Abstract
The overall objective of this study was to determine the association between hemoglobin (HGB) and bone mineral density (BMD) in the lumbar and thoracic spine of adults aged ≥ 18 years. This cross-sectional study utilized the non-institutionalized US population from the National Health and Nutrition Survey (NHANES) as the sample source. A multiple linear regression model was used to assess the relationship between HGB and BMD in the lumbar and thoracic spine, with analysis of subgroups conducted according to sex and race. Smooth curve fitting was performed to explore the potential nonlinear relationship. When nonlinearity was found, we further constructed a weighted two-piecewise linear regression model and used a recursive algorithm to calculate the inflection point. After accounting for relevant confounding variables, HGB was found to be negatively associated with lumbar spine BMD in multiple regression models. However, in the subgroup analyses stratified by sex and race, the relationship between HGB and thoracic spine BMD and lumbar spine BMD was only found in women and other races and races that were not recorded. In Non-Hispanic Asian subjects, the relationship between HGB and BMD in the lumbar spine and thoracic spine showed a U-shaped curve. In addition, the relationship between HGB and BMD in the lumbar spine formed an inverted U-shaped curve among participants in other races and those whose race was not reported. Our study shows that HGB has a non-linear relationship with lumbar and thoracic BMD. Further studies are required to elucidate the mechanisms underlying this association.
Similar content being viewed by others
Introduction
Osteoporosis, which most commonly occurs in the hip, spine, and wrist, not only puts people at an increased risk of fragility and fracture but also has a substantial impact on their families and the society1,2. Bone mineral density (BMD) evaluated using the Dual energy X-ray absorptiometry (DXA)3, accurately captures bone mass and is the gold standard for diagnosing osteoporosis, as well as being the best predictor of osteoporotic fracture risk4. BMD is affected by several factors, including sex, age, genetic factors, nutrition, hormone levels, physical activity, smoking, and alcohol consumption5. A recent study by Korkmaz et al. showed that anemia was an independent risk factor for low BMD in postmenopausal women6.
Hemoglobin (HGB) is found in erythrocytes, and its role is to combine with oxygen and transport it from the lungs to tissues7. Anemia, characterized by 'low hemoglobin concentration', is a major public health problem prevalent in low-, middle-, and high-income countries worldwide8, affecting nearly a quarter of the world's population and predominantly affecting women and pre-school children9. Low levels of HGB are recognized as risk factors for osteoporosis, falls, fractures, and physical decline in the elderly population10,11.
Zhou et al. conducted a study on 1238 volunteers from Anhui, China, and obtained a U-shaped curve for the relationship between BMD and HGB in healthy men12. In contrast, a positive correlation was found between HGB levels and BMD in patients with chronic obstructive pulmonary disease, chronic kidney disease, sickle cell anemia, and inflammatory bowel disease13,14,15,16,17. In addition, Zarei et al. measured BMD and bone mineral content in the lumbar spine and femoral neck of 21 patients over 10 years of age with hemoglobin H disease and concluded that the prevalence of BMD in the lumbar spine and femoral neck region in patients with hemoglobin H disease was significantly lower than the expected age range compared to healthy individuals18. Cui et al. adjusted for age, duration of diabetes, body mass index, and alanine aminotransferase to conclude that anemia was associated with osteoporosis in patients with type 2 diabetes mellitus, irrespective of sex19. Research on the relationship between HGB and BMD has been limited by the selection of study populations and sample sizes, and the findings are inconsistent. Furthermore, only a few studies have thoroughly investigated the association between HGB and thoracic and lumbar BMD.
Therefore, this cross-sectional study was conducted using the 2013–2018 National Health and Nutrition Survey (NHANES) as a sample source to determine the association between HGB and BMD in the lumbar and thoracic spine in adults aged ≥ 18 years.
Materials and methods
Study population
Data for the analysis were obtained from the NHANES (2013–2018), a large, comprehensive, and regularly updated probability sample of the non-institutionalized U.S. population20,21,22.
The study population consisted of participants aged ≥ 18 years who had complete data on HGB and lumbar and thoracic spine BMD. The NCHS Ethics Review Committee approved the implementation of the NHANES, and informed consent was obtained from all participants. The study adhered to the relevant guidelines and regulations. The encryption procedure was uniform to make it possible to link complaints belonging to the same patient in the NHANES. Detailed documentation of the ethics application and written informed consent are available on the NHANES website23,24,25.
Data collection
The following information was collected by two researchers (XZH and SQL):
Demographic data [gender, age, race, annual household income and education level].
Examination data [BMD of the lumbar spine and the thoracic spine (mg/cm2), systolic blood pressure (SBP) (mmHg), diastolic blood pressure (DBP) (mmHg), body mass index (BMI) (kg/m2)].
Laboratory data [HGB (g/dL), red blood cell count (× 106 cells/μL), albumin creatinine ratio (mg/g), albumin (g/L), alkaline phosphatase (ALP)(IU/L), alanine aminotransferase (ALT)(U/L), blood urea nitrogen (mmol/L), total calcium (mg/dL), iron (umol/L), creatinine phosphor kinase (CPK)(IU/L), glucose (mmol/L), lactate dehydrogenase (LDH)(IU/L), phosphorus (mmol/L), potassium (mmol/L), total bilirubin (umol/L), total protein (g/L), creatinine (umol/L), uric acid (umol/L), blood cadmium (μg/L),blood lead (μg/L),triglyceride (TG) (mmol/L),low-density lipoprotein cholesterol (LDL-C)(mmol/L), high-density lipoprotein cholesterol (HDL-C)(mmol/L) and cholesterol level (mmol/L)].
Questionnaire data [alcohol status (average alcohol drinks/day in the past 12 months), smoking status, diabetes (Has a doctor told you that you have diabetes?), rheumatoid arthritis (RA) (Which type of arthritis was it?), chronic obstructive pulmonary disease (COPD) (Ever told you had copd?), cancer or malignancy (Ever told you had cancer or malignancy?)].
Weight value [Depending on the rules for selecting weight values offered on the NHANES website, “Full Sample Two-Year Mobile Examination Center Exam Weight (WTMEC2YR)” was Selected as representative weighting value].
For the selection of confounding variables, we followed the following principles: (1) based on clinical significance, we selected indicators related to HGB and BMD; (2) from the perspective of statistical methodology, we chose to include factors that have an impact greater than 10% on the results; (3) based on the above two points, confounding factors were selected according to the biological point of view.
Treatment of missing values for variables: (1) the missing values of continuous variables were replaced with mean values; (2) the missing values of a categorical variable were separated into a "not recorded" group.
Evaluation of variables
HGB was measured at the NHANES Mobile Examination Centers (MECs). A Beckman Coulter DxH 800 instrument was used to obtain a complete blood count (CBC) on blood specimens and provide a distribution of blood cells for all participants. Transmittance of light at 525 nm through a lysed WBC solution in the HGB cuvette was compared to the transmittance of the same light through a reagent blank. The system converted this ratio to an HGB value using a calibration factor. The weight (mass) of HGB was determined by the degree of absorbance recorded from the photocurrent transmittance expressed in g/dl. A detailed description of the measurement of HGB is mentioned in the Laboratory Method Files section on the NHANES website26.
The low-level X-rays of the Hologic Discovery model A densitometer (Hologic, Inc., Bedford, Massachusetts) with Apex 3.2 software was used to scan the patient's body to estimate BMD under standard operating conditions by radiographers who participated in the DXA examination; the entrance dose of the examinee for a whole-body scan was less than 1 mR1 (a standard X-ray is approximately 35 R). More information regarding the measurement of the DXA examination protocol can be obtained from the NHANES website27.
Information about age, gender, race, education level, annual household income, alcohol status, smoking status, diabetes, RA, and COPD was provided to the participants by trained interviewers using a computer-assisted personal interview (CAPI) system. Data for serum albumin, blood urea nitrogen, uric acid, phosphorus, ALP, ALT, iron, CPK, glucose, LDH, potassium, total bilirubin, total protein, creatinine and calcium were obtained from standard biochemical profile analysis using a Beckman Synchron LX20. Total cholesterol were analyzed using a Roche Modular P chemistry analyzer (enzymatic method). Metals determination for blood cadmium and blood lead was performed by inductively coupled plasma mass spectrometry on whole blood samples from the Laboratory Sciences Department of the National Center for Environmental Health. Triglycerides (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C).The details of the variables mentioned above are available on the NHANES website28 and Appendix 1.
Statistical analysis
We used the weighted chi-square test to calculate the differences between the classification variables and a weighted linear regression model for continuous variables. Finally, the means (continuous variables) or proportions (categorical variables) were used to describe the baseline characteristics of all participants included in the study. Weighted multiple linear regression analysis was used to calculate the relationship between HGB and BMD. Following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines29, we calculated the unadjusted, minimally adjusted, and fully adjusted results. To further investigate the relationship between HGB and BMD of the thoracic and lumbar spine in different populations, a subgroup analysis was performed by separating the participants by age and sex. Additionally, smooth curve fitting was performed to explore the nonlinear relationship. Accordingly, we further constructed a weighted two-piecewise linear regression model and used a recursive algorithm to calculate the inflection point.
P values < 0.05 were defined as significant. All analyses were undertaken by EmpowerStats (version: 2.0. X&Y Solutions, Inc, Boston, MA. http://www.empowerstats.com) and R software, v.3.4.3 (Vienna, Austria: R Foundation for Statistical Computing, 2016 http://www.R-project.org).
Ethics approval
All analyses were based on data of the National Health and Nutrition Examination Survey (NHANES). And all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics review board of the National Center for Health Statistics. The National Center for Health Statistics Ethics Review Board protocol numbers are Protocol #2011-17 (NHANES 2013–2014), Continuation of Protocol #2011-17 (NHANES 2015–2016), Continuation of Protocol #2011-17 and #2018-01 (NHANES 2017–2018), respectively. The detailed information located on the NHANES website.
Consent for publication
All participating authors give their consent for this work to be published.
Results
Participant selection and general characteristics
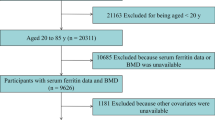
Initially, the information of 29,400 participants was extracted from the NHANES database for 2013–2018. After excluding individuals without HGB data (n = 5211) and BMD data (n = 10,942), under 18 years of age (n = 4133), and with RA, COPD, cancer, or any other malignancy (n = 623), a total of 8491 participants were included in this study (Fig. 1).
The weighted socio-demographic and medical characteristics of the participants are presented in Table 1. The mean age of these participants was 37.8 ± 12.1 years, of which 52.5% were male and 47.2% were female, with non-Hispanic whites being the most numerous. There were significant differences in sex, age, race, annual household income, education level, SBP, DBP, BMI, red blood cell count, albumin, ALP, ALT, blood urea nitrogen, total calcium, iron, CPK, creatinine, glucose, LDH, phosphorus, total bilirubin, total protein, uric acid, albumin creatinine ratio, blood cadmium, blood lead, phosphorus, potassium, triglyceride, LDL-C, HDL-C, total cholesterol, alcohol status, smoking status and diabetes across the different HGB groups (quartiles, Q1 to Q4).
Relationship between HGB and BMD
To explore the relationship between HGB and BMD, we constructed three weighted univariate and multivariate linear regression models: Model 1, unadjusted; Model 2, adjusted for sex, age, and race; and Model 3, adjusted for the covariates in Table 1.
After fully adjusting for confounders, HGB was negatively correlated with lumbar spine BMD (β = − 0.0416, P = 0.031873) (Table 2, Fig. 2). However, the negative correlation between HGB and thoracic spine BMD was not statistically significant (β = − 0.0127, P = 0.362458) (Table 2, Fig. 3). In fully adjusted subgroup analyses stratified by sex and race, the negative association between HGB and thoracic spine BMD and lumbar spine BMD was only present in females and other races and races that were not recorded (Tables 3 and 4).
Correlation between hemoglobin and thoracic bone mineral density. (a) Each black point represents one sample. (b) The area between the two blue dashed lines is represented as a 95% CI. The area between the lines is expressed as 95% CI. Each point represents the size of the hemoglobin and is connected into a continuous line. Age, sex, race, annual household income, education level, SBP, DBP, BMI, red blood cell count, albumin, ALP, ALT, blood urea nitrogen, total calcium, iron, CPK, creatinine, glucose, LDH, phosphorus, total bilirubin, total protein, uric acid, albumin creatinine ratio, blood cadmium, blood lead, phosphorus, potassium, triglyceride, LDL-C, HDL-C, total cholesterol, alcohol status, smoking status and diabetes were adjusted.
Correlation between hemoglobin and lumbar bone mineral density. (a) Each black point represents one sample. (b) The area between the two blue dashed lines is represented as a 95% CI. The area between the lines is expressed as 95% CI. Each point represents the size of the hemoglobin and is connected into a continuous line. Age,sex, race, annual household income, education level, SBP, DBP, BMI, red blood cell count, albumin, ALP, ALT, blood urea nitrogen, total calcium, iron, CPK, creatinine, glucose, LDH, phosphorus, total bilirubin, total protein, uric acid, albumin creatinine ratio, blood cadmium, blood lead, phosphorus, potassium, triglyceride, LDL-C, HDL-C, total cholesterol, alcohol status, smoking status and diabetes were adjusted.
In addition, fully adjusted smoothened plots showed a non-linear relationship between HGB and BMD in the lumbar and thoracic spine after stratification by sex or race (Figs. 4 and 5). Among participants of Non-Hispanic Asian, BMD in the thoracic spine decreased with HGB until the turning point (turning point: HGB 14.9 g/dL). Similarly, there was a turning point between HGB and BMD in the lumbar spine (turning point: HGB 15.6 g/dL). There is also a turning point in other races and races that were not recorded (turning point: HGB 13.4 g/dL) (Table 5).
The hemoglobin and bone mineral density relationship, stratified by gender. Age, race, annual household income, education level, SBP, DBP, BMI, red blood cell count, albumin, ALP, ALT, blood urea nitrogen, total calcium, iron, CPK, creatinine, glucose, LDH, phosphorus, total bilirubin, total protein, uric acid, albumin creatinine ratio, blood cadmium, blood lead, phosphorus, potassium, triglyceride, LDL-C, HDL-C, total cholesterol, alcohol status, smoking status and diabetes were adjusted. (a) Thoracic BMD; (b) Lumbar BMD. Red line: Male; Blue line: Female.
The hemoglobin and bone mineral density relationship, stratified by race. Age, sex, annual household income, education level, SBP, DBP, BMI, red blood cell count, albumin, ALP, ALT, blood urea nitrogen, total calcium, iron, CPK, creatinine, glucose, LDH, phosphorus, total bilirubin, total protein, uric acid, albumin creatinine ratio, blood cadmium, blood lead, phosphorus, potassium, triglyceride, LDL-C, HDL-C, total cholesterol, alcohol status, smoking status and diabetes were adjusted. (a) Thoracic BMD; (b) Lumbar BMD. Red line: Mexican American; Yellow line: Other Hispanic; Green line: Non-Hispanic White; Light blue line: Non-Hispanic Black; Dark blue line: Non-Hispanic Asian; Purple line: Other Races and Not recorded.
Overall, the relationship between HGB and BMD in the lumbar spine and BMD in the thoracic spine in Non-Hispanic Asian showed a U-shaped curve. In other races and races that were not recorded, there was an inverted U-shaped curve between HGB and lumbar BMD.
Discussion
The main goal of this study was to clarify whether HGB is correlated with BMD in the lumbar and thoracic spine. In our comprehensive adjusted model multiple linear regression analysis, we found that HGB was negatively correlated with lumbar BMD, and the completely adjusted smoothed curve fits showed a non-linear correlation. When stratified by sex and race, the relationship between HGB and thoracic spine BMD and lumbar spine BMD was only found in women and other races and races that were not recorded. In Non-Hispanic Asian subjects, the relationship between HGB and BMD in the lumbar spine and thoracic spine showed a U-shaped curve. In addition, the relationship between HGB and BMD in the lumbar spine formed an inverted U-shaped curve among participants in other races and those whose race was not reported.
Similarly, a cross-sectional study involving 3626 Korean participants found that HGB levels were inversely associated with low BMD in the lumbar spine among non-anemic adults30. Furthermore, in a recently published study, BMD was negatively correlated with HGB levels in younger and older women31. imilar results were obtained by Cesari et al. in a survey of 950 older adults32. Nevertheless, a study examining the association between serum HGB levels, BMD, and fracture risk using estimated scores from the Fracture Risk Assessment Tool (FRAX) in 662 male patients concluded that HGB was positively associated with BMD but negatively associated with the risk of hip fracture and major osteoporotic fracture33. It is possible that age and ethnic differences in the study population, or the limited sample size, may have influenced the results. Based on this, the cross-sectional study has a broad and large sample size, targeting both males and females aged 18 years and older, and provides subgroup analysis across gender and race.
The mechanism of the link between HGB and BMD is not yet clear; however, based on the theory that both osteoblasts and cells of the hematopoietic microenvironment that are responsible for maintaining hematopoietic tissue have a common progenitor, namely mesenchymal stem cells (MSC)34, Gurevitch et al. proposed a hypothesis that the differentiation pathways of osteogenesis and the hematopoietic microenvironment compete with each other, with osteogenic stimulation predominating during the growth phase of the organism. Nonetheless, after maturation, there is a gradual increase in differentiation of MSCs towards cells of the hematopoietic microenvironment and a decrease in intraosseous differentiation. This subsequently leads to a reduction in bone mass and enlargement of the bone marrow cavity in hematopoietically active cancellous bones35. They speculated that continuous overproduction of blood cells leads to excessive depletion of the hematopoietic system and is a non-negligible component in the etiology of osteoporosis. Blood loss promotes the proliferation of hematopoietic progenitor cells, leading to an increase in the number of hematopoietic cells including osteoblasts, which enhances bone tissue resorption. In addition, a reduction in blood volume stimulates bone development and increases the number of osteoblasts, thereby promoting new bone formation36. It has also been shown that acute bleeding stimulates the secretion of bone morphogenetic protein 2 and BMP6 by hematopoietic stem cells, thereby driving MSC differentiation along the osteogenic pathway37.
This cross-sectional study not only confirmed the association between HGB and BMD, but also provided substantial clinical significance. The U-shaped curve in the relationship between HGB and BMD in the Non-Hispanic Asian population implies that BMD in the thoracic and lumbar spine may be quietly decreasing as HGB levels approach 14.9 (g/dL) or 15.6 (g/dL) levels. More notably, however, the inverted U-shaped curve in the relationship between HGB and BMD of the lumbar and thoracic spine in people of other races and unreported races demonstrates that for these patients, clinicians should be aware of low levels of HGB while being alert to the risk of reduced bone mass and the need for close monitoring of BMD and early intervention.
This study analyzed NHANES 2013–2018 data conducted by the U.S. Centers for Disease Control and Prevention. Owing to the rigorous design of the NHANES database, the accuracy of the data requires the completion of a large sample size and stratified analysis for this study. It is regrettable that for some confounding factors that may affect the results, such as chronic kidney disease, chronic inflammatory disease, long-term infection, and the use of certain drugs, due to the lack of relevant information in the 2013–2018 NHANES database, this study cannot describe the current cases in the study. Nonetheless, this study relied on a cross-sectional design; therefore, it was not possible to ascertain a causal relationship between BMD and HGB.
Conclusions
Our study shows that HGB has a non-linear relationship with lumbar and thoracic BMD. Further studies are required to elucidate the mechanisms underlying this association.
Data availability
The datasets obtained and analysed during the current study are available in the NHANES [https://www.cdc.gov/nchs/nhanes/index.htm].
Abbreviations
- HGB:
-
Hemoglobin
- BMD:
-
Bone mineral density
- NHANES:
-
National Health and Nutrition Survey
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BMI:
-
Body mass index
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- CPK:
-
Creatine phosphor kinase
- TG:
-
Triglyceride
- LDH:
-
Lactate dehydrogenase
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- RA:
-
Rheumatoid arthritis
- COPD:
-
Chronic obstructive pulmonary disease
- MECs:
-
Mobile examination centers
- CBC:
-
Complete blood count
- DXA:
-
Duel energy X-ray absorptiometry
- CAPI:
-
Computer-assisted personal interview
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- FRAX:
-
Fracture Risk Assessment Tool
- MSC:
-
Mesenchymal stem cell
References
Hauk, L. Treatment of low BMD and osteoporosis to prevent fractures: Updated guideline from the ACP. Am. Fam. Physician. 97(5), 352–353 (2018).
Falaschi, P., Marques, A. & Giordano, S. Osteoporosis and fragility in elderly patients. In Orthogeriatrics: The Management of Older Patients with Fragility Fractures (eds Falaschi, P. & Marsh, D.) 35–52 (Springer, 2021).
Blake, G. M. & Fogelman, I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad. Med. J. 83(982), 509–517 (2007).
Schurman, L. et al. Guidelines for the diagnosis, prevention and treatment of osteoporosis, 2012. Medicina 73(1), 55–74 (2013).
Lane, N. E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 194(2 Suppl), S3-11 (2006).
Korkmaz, U. et al. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur. J. Intern. Med. 23(2), 154–158 (2012).
Farid, Y., Bowman, N. S. & Lecat, P. Biochemistry, Hemoglobin Synthesis (StatPearls Publishing, 2021).
Balarajan, Y., Ramakrishnan, U., Ozaltin, E., Shankar, A. H. & Subramanian, S. V. Anaemia in low-income and middle-income countries. Lancet (London, England). 378(9809), 2123–2135 (2011).
Crea, F. et al. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur. Heart J. 40(29), 2455–2462 (2019).
Bani Hassan, E. et al. Hemoglobin levels are low in sarcopenic and osteosarcopenic older persons. Calcif. Tissue Int. 107(2), 135–142 (2020).
Hirani, V. et al. Low hemoglobin concentrations are associated with sarcopenia, physical performance, and disability in older australian men in cross-sectional and longitudinal analysis: The concord health and ageing in men project. J. Gerontol. A Biol. Sci. Med. Sci. 71(12), 1667–1675 (2016).
Zhou, Y. et al. Implications of gender-based variabilities in bone mineral density and hemoglobin levels. BMC Musculoskelet. Disord. 22(1), 645 (2021).
Shirazi, K. M. et al. Bone density and bone metabolism in patients with inflammatory bowel disease. Saudi J. Gastroenterol. 18(4), 241–247 (2012).
Miller, R. G. et al. High prevalence and correlates of low bone mineral density in young adults with sickle cell disease. Am. J. Hematol. 81(4), 236–241 (2006).
Sarrai, M., Duroseau, H., D’Augustine, J., Moktan, S. & Bellevue, R. Bone mass density in adults with sickle cell disease. Br. J. Haematol. 136(4), 666–672 (2007).
Aggarwal, H. K., Jain, D., Yadav, S. & Kaverappa, V. Bone mineral density in patients with predialysis chronic kidney disease. Ren. Fail. 35(8), 1105–1111 (2013).
Rutten, E. P., Franssen, F. M., Spruit, M. A. & Wouters, E. F. Anemia is associated with bone mineral density in chronic obstructive pulmonary disease. COPD 10(3), 286–292 (2013).
Zarei, T. et al. Evaluation of bone mineral density in patients with hemoglobin H disease. Ann. Hematol. 95(8), 1329–1332 (2016).
Cui, R., Zhao, Z., Fei, Z., Li, Y. & Gao, W. Anemia is related to osteoporosis in Chinese type 2 diabetic patients. Arch. Osteoporos. 16(1), 161 (2021).
CDC (2013) National Health and Nutrition Examination Survey, NHANES 2013–2014. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013. Accessed 3 Jan 2022
CDC (2015) National Health and Nutrition Examination Survey, NHANES 2015–2016. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2015. Accessed 3 Jan 2022
CDC (2017) National Health and Nutrition Examination Survey, NHANES 2017–2018. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017. Accessed 3 Jan 2022
CDC (2013) NHANES 2013–2014 Brochures and Consent Documents. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Documents.aspx?BeginYear=2013. Accessed 3 Jan 2022
CDC (2015) NHANES 2015–2016 Brochures and Consent Documents. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Documents.aspx?BeginYear=2015. Accessed 3 Jan 2022
CDC (2017) NHANES 2017–2018 Brochures and Consent Documents. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Documents.aspx?BeginYear=2017. Accessed 3 Jan 2022
National Health and Nutrition Examination Survey 2017–2018 Data Documentation, Codebook, and Frequencies Complete Blood Count with 5-Part Differential (CBC_J). https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/CBC_J.htm. Accessed 3 Jan 2022
National Health and Nutrition Examination Survey 2017–2018 Data Documentation, Codebook, and Frequencies Dual-Energy X-ray Absorptiometry - Whole Body (DXX_J). https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/DXX_J.htm. Accessed 3 Jan 2022
CDC (2017) NHANES 2017–2018 Laboratory Methods. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2017. Accessed 3 Jan 2022
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (London, England). 370(9596), 1453–1457 (2007).
Kim, H. S., Park, H. M., Lee, H. S. & Lee, Y. J. Hemoglobin levels and low bone mineral density in non-anemic older adults: Secondary analysis of the Korean National Health and Nutrition Examination Survey. Exp. Gerontol. 126, 110706 (2019).
Chuang, T.L., Koo, M., Chuang, M.H., Wang, Y.F. Bone mineral density and hemoglobin levels: Opposite associations in younger and older women. Int. J. Environ. Res. Public Health. 18(10) (2021).
Cesari, M. et al. Bone density and hemoglobin levels in older persons: Results from the InCHIANTI study. Osteoporosis Int. 16(6), 691–699 (2005).
Chuang, M. H., Chuang, T. L., Koo, M. & Wang, Y. F. Low hemoglobin is associated with low bone mineral density and high risk of bone fracture in male adults: A retrospective medical record review study. Am. J. Mens Health 13(3), 1557988319850378 (2019).
Ho, M. S., Medcalf, R. L., Livesey, S. A. & Traianedes, K. The dynamics of adult haematopoiesis in the bone and bone marrow environment. Br. J. Haematol. 170(4), 472–486 (2015).
Gurevitch, O., Slavin, S. & Feldman, A. G. Conversion of red bone marrow into yellow—Cause and mechanisms. Med. Hypotheses 69(3), 531–536 (2007).
Gurevitch, O. & Slavin, S. The hematological etiology of osteoporosis. Med. Hypotheses 67(4), 729–735 (2006).
Adams, G. B. & Scadden, D. T. A niche opportunity for stem cell therapeutics. Gene Ther. 15(2), 96–99 (2008).
Acknowledgements
We would like to acknowledge the following financial support: the Shandong University of Chinese Medicine Research and Innovation Team (No. 220318); the Shandong Province Famous Old Chinese Medicine Experts Inheritance Studio Construction Project (No. 22201906). We thank the NHANES database for sharing the data.
Funding
This work was supported by grants from the Shandong University of Chinese Medicine Research and Innovation Team (No. 220318); the Shandong Province Famous Old Chinese Medicine Experts Inheritance Studio Construction Project (No. 22201906).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported and agreed to be accountable for all aspects of the work. H.J. and L.E.Q. designed the experiments. L.E.Q., H.X.Z., and L.S.Q. collected and analyzed data. L.E.Q. and H.X.Z. prepared the initial draft of the manuscript. L.H., L.E.Q. and H.J. gave critical feedback during the study or during the submission of the manuscript. All authors provided final approval of the version to be submitted and agreed on the journal for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, E., Hou, X., Liu, S. et al. Association of hemoglobin levels with bone mineral density for adults over 18 years of age: a cross-sectional study. Sci Rep 12, 9975 (2022). https://doi.org/10.1038/s41598-022-13973-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13973-w
- Springer Nature Limited
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.