore B. von Wullerstorf-Urbair. Zoologischer Theil, 2(1) (B). Wien i-vi, 1–388 (1868).
Alexander, C. P. New or little-known Tipulidae from eastern Asia (Diptera). LI. Philipp. J. Sci. 92, 205–246 (1963).
Enderlein, G. Eine neue Fliegengattung von den Falklands-Inseln. 9. Beitrag zur Kenntnis der antarktischen Landarthropoden. Zoologischer Anzeiger 29, 69–72 (1906).
Alexander, C.P. New or little-known Tipulidae (Diptera). XXII. Australas. Species Ann. Mag. Nat. Hist. 13, 499–523 (1924).
Acknowledgements
We warmly thank Mr. Eric Dépré (Aigrefeuille d’Aunis) for the donation of the type specimen from Cadeuil. Financial support for field studies and collection of Charentese amber was provided by French National Research Agency grant BLAN07-1-184190 (project AMBRACE to D. Néraudeau, University of Rennes). This research was supported by the project of the National Science Centre, Poland, Grant No. 2016/23/B/NZ8/ 00936.
Funding
Open Access funding enabled and organized by University of Rzeszów.
Author information
Authors and Affiliations
Contributions
I.K.-K. conceived and designed the study, led data analysis, made the photographs and drawings, and drafted the manuscript; I.K.-K. and V.P. performed data analysis, made graphical figures, and wrote the manuscript; W.K. performed data analysis and corrected the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kania-Kłosok, I., Perrichot, V. & Krzemiński, W. Cretaceous Antodicranomyia (Diptera: Limoniidae) and their paleohabitat. Sci Rep 12, 10167 (2022). https://doi.org/10.1038/s41598-022-14182-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14182-1
- Springer Nature Limited