Abstract
In our previous studies, we found that blue light has a lethal effect on various insect species and demonstrated that the most effective wavelength to control the hygiene pest, the mosquito, Culex pipiens form molestus (Diptera: Culicidae), is ~ 420 nm through all developmental stages. The genera Aedes and Culex include many globally crucial hygiene pest species that transmit serious diseases to humans and animals. However, effective lethal wavelengths have been shown to differ among insect species. In this study, we investigated the lethal effects of blue light on the Asian tiger mosquito, Aedes albopictus, using light-emitting diodes. Blue-light irradiation had a lethal effect on the larvae, pupae, and adults of Ae. albopictus. In particular, the 417-nm blue-light wavelength had a strong lethal effect on the larvae, showing 100% mortality before pupation at the photon flux density of 10 × 1018 photons·m−2·s−1. In contrast, no blue-light wavelength had a lethal effect on the eggs. Moreover, the 417-nm wavelength had the strongest effect on the pupae among the tested blue-light wavelengths. Our findings indicate that ~ 420 nm is the most promising blue-light wavelength to control populations of Ae. albopictus and C. pipiens f. molestus.
Similar content being viewed by others
Introduction
In our previous studies, we have found that irradiation with blue light (400–500 nm) has a lethal effect on various insect species1,2. This effect is considered to be useful for the development of clean and safe pest control techniques. In fact, light-emitting diode (LED) devices that emit blue light have recently begun to be applied to control insect pests in some food-processing facilities3. Therefore, we investigated the applicability of blue light toxicity to insects for reducing mosquito populations.
Mosquitoes are one of the most dangerous animals because their bites not only cause skin irritation but also may spread a variety of deadly diseases. In addition, for most of the arboviral diseases transmitted by mosquitoes, there is no preventive vaccine or medicinal treatment4. Therefore, mosquito-vector controls play a key role in reducing disease transmission5. Larvicides that can kill immature stages of mosquitoes are used to reduce adult mosquito populations. However, the use of larvicides has certain disadvantages, such as the development of insecticide resistance and disruption of the aquatic ecosystem. Therefore, eco-friendly control techniques that can kill immature stages of mosquitoes need to be developed.
In mosquitoes, we have previously demonstrated the lethal effect of blue light on Culex pipiens form molestus1,6. The mosquito vectors mainly belong to three genera, Aedes, Anopheles, and Culex7. In C. pipiens f. molestus, the most toxic blue-light wavelength is ~ 420 nm through all developmental stages6. However, in insects, effective blue-light wavelengths are species- and growth stage-specific1,2,8. Therefore, in this study, we investigated blue-light wavelengths highly toxic to each developmental stage of the Asian tiger mosquito, Aedes albopictus.
Aedes mosquitoes, especially those belonging to two species, i.e. Ae. aegypti and Ae. albopictus, bear relevance to human public health as vectors of many viral diseases including dengue, Zika, chikungunya, yellow fever, and Rift Valley fever9. The global distribution of dengue fever, particularly, has rapidly grown in recent decades, with approximately 390 million people estimated to be infected each year10,11. In addition, climate change is expected to exacerbate the risk of the global expansion of Aedes-borne virus transmission12,13,14,15,16,17,18.
Aedes albopictus originally originated from Southeast Asia, China, and Japan but has expanded its habitat to Europe, Western Africa, Southern Africa, and North and South America in the last 3–4 decades19,20,21,22. It is a vector of at least 26 diseases, including the above-mentioned diseases23,24,25,26,27. In Japan, the annual number of imported dengue cases has increased over the years, reaching 200 in recent years28. Tokyo experienced an outbreak of autochthonous dengue fever transmitted by Ae. albopictus in 2014. Therefore, a pyrethroid insecticide against adult mosquitoes and an insect growth regulator against larval mosquitoes were applied in the parks where mosquitoes were inspected for dengue virus presence29. In addition, water drainage from ponds and rainwater inlets and fountain cleaning in parks have been carried out every year since then29. However, insecticide resistance has developed in some mosquito species30,31,32, partly because of the expression of the knockdown resistance (kdr) gene that prevents the binding of pyrethroids to the sodium channel33. Aedes albopictus populations expressing the kdr gene have been found in Singapore, China, and the United States33,34. The widespread use of insecticides has led to the spread of kdr mutations in mosquito populations35.
In this study, we demonstrated the application of a blue-light wavelength for killing Ae. albopictus as a promising alternative to insecticide application, which can reduce the populations of Aedes mosquitoes, major arbovirus vectors, using light. It has been revealed that ~ 440- and ~ 470-nm blue lights show higher toxicity to D. melanogaster than that at ~ 380-nm UVA1. Moreover in C. pipiens f. molestus, the toxicity of ~ 420 nm blue light is shown to be higher than that of ~ 380 nm UVA6. Therefore, in this study, we also investigated the toxicity of ~ 380-nm UVA to Ae. albopictus for comparison.
Result
Lethal effect of blue-light irradiation on Ae. albopictus eggs
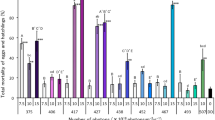
The mortalities of Ae. albopictus eggs continuously irradiated with blue-light wavelengths of 407, 417, 438, 454, and 467 nm at 10 × 1018 photons·m−2·s−1 were significantly lower than the mortalities of those kept under dark conditions (DD) (Fig. 1). However, the mortalities of the eggs irradiated with 417, 454, and 467 nm at 15 × 1018 photons·m−2·s−1 did not significantly differ from the mortalities of those kept under DD. Continuous irradiation with the wavelengths of 379 nm (UVA) and 497 nm at 10 × 1018 photons·m−2·s−1 resulted in egg mortalities similar to those obtained under DD. The egg mortalities under continuous white light condition (LL) did not significantly differ from those irradiated with all wavelengths of light tested, except for 379 and 497 nm at 10 × 1018 photons·m−2·s−1 (Fig. 1).
Mortality of Aedes albopictus irradiated with blue light during the egg stage. Data represent the means ± standard errors. Asterisks above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and control [dark condition (DD)] (Steel test: *p < 0.05, **p < 0.01, ***p < 0.001). Daggers above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and the control [continuous white light condition (LL)] (Steel test: †p < 0.05). Bars with the same letters are not significantly different (Steel–Dwass test, p > 0.05). Ten replications (20 eggs per replicate) were conducted.
Lethal effect of blue-light irradiation on the larval to pupal stages of Ae. Albopictus
Continuous irradiation with 379-nm UVA light and 407- and 417-nm blue light at a photon density of 5 × 1018 photons·m−2·s−1 during the larval to pupal stages significantly increased the total mortalities of larvae and pupae compared with those under DD (Fig. 2). In particular, at 5 × 1018 photons·m−2·s−1, 379- and 417-nm wavelengths had strong lethal effects, resulting in 85% and 66% total mortalities (84% and 66% larval mortalities), respectively (Fig. 2; Supplementary Fig. S1). However, at 10 × 1018 photons·m−2·s−1, these two wavelengths resulted in 100% larval mortalities (Supplementary Fig. S1). The 438-nm blue-light wavelength also had a strong lethal effect at 10 × 1018 photons·m−2·s−1, showing 95% larval and total mortalities, although it did not have a significant lethal effect at 5 × 1018 photons·m−2·s−1. Irradiation with 407-nm and 454-nm blue-light wavelengths at 10 × 1018 photons·m−2·s−1 had almost similar lethal effects resulting in 72% and 66% total mortalities (72% and 65% larval mortalities), respectively. The 467-nm blue light resulted in a significant lethal effect, with 42% total and 36% larval mortality, only at 10 × 1018 photons·m−2·s−1. The 497-nm blue light, however, produced no significant lethal effect even at 10 × 1018 photons·m−2·s−1. The mortality under LL was similar to that irradiated with 497-nm blue light at 10 × 1018 photons·m−2·s−1 (Fig. 2; Supplementary Fig. S1). Irradiation with blue light to larval feed did not influence the larval and pupal mortalities (Fig. S2).
Mortality of Aedes albopictus irradiated with blue light during the larval to pupal stages. Data represent the means ± standard errors. Asterisks above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and control [dark condition (DD)] (Steel test: *p < 0.05, **p < 0.01, ***p < 0.001). Daggers above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and the control [continuous white light condition (LL)] (Steel test: †p < 0.05, †††p < 0.001). Bars with the same letters are not significantly different (Steel–Dwass test, p > 0.05). Ten replications (10 larvae per replicate) were conducted.
Lethal effect of blue-light irradiation on Ae. albopictus pupae
Irradiation with the 417-nm blue light produced the strongest lethal effect on the pupae among the tested blue-light wavelengths (Fig. 3). At 15 × 1018 photons·m−2·s−1, the mortality of the pupae irradiated with the 417-nm blue light (87% mortality) was higher than that of the pupae irradiated with the 379-nm UVA light (67% mortality), whereas at 10 × 1018 photons·m−2·s−1, the mortality obtained with 379-nm wavelength (50% mortality) was higher than that obtained with 417-nm wavelength (27% mortality). Only the 417-nm blue light had a significant lethal effect at 10 × 1018 photons·m−2·s−1 among the tested blue-light wavelengths. The 407-nm blue light also had a significant lethal effect with only 28% mortality at 15 × 1018 photons·m−2·s−1. The other blue-light wavelengths had no significant lethal effect even at 15 × 1018 photons·m−2·s−1.
Mortality of Aedes albopictus irradiated with blue light during the pupal stage. Data represent the means ± standard errors. Asterisks above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and control [dark condition (DD)] (Steel test: *p < 0.05, ***p < 0.001). Daggers above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and the control [continuous white light condition (LL)] (Steel test: ††p < 0.01, †††p < 0.001). Bars with the same letters are not significantly different (Steel–Dwass test, p > 0.05). Ten replications (10 pupae per replicate) were conducted.
Lethal effect of blue-light irradiation on Ae. albopictus adults
The mean adult longevity values of males and females kept under DD were 36.02 and 47.28 days and those kept under LL were 25.40 days and 38.14 days, respectively (Supplementary Fig. S3). In contrast, the mean adult longevity values of males and females irradiated with UVA or blue light were 7.62–14.06 and 9.88–19.26 days, respectively, being significantly different from those under LL and DD. The reduction rate of longevity (RRL) values of the males and females irradiated with the 379-nm UVA light (78.85% and 79.10%, respectively) were the highest followed by the RRL values of those irradiated with the 417-nm (71.68% and 68.40%, respectively) and 438-nm blue-light wavelengths (68.74% and 64.59%, respectively) (Fig. 4). The RRL values of males and females irradiated with the 407-nm (63.46% and 59.26%, respectively), 454-nm (62.30% and 59.86%, respectively), and 467-nm wavelengths (60.97% and 62.35%, respectively) were almost similar.
Reduction rate of longevity (RRL) of Aedes albopictus adults irradiated with blue light. Data represent the means ± standard errors. Asterisks above the bars indicate significant differences between the treatments (UVA and blue light irradiation) and control [dark condition (DD)] (Steel test, ***p < 0.001). Bars with the same letters are not significantly different (Steel–Dwass test, p > 0.05). Fifty replications (5 adults × 10 Petri dishes) were conducted.
Discussion
Continuous white-light exposure (LL) showed no lethal effect on the larvae and pupae of Ae. albopictus. Therefore, the lethal effects of UVA and blue light on them presumably did not result from the disturbance of circadian rhythms. On the other hand, longevities of adults under LL were shorter than those under DD. The reduction in adult longevity under LL compared with that under DD may be attributed to the disturbance of circadian rhythms. However, the longevities of adults irradiated with UVA or blue light were shorter than those under LL. These results indicate that UVA and blue-light irradiation are lethal to the adults as well as the larvae and pupae.
In some insects, such as Drosophila melanogaster and Galerucella grisescens, the toxic blue-light wavelengths change with growth2,8. In contrast, the present study showed that, in Ae. albopictus, the most effective wavelength did not vary among developmental stages, in a manner similar to C. pipiens f. molestus1,6. Moreover, in our previous studies, we reported that effective blue-light wavelengths are species-specific in insects1,2,6. In Ae. albopictus, the most toxic blue-light wavelength for all stages, except for eggs, was the same as that for C. pipiens f. molestus, i.e. ~ 420 nm.
In our previous study, we indicated that reactive oxygen species (ROS) influence the lethal effect of blue light8. In mammals, the ROS generated by blue-light irradiation damage their retina cells36,37. Therefore, we speculated that the ROS produced by the absorption of specific blue-light wavelengths in insect tissues damages them and kills the insects1. It is likely that the absorption mechanism of the blue light having a lethal effect is common to C. pipiens f. molestus and Ae. albopictus through all developmental stages except for eggs. For example, mitochondria are known to specifically absorb ~ 420-nm light, and this absorption is attributed to the presence of porphyrins38. In addition, it has been reported that the absorption of wavelengths of ~ 420 nm by porphyrins interacts with triplet oxygen molecules to form radicals and ROS, leading to cell death39,40,41,42,43. The high lethal effect of ~ 420 nm on Ae. albopictus may be caused by the ROS produced by absorption of this wavelength of blue light by mitochondria.
Unlike the findings on C. pipiens f. molestus eggs, no blue-light wavelength exhibited a lethal effect on the eggs of Ae. albopictus. The difference in lethal effects on the eggs of both species may be related to the colours of their eggs. The eggshell colour of C. pipiens f. molestus is light brown, whereas that of Ae. albopictus is black. Therefore, the blue light is thought to easily reach the inner tissues of the eggs through the eggshell in C. pipiens f. molestus but not in Ae. albopictus. Furthermore, the mortalities of Ae. albopictus eggs irradiated with the blue-light wavelengths of 407–467 nm were significantly lower at the photon density of 10 × 1018 photons·m−2·s−1 than under DD. On the other hand, there was no significant difference in egg mortalities between LL and 407- to 467-nm blue-light irradiation. The cause of the improvement of the survival rate of eggs irradiated with blue light or LL remains unclear. The presence of adequate light conditions (photon density and wavelength) may have a positive effect on the growth of Ae. albopictus eggs.
Our findings indicate that immature populations of Ae. albopictus may be reduced by irradiation with ~ 420-nm blue light. Irradiation with 417-nm blue light at 10 × 1018 photons·m−2·s−1 resulted in 100% mortality in the larvae of Ae. albopictus. Therefore, Ae. albopictus adult populations can be reduced if the larvae are reliably exposed to ~ 420-nm blue light at photon densities higher than 10 × 1018 photons·m−2·s−1. Aedes albopictus can take advantage of any natural or artificial water reserve, and the eggs can withstand long-term drying in the diapause phase44. Water-holding containers, such as tyres, plastic buckets, plastic drums, and flowerpots, are their preferred field habitat45,46. We can prevent the immature stages of the mosquitoes from inhabiting these containers by removing water from them or by covering the containers with sheets. However, the mosquitoes also inhabit artificial waterbodies, such as ditches, reservoirs, and ponds in cemeteries, residential sites, and parks45,47, where it is difficult to drain water. The lethal effect of blue light may be useful for reducing mosquito populations in these sites. Irradiation of the water surface of artificial waterbodies using ~ 420-nm wavelength LED or laser diode (LD) devices (e.g. panel light type, tube light type, and floodlight type devices) could presumably be a practical application of the lethal effect of blue light on Ae. albopictus. Mosquito control using blue light is considered a highly safe and eco-friendly alternative to insecticides. The toxicity of blue light to biological organisms is much lower than that of UV, and blue-light irradiation generates no residuals. However, blue light irradiation induces injury in mammalian retina cells as mentioned above. Therefore, it is necessary that the installation is performed appropriately such that humans are prevented from directly looking at the light source. In addition, it is necessary to place the light source such that the negative effect on non-target organisms is prevented as well.
The distance between light source and mosquitoes in this study was ~ 200 mm. However, the distance under the field conditions is likely to be greater than that in the laboratory. The values of photon flux densities on irradiated surfaces are important for the lethal effect on insects. Therefore, it is necessary to design light sources (e.g. the characteristics of light distribution and LED output power) that can irradiate target insects at higher than 10 × 1018 photons·m−2·s−1 from the distance required for practical use. In addition, it is necessary to confirm the lethal effect of light source designs in a semi-field test.
Aedes albopictus is one of the most widespread Diptera species in the world44. They occur in urban areas as well as rural areas47 and transmit various arboviruses23. The worldwide distribution of arboviruses, such as dengue, chikungunya, and Zika viruses, is expanding because of globalised traffic (e.g. air passengers) and trade48,49. Therefore, in many regions, the outbreak risks of these diseases are expected to increase further in the future partly because of the impact of climate change. The lethal effect of blue light on mosquitoes, if put into practical use, will be able to contribute to the control of the outbreak of these diseases.
Methods
Insects
Fifty adult females of Ae. albopictus were collected from Nishitokyo (35.43° N, 139.32° E, Tokyo, Japan) to establish a colony that was maintained in our laboratory (Graduate School of Agricultural Science, Tohoku University, Sendai, Japan). Eggs, larvae, and pupae were reared in a plastic container (150 mm diameter × 91 mm height) containing 250 mL of tap water decalcified for 48 h, with a constant supply of fishery feed for trout juveniles (EC 1C, Feed One Co., Ltd., Yokohama, Japan). Adults were maintained in a mesh cage (300 mm × 300 mm × 300 mm) containing an Erlenmeyer flask (50 mL) and a plastic cup (30 mm diameter × 35 mm height). Cotton wool soaked with 10% honey solution (50 mL) was placed in the flask (food substitute), and a filter paper soaked with water was placed in the cup (oviposition substrate). Adult females were fed human blood by offering the author’s (Taniyama) arm every three days after five days of the emergence. The blood was given to mosquitoes until they left the arm. The blood of only Taniyama was given to the mosquitoes to avoid the influence of different blood on the survival of eggs and larvae. The oviposition substrate was placed in the cage five days after the first sucking of blood. Subsequently, the oviposition substrate with eggs deposited by females was taken out from the cage after five days and put into the water in the above-mentioned plastic container. In the cage, fresh oviposition substrate was placed. All stages were maintained in a constant-temperature room at 25 ± 1 °C and ~ 60% relative humidity under a 16 h:8 h (light:dark) photoregime. The offspring from more than six generations of the above colony were used for the present study.
LED light irradiation
LED lighting units (IS-mini®, ISL-150 × 150 Series; CCS Inc., Kyoto, Japan; light emission surface: 150 mm × 150 mm; arrangement: 360 LEDs were equally arranged on a panel; LED type: φ 3 mm plastic mould) with power supply units (ISC-201–2; CCS Inc., Kyoto, Japan) were used for light irradiation. Insects were irradiated with LED light in a multi-room incubator (LH-30CCFL-8CT; Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The emission spectra and the number of photons (photons·m−2·s−1) were measured using a high-resolution spectrometer (HSU-100S; Asahi Spectra Co., Ltd., Tokyo, Japan; numerical aperture of the fibre: 0.2) in a dark room. The number of photons was adjusted by current control using a power supply unit. During measurements, the distance between the LED lighting unit and the spectrometer sensor was set to be approximately equal to the same distance as between the insects and the LED lighting unit in the incubator. Because the mosquitoes were irradiated through a glass plate or glass lid, the same plate or lid was placed between the light source and sensor during measurement. The number of photons was measured five times before and after each experiment. The average values of the number of photons in each experiment are shown in Supplementary Tables S1–S4.
Lethal effect of blue-light irradiation on Ae. albopictus eggs
Eggs were collected from the stock cultures within 1 h of deposition and were placed (n = 20) in water (12 mL) in a glass Petri dish (50 mm diameter × 15 mm height). The Petri dish was placed in a multi-room incubator (25 ± 1 °C) and irradiated with LED light at wavelengths of 379, 407, 417, 438, 454, 467, and 497 nm with set values of 10 × 1018 or 15 × 1018 photons·m−2·s−1 for 20 days. The distance between the light source and eggs (i.e. the water surface in the Petri dish) was 214 mm. The number of hatchlings (larvae) was counted every day to determine egg mortality. The hatchlings were removed from the Petri dish every day. In the control treatment, the eggs were maintained under LL {i.e. continuous white light condition with a cold cathode fluorescent lamp (CCFL, wavelength range: 250–1000 nm, photon flux density: 0.06 × 1018 photons·m−2·s−1, Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan)} and DD (i.e. no irradiation) for 20 days, and egg mortality was determined as described above. In the LL control, the mosquitoes were irradiated at 0.06 × 1018 photons·m−2·s−1 because this is the maximum photon flux density produced by the CCFL. The photon flux density of the CCFL used for the control was much lower than those of the LEDs. However, white light contains UV and blue light because it comprises a wide range of wavelengths (250–1000 nm). If mosquitoes are irradiated with white light at a high photon density, they will be killed by the UV and blue light contained in white light. Therefore, the photon flux density used for LL conditions is adequate for investigating whether disruption of the circadian rhythm by continuous light conditions (not UV and blue light energy) affects the lifespan of an insect. The average number of photons and emission spectra of the CCFL are shown in Supplementary Tables S1–S4 and Supplementary Figure S4, respectively. Ten replications (dishes) were performed for each dose of each light wavelength.
Lethal effect of blue-light irradiation on the larval to pupal stages of Ae. Albopictus
The method used to irradiate larval to pupal stages was the same as that in our previous study6. Hatchlings were collected from the stock cultures within 12 h of hatching and were placed (n = 10) in water (50 mL) in polyethylene terephthalate (PET) ice-cream cups (60 mm diameter × 38 mm height, Risupack, Co., Ltd., Aichi, Japan). Fishery feed for trout juveniles (EC 1C, Feed One Co., Ltd., Yokohama, Japan) (0.01 g) was supplied in the cups on the first day of irradiation, and subsequently, 0.03 g was added five days after the start of the irradiation. Rearing protocols for Aedes mosquitoes recommend feeding small quantities of food every 24–48 h to avoid mortality due to either biofilm development or starvation. However, feeding interrupts continuous irradiation. Therefore, the above-mentioned feeding methods were adopted in this study after an investigation of the appropriate feeding intervals and the amount of food required to avoid biofilm development or starvation. The cups were each covered with a glass plate, placed in the multi-room incubator (25 ± 1 °C), and irradiated with LED light at the same wavelengths as mentioned above, with set values of 5 × 1018 or 10 × 1018 photons·m−2·s−1. The distance between the light source and the larvae or pupae (i.e. the water surface in the cup) was 203 mm. The larvae in the cups were irradiated until they had all died or developed to the adult stage. The number of pupated larvae and emerging adults were counted to determine the larval and pupal mortalities, respectively. In the control treatment, mosquitoes were maintained under LL and DD until they had all died or developed to the adult stage, after which the larval and pupal mortalities were determined using the same method as described above. Ten replications (cups) were performed for each dose of each light wavelength.
The contribution of blue-light exposed feed to larval mortality was also investigated. The fishery feed for trout juveniles (0.01 g) in a cup with 50 ml of water was irradiated with 417-nm light at set values of 10 × 1018 photons·m−2·s−1 for 3 days. After the irradiation, ten hatchlings within 12 h after hatching were placed in the cup, and were maintained under DD. After three days, the larvae were moved to a new cup with 0.03 g feed irradiated with 417-nm light at the same photon flux density for five days. Each of the cups were covered with a glass plate and placed in a multi-room incubator (25 ± 1 °C). All larvae in the cups were maintained under DD until all of them either died or developed to the adult stage. The number of pupated larvae and emerged adults were counted to determine the larval and pupal mortalities, respectively. Five replications (cups) were performed.
Lethal effect of blue-light irradiation on Ae. albopictus pupae
Pupae were collected from the stock cultures within 4 h of pupation and were placed (n = 10) in water (50 mL) in PET ice-cream cups (60 mm diameter × 38 mm height). The cups were each covered with a glass plate, were placed in the multi-room incubator (25 ± 1 °C), and irradiated with LED light at the same wavelengths as mentioned above with the set values of 10 × 1018 or 15 × 1018 photons·m−2·s−1. The distance between the light source and the pupae (i.e. the water surface in the cup) was 203 mm. The pupae in the cups were irradiated until they had all died or developed to the adult stage. The number of emerging adults were counted to calculate the pupal mortalities. In the control treatment, the pupae were maintained under LL and DD until they had all died or developed to the adult stage, after which the pupal mortalities were determined using the above-described method. Ten replications (cups) were performed for each dose of each light wavelength.
Lethal effect of blue-light irradiation on Ae. albopictus adults
The method used to irradiate adults was the same as that in our previous study6. Adults were collected from the plastic rearing containers within 12 h of emergence, and their pairs (n = 5 pairs) were released onto circular cotton pads (10 g, diameter 90 mm) soaked with 5% honey solution (10 mL) in a glass Petri dish (60 mm diameter × 90 mm height). The Petri dish was placed in a multi-room incubator equipped with an LED lighting unit. The distance between the light source and the adults (i.e. the bottom of the Petri dish) was 225 mm. The adults were irradiated with different wavelengths of light at 25 ± 1 °C until all of them died. We counted the number of dead adults every day and replaced the Petri dish containing the honey water every 2 days. Lethal effects of irradiation at the set values of 15 × 1018 photons·m−2·s−1 were compared among the six wavelengths [379, 407, 417, 438, 454, and 467 nm]. In the test for adults, a high lethal effect was not obtained even at 15 × 1018 photons·m−2·s−1; therefore, the effect at 10 × 1018 photons·m−2·s−1 was not investigated. In the control treatment, the longevities of the mosquitoes maintained under LL and DD (no irradiation) were determined. The lethal effect was determined using the RRL values, which were calculated as follows:
where LD and LI represent the average longevities of nonirradiated (DD) and irradiated adults, respectively. Fifty replications (five pairs adults × 10 Petri dishes) were performed for each dose of each light wavelength.
Statistical analysis
Comparisons of mortalities and RRLs between irradiation and control treatments and among the studied wavelengths were analysed using the Steel and Steel–Dwass tests, respectively. The investigation of the contribution of blue-light exposed feed to larval mortality was performed using the Mann–Whitney U test. The calculations were performed using R version 4.0.250.
Ethical approval
This study is exempt from the need for ethical approval under local law as per advice received from the Ethical Review Committee, Graduate School of Agricultural Science, Tohoku University.
Data availability
The datasets are available from the corresponding author on a reasonable request.
References
Hori, M., Shibuya, K., Sato, M. & Saito, Y. Lethal effects of short- wavelength visible light on insects. Sci. Rep. 4, 7383. https://doi.org/10.1038/srep07383,Pubmed:25488603 (2014).
Hori, M. & Suzuki, A. Lethal effect of blue light on strawberry leaf beetle, Galerucella grisescens (Coleoptera: Chrysomelidae). Sci. Rep. 7, 2694. https://doi.org/10.1038/s41598-017-03017-z (2017).
Hori, M. Lethal effect of blue light on insects and its application to pest control (in Japanese). Jpn. J. Pestic. Sci. 43, 109–116. https://doi.org/10.1584/jpestics.W18-32 (2018).
Qureshi, A. I. Chapter 2. https://doi.org/10.1016/B978-0-12-812365-2.00003-2 (Academic, 2018) in Mosquito-Borne Diseases in Zika Virus Disease 27–45 (2018).
Saha, P. et al. Prevalence of kdr mutations and insecticide susceptibility among natural population of Aedes aegypti in West Bengal. PLoS ONE 14, e0215541. https://doi.org/10.1371/journal.pone.0215541 (2019).
Taniyama, K., Saito, Y. & Hori, M. Lethal effect of blue light on the developmental stages of the urban mosquito, Culex pipiens form molestus (Diptera: Culicidae). Appl. Entomol. Zool. 56, 319–325. https://doi.org/10.1007/s13355-021-00737-7 (2021).
Tandina, F. et al. Mosquitoes (Diptera: Culicidae) and mosquito-borne diseases in Mali, West Africa. Parasit. Vectors 11, 467. https://doi.org/10.1186/s13071-018-3045-8 (2018).
Shibuya, K., Onodera, S. & Hori, M. Toxic wavelength of blue light changes as insects grow. PLoS ONE 13, e0199266. https://doi.org/10.1371/journal.pone.0199266 (2018).
Fontenille, D. & Powell, J. R. From anonymous to public enemy: How does a mosquito become a feared arbovirus vector?. Pathogens 9, 265. https://doi.org/10.3390/pathogens9040265 (2020).
Messina, J. P. et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 22, 138–146. https://doi.org/10.1016/j.tim.2013.12.011 (2014).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507. https://doi.org/10.1038/nature12060 (2013).
Oliveira, S., Rocha, J., Sousa, C. A. & Capinha, C. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Sci. Rep. 11, 9916. https://doi.org/10.1038/s41598-021-89096-5 (2021).
Proestos, Y. et al. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2013.0554 (2015).
Campbell, L. P. et al. Climate change influences on global distributions of dengue and Chikungunya virus vectors. Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2014.0135 (2015).
Kamal, M., Kenawy, M. A., Rady, M. H., Khaled, A. S. & Samy, A. M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLOS ONE 13, e0210122. https://doi.org/10.1371/journal.pone.0210122 (2018).
Ryan, S. J., Carlson, C. J., Mordecai, E. A. & Johnson, L. R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLOS Negl. Trop. Dis. 13, e0007213 (2019).
Iwamura, T., Guzman-Holst, A. & Murray, K. A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 11, 2130. https://doi.org/10.1038/s41467-020-16010-4 (2020).
Kramer, I. M. et al. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci. Total Environ. 778, 146128. https://doi.org/10.1016/j.scitotenv.2021.146128 (2021).
Reiter, P. & Darsie, R. F. Jr. Aedes albopictus in Memphis, Tennessee, (USA): An achievement of modern transportation?. Mosq. News 44, 396–399 (1984).
Sprenger, D. & Wuithiranyagool, T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 2, 217–219 (1986).
Paupy, C., Delatte, H., Bagny, L., Corbel, V. & Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 11, 1177–1185. https://doi.org/10.1016/j.micinf.2009.05.005 (2009).
Medlock, J. M. et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 12, 435–447. https://doi.org/10.1089/vbz.2011.0814 (2012).
Gratz, N. G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18, 215–227. https://doi.org/10.1111/j.0269-283X.2004.00513.x (2004).
Benedict, M. Q., Levine, R. S., Hawley, W. A. & Lounibos, L. P. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 7, 76–85. https://doi.org/10.1089/vbz.2006.0562 (2007).
Wong, P. S. J., Li, M. Z., Chong, C. S., Ng, L. C. & Tan, C. H. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLOS Negl. Trop. Dis. 7, e2348. https://doi.org/10.1371/journal.pntd.0002348 (2013).
Liu, Z. et al. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus mosquitoes as Zika virus vectors, China. Emerg. Infect. Dis. 23, 1085–1091 (2017).
Smartt, C. T. et al. Evidence of Zika virus RNA fragments in Aedes albopictus (Diptera: Culicidae) field-collected eggs from Camaçari, Bahia, Brazil. J. Med. Entomol. 54, 1085–1087. https://doi.org/10.1093/jme/tjx058 (2017).
Tsuda, Y. et al. Biting density and distribution of Aedes albopictus during the September 2014 outbreak of dengue fever in Yoyogi park and the vicinity of Tokyo metropolis Japan. Jpn. J. Infect. Dis. 69, 1–5. https://doi.org/10.7883/yoken.JJID.2014.576 (2016).
Kori, M. et al. The 2014 autochthonous dengue fever outbreak in Tokyo: A case series study and assessment of the causes and preventive measures. Respir. Med. Case Rep. 31, 101246. https://doi.org/10.1016/j.rmcr.2020.101246 (2020).
Hamdan, H., Sofian-Azirun, M., Nazni, W. A. & Lee, H. L. Insecticide resistance development in Culex quinquefasciatus (Say), Aedes aegypti (L) and Aedes albopictus (Skuse) larvae against Malathion, permethrin and temephos. Trop. Biomed. 22, 45–52 (2005).
Casimiro, S., Coleman, M., Mohloai, P., Hemingway, J. & Sharp, B. Insecticide resistance in Anopheles funestus (Diptera: Culicidae) from Mozambique. J. Med. Entomol. 43, 267–275 (2006).
Kasai, S. et al. Insecticide resistance in potential vector mosquitoes for West Nile virus in Japan. J. Med. Entomol. 44, 822–829 (2007).
Kasai, S. et al. First detection of a putative knockdown resistance gene in major mosquito vector Aedes albopictus. Jpn. J. Infect. Dis. 64, 217–221 (2011).
Xu, J. et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus. PLOS Negl. Trop. Dis. 10, e0004696. https://doi.org/10.1371/journal.pntd.0004696 (2016).
Vu, T. X., Andrianov, B. V., Vu, D. C. & Goryacheva, I. I. qPCR identification of the kdr allele F1534C in voltage-gated sodium channel gene (vgsc) of the major mosquito vectors Aedes aegypti and Aedes albopictus in Northern and Central Vietnam. Russ. J. Genet. 56, 460–469. https://doi.org/10.1134/S1022795420040158 (2020).
Wu, J., Seregard, S. & Algvere, P. V. Photochemical damage of the retina. Surv. Ophthalmol. 51, 461–481. https://doi.org/10.1016/j.survophthal.2006.06.009 (2006).
Kuse, Y., Ogawa, K., Tsuruma, K., Shimazawa, M. & Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 4, 5223. https://doi.org/10.1038/srep05223 (2014).
Kam, J. H., Hogg, C., Fosbury, R., Shinhmar, H. & Jeffery, G. Mitochondria are specifically vulnerable to 420nm light in drosophila which undermines their function and is associated with reduced fly mobility. PLoS ONE 16, e0257149. https://doi.org/10.1371/journal.pone.0257149 (2021).
Pineiro, M. et al. Photoacoustic measurements of porphyrin triplet-state quantum yields and singlet-oxygen efficiencies. Chem. Eur. J. 4, 2299–2307 (1998).
Bonnett, R. et al. Triplet states of porphyrin esters. J. Chem. Soc. Faraday Trans. 1(76), 852–859. https://doi.org/10.1039/F19807600852 (1980).
Kou, J., Dou, D. & Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 8, 81591–81603 (2017).
Marie, M. et al. Phototoxic damage to cone photoreceptors can be independent of the visual pigment: The porphyrin hypothesis. Cell Death Dis. 11, 711. https://doi.org/10.1038/s41419-020-02918-8 (2020).
Berg, K. et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 218, 133–147. https://doi.org/10.1111/j.1365-2818.2005.01471.x (2005).
Obregón, R., Flores, E. & Jordano, D. First report of the Asian tiger mosquito, Aedes (Stegomyia) albopictus Skuse, 1984 (Diptera, Culicidae) in Cordoba (southern Spain). New challenges for the administration and citizens of Cordoba. J. Eur. Mosq. Control Assoc. 37, 29–33 (2019).
Rahman, Md. S. et al. Entomological survey for identification of Aedes larval breeding sites and their distribution in Chattogram, Bangladesh. Beni-Suef Univ. J. Basic Appl. Sci. 10, 32. https://doi.org/10.1186/s43088-021-00122-x (2021).
Surendran, S. N. et al. Aedes larval bionomics and implications for dengue control in the paradigmatic Jaffna peninsula, northern Sri Lanka. Parasit. Vectors 14, 162. https://doi.org/10.1186/s13071-021-04640-6 (2021).
Champion, S. R. & Vitek, C. J. Aedes aegypti and Aedes albopictus habitat preferences in South Texas, USA. Environ. Health Insights 8, 35–42. https://doi.org/10.4137/EHI.S16004 (2014).
Semenza, J. C. et al. International dispersal of dengue through air travel: Importation risk for Europe. PLOS Negl. Trop. Dis. 8, e3278. https://doi.org/10.1371/journal.pntd.0003278 (2014).
Solimini, A. G., Manica, M., Rosà, R., Della Torre, A. & Caputo, B. Estimating the risk of Dengue, chikungunya and Zika outbreaks in a large European city. Sci. Rep. 8, 16435. https://doi.org/10.1038/s41598-018-34664-5 (2018).
R developmental core team. The R project for statistical computing. http://www.r-project.org, (2020).
Acknowledgements
This study was supported by the Japan Society for the Promotion of Science (JSPS) through KAKENHI Grant Numbers 17K19254 and 18H03946 and the JSPS Core-to-Core Program (Advanced Research Networks), titled “Establishment of international agricultural immunology research-core for a quantum improvement in food safety”. We wish to thank Dr. Tuno (Graduate School of Natural Science and Technology, Kanazawa University) for providing kind advice.
Author information
Authors and Affiliations
Contributions
M.H. conceived the study and designed experiments. K.T. performed the experiments. M.H. and K.T. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taniyama, K., Hori, M. Lethal effect of blue light on Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Sci Rep 12, 10100 (2022). https://doi.org/10.1038/s41598-022-14096-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14096-y
- Springer Nature Limited
This article is cited by
-
Negative phototaxis of jumping cocooned parasitoid wasp larvae against short wavelengths and physicochemical properties of the cocoon shell
Scientific Reports (2023)
-
Lethal effect of blue light on Liposcelis bostrychophila (Psocoptera: Liposcelididae)
Applied Entomology and Zoology (2023)