Abstract
Steroids affect bone health causing osteoporosis and fractures. The study aims to compare dual-release hydrocortisone (DR-HC) and conventional steroids on bone metabolism in patients with primary adrenal insufficiency (PAI). Thirty-five patients with PAI on conventional steroids (group A) and 35 patients switched to DR-HC (group B), consecutively referred at our hospital, were evaluated at baseline and after 18, 36 and 60 months of treatment. After 60 months of follow-up, patients in group A had a significant increase in body mass index (p = 0.004) and waist circumference (WC) (p = 0.026) and a significant decrease in osteocalcin (p = 0.002), bone alkaline phosphatase (p = 0.029), lumbar spine bone mass density (BMD) T and Z scores (p < 0.001 and p = 0.001, respectively) and vertebral fractures rate (p = 0.021) than baseline. By contrast, patients in group B had a significant decrease in WC (p = 0.047) and increase in bone alkaline phosphatase (p = 0.019), lumbar spine BMD T score (p = 0.032), femoral neck BMD T and Z scores (p = 0.023 and p = 0.036, respectively) than baseline. Long-term conventional steroid replacement therapy is associated with a decrease in BMD, notably at lumbar spine, and increase in vertebral fractures rate. By contrast, DR-HC treatment is associated with improvement of BMD.
Similar content being viewed by others
Introduction
Steroid-induced osteoporosis is the most common cause of secondary osteoporosis and the most common iatrogenic cause of the disease1. Patients with adrenal insufficiency (AI) need chronic steroid replacement therapy, exposing them to an increased risk of comorbidities2. Conventional steroid treatment is associated with bone fragility and vertebral fractures3. The negative impact of steroids on bone is well known, being related to a number of factors, such as the dose and type of steroid used, the duration of therapy, and the cumulative dose4, but also age, white race, female gender, smoking and alcohol can have an impact on bone loss5. Generally, steroids can induce bone loss in the short term up to one year of treatment with a loss of bone mass density (BMD) of 6–12% and in the long term with a BMD loss of 3% per year1. The risk significantly decreases when the steroid treatment is interrupted5. The risk of osteoporosis and fractures is increased for replacement steroid doses more than 30 mg/day of hydrocortisone or equivalent6 or for prednisone treatment, even at low doses7.
By contrast, novel formulations of hydrocortisone (HC), and specifically dual-release HC (DR-HC), are associated with improvement of BMD8.
The objective of the current study was to compare the effects of DR-HC and conventional steroids in patients with primary AI (PAI), on bone metabolism, fracture rate and BMD at lumbar spine and femoral neck during 60 months of follow-up.
Materials and methods
Study participants
We evaluated data from 70 consecutive patients with PAI on conventional steroid treatment in a real-life study. Patients were consecutively referred to the Division of Endocrinology of Palermo University from January 2012 to December 2020. Patients were on conventional steroid treatment (cortisone acetate and HC), administered twice or three times a day. Thirty-five patients, 15 males and 20 females, maintained conventional steroid therapy (17 cortisone acetate and 18 HC) (group A) while the other 35, (16 who were on cortisone acetate and 19 who were on HC), 11 males and 24 females, were switched from conventional steroid treatment to DR-HC (group B) administered orally in the morning in a fasting state. Patients had a 60-month follow-up. In group A there were 5 women in menopause, while in group B there were 6 women in menopause. Exclusion criteria were the following: age ≤ 18 years, secondary AI (SAI), treatment with other steroids (prednisone), pregnancy, breastfeeding, premature ovarian failure, hypoparathyroidism, hyperparathyroidism, treatment with estrogens and underweight (BMI < 18.5 kg/m2). The switch to DR-HC was judged to be appropriate on clinical grounds in those patients who complained of fatigue and weakness, presented hyponatraemia (< 134 mmol/L) or hypoglycaemia (≤ 2.78 mmol/L) or showed more than two comorbidities such as diabetes, osteoporosis/osteopenia, arterial hypertension and central obesity. The switch from HC to DR-HC was made with an equivalent dose, while the dose was reduced from cortisone acetate to DR-HC taking into consideration the minor steroid activity of cortisone acetate compared to HC and patients’ clinical characteristics.
PAI was diagnosed as recommended by international guidelines9.
In detail, among the total of 70 patients, 42 had autoimmune polyglandular syndrome (APS), while 28 had isolated autoimmune AI. Among patients with APS, 26 had combined Addison’s disease and autoimmune thyroid disease, 6 had combined Addison’s disease, type 1 diabetes mellitus and autoimmune hypothyroidism and 10 had combined Addison’s disease, autoimmune hypothyroidism and celiac disease. Patients with celiac disease were on a stable gluten-free diet. All patients with PAI were also on stable treatment with fludrocortisone (0.05–0.1 mg/day, once). Patients with hypothyroidism were treated with levo-thyroxine at the average dose of 1.2–1.5 mcg/kg. Patients with type 1 diabetes were on basal-bolus treatment on flash blood glucose monitoring. Five postmenopausal women had been treated with DHEA for a ranging period of 6–18 months, before being included in the study.
During the 60-month treatment period, the conventional steroid and the DR-HC doses were changed based on the physician’s judgement of a patient’s need in both groups of patients (Table 1). Each patient received instructions for treatment in special or emergency situations. Patients treated with DR-HC were instructed to add a rescue dose of HC during an intercurrent illness or stress (5 or 10 mg according to severity of stress and symptoms). Overall, 8 patients had to take a rescue dose of HC, 5 of them less than 10 times and 3 of them from 20 to 30 times during the 60-month period.
The current study was carried out in accordance with the recommendations of the Paolo Giaccone Policlinico ethics committee, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Paolo Giaccone Policlinico ethics committee (protocol 06/2021).
Study design
At baseline and after 18, 36 and 60 months of conventional steroid and DR-HC treatment, clinical and bone metabolic parameters were evaluated.
Anthropometric parameters such as BMI and waist circumference (WC), measured at the midpoint between the lower rib and the iliac crest, were evaluated. In addition, sodium, potassium, serum 25hydroxyvitamin-D (vitaminD), parathyroid hormone, calcium, phosphorus, creatinine, osteocalcin and bone alkaline phosphatase were assayed.
The blood sample was taken about 2 h after steroid administration (patients took the dose in the morning on waking) to avoid patients experiencing fatigue or other symptoms due to delayed intake of the drug.
In both groups, hypovitaminosis D was observed at baseline and a pharmacological supplementation was started in 26 patients of group A and 25 of group B, at the mean dose of 800 UI/day and maintained during the follow-up. Hypovitaminosis D was defined as a serum 25-hydroxy vitamin D level below the normal range (< 30 ng/ml). All patients supplemented with vitamin D reached the threshold of 30 ng/ml.
BMD was measured by DXA at lumbar spine and femoral neck (Hologic Horizon Inc., QDR-4500 W Waltham, MA) at baseline and after 18, 36 and 60 months of follow-up.
In patients aged 50 or more, BMD was expressed as the T-score, comparing the results with those obtained in a sex-matched Caucasian population at the peak of bone mass. A T-score less than or equal to − 2.5 SD at the neck or spine was defined as osteoporosis, whereas osteopenia was defined as a T-score between − 1 and − 2.5 SD. In patients younger than 50 years, the results were expressed as a/the Z-score, comparing the results with those obtained in an age and sex-matched Caucasian population. A Z-score of − 2.0 SD or lower was used to define a BMD “below the expected range for age”10. The coefficients of variation in the DXA measurements for BMD, bone mineral content (BMC) and area were 0.61%, 2.98% and 2.89%, respectively.
We also evaluated rib, femoral neck and hip fractures rate during the follow-up in both groups.
Assays
Sodium, potassium, serum 25hydroxyvitamin-D (vitamin-D), parathyroid hormone, calcium, phosphorus, creatinine, osteocalcin and bone alkaline phosphatase were measured with standard methods (Modular P800, Roche, Milan) at our hospital centralized laboratory. The intra- and interassay coefficients of variation were the following: Sodium 0.5% to 1.2% and 1.28% to 1.44%, respectively; potassium 1% to 2.1% and 2.2% to 3.08%, respectively; vitaminD 0.9% to 1.6% and 1.7% to 2.56%, respectively, parathyroid hormone 1.2% to 2.6% and 2.8% to 3.9%, respectively; calcium 1.8% to 3.5% and 3.75% to 4.23%, respectively; phosphorus 1.6% to 3.3% and 3.5% to 4.2%, respectively; creatinine 1.7% to 2.9% and 3.3% to 4.6%, respectively; osteocalcin 1.9% to 3.3% and 3.6% to 4.9%, respectively; bone alkaline phosphatase 2.7% to 3.6% and 3.8% to 5.2%, respectively.
Statistical analysis
The Statistical Packages for Social Science SPSS version 19 (SPSS, Inc., IBM, New York, USA) were used for data analysis. The normality of quantitative variables was tested with the Shapiro-Wilk test. The baseline characteristics of the groups were presented as mean ± SD for continuous variables, while the rates and proportions were calculated for categorical data. The differences between groups were performed using ANOVA for quantitative variables and the χ2-test for categorical variables. A comparison between numerical variables at baseline, 18-, 36- and 60-month follow-up was performed with the Friedman analysis. In addition, multiple linear regression analysis was performed to identify independent predictors of the dependent variables lumbar spine and femoral neck T and Z scores and of dependent variable fracture. A p value < 0.05 was considered statistically significant.
Results
The baseline characteristics of all patients with PAI and subgroups (A and B) are shown in Table 3. During the 60-month period of observation, 4 out of 35 patients had an adrenal crisis in group A and none in the group B.
At baseline, patients in group B had a higher frequency of osteoporosis/osteopenia (p = 0.001) and visceral obesity (p = 0.020), higher WC values (p = 0.048) and lower osteocalcin levels (p = 0.004) than group A (Table 2).
After 60 months of follow-up, patients in group A had a significant increase in BMI (p = 0.004), WC (p = 0.026), vitamin D (p = 0.005), and a significant decrease in osteocalcin (p = 0.002), bone alkaline phosphatase (p = 0.024), lumbar spine BMD T and Z scores (p < 0.001 and p = 0.001, respectively) compared to baseline (Table 3). By contrast, patients in group B had a significant decrease in WC (p = 0.047) and increase in vitamin D (p < 0.001), bone alkaline phosphatase (p = 0.019) lumbar spine BMD T score (p = 0.032), femoral neck BMD T and Z scores (p = 0.023 and p = 0.036, respectively) compared to baseline (Table 3). In addition, at 60 months of follow-up we observed significantly higher values of WC (p = 0.045) and bone alkaline phosphatase (p < 0.001) and significantly lower values of osteocalcin (p = 0.001), lumbar spine and femoral neck Z scores (p = 0.045 and p = 0.047, respectively) in group A compared to group B (Table 3).
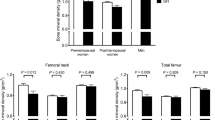
A significant trend of decrease in BMD lumbar spine T and Z scores was observed in group A, while in group B we observed a significant trend of increase during the follow-up (Fig. 1A,B). In addition, a significant trend of increase was also observed in the BMD femoral neck T and Z scores of group B during the follow-up, while no differences were observed in group A (Fig. 1C,D).
(A) Changes in BMD lumbar spine T score from baseline to 60 months for patients of groups A and B. *p < 0.05, **p < 0.01; ***p < 0.001 versus baseline using the Friedman analysis. Data are means (S.D.). (B) Changes in BMD lumbar spine Z score from baseline to 60 months for patients of groups A and B. *p < 0.05, **p < 0.01; ***p < 0.001 versus baseline using the Friedman analysis. Data are means (S.D.). (C) Changes in BMD femoral neck T score from baseline to 60 months for patients of groups A and B. *p < 0.05, **p < 0.01; ***p < 0.001 versus baseline using the Friedman analysis. Data are means (S.D.). (D) Changes in BMD femoral neck Z score from baseline to 60 months for patients of groups A and B. *p < 0.05, **p < 0.01; ***p < 0.001 versus baseline using the Friedman analysis. Data are means (S.D.).
Multivariate analysis showed that the duration of disease was negatively independently associated with T scores of femoral neck (β = − 0.542, p = 0.017) and lumbar spine (β = − 0.423; p = 0.043) at 60 months. The daily steroid dose per body surface was negatively independently associated with Z scores of femoral neck (β = − 0.997; p = 0.048) and lumbar spine (β = − 0.768; p = 0.045) at 60 months (Fig. 2). The rate of vertebral fractures in group A was also positively independently associated with daily steroid dose per body surface (β = 0.875; p = 0.048) (Table 4).
(A) Correlation between T score at lumbar spine and duration of disease at 60 months of follow-up. (B) Correlation between T score at femoral neck and duration of disease at 60 months of follow-up. (C) Correlation between Z score at lumbar spine and steroid daily dose per body surface at 60 months of follow-up. (D) Correlation between Z score at femoral neck and steroid daily dose per body surface at 60 months of follow-up.
Discussion
In the current study, we demonstrated that patients on conventional steroid treatment had a significant decrease in lumbar spine BMD T and Z scores, with increased rate of fractures in the 5-year period of observation. This resulted in a significant association between daily steroid dose per body surface and BMD and between duration of disease and BMD, while fracture rate was associated with the steroid dose per body surface. By contrast, DR-HC was associated with improvement in BMD and no new cases of fractures.
Steroids induce osteoporosis by several mechanisms such as inhibition of osteoblast activity, stimulation of osteoclast activity and inhibition of calcium absorption, stimulating renal excretion11. Steroids act not only by decreasing BMD, but also by altering the structure of the bone, favouring the risk of fracture12. Generally, there is a rapid fall in BMD due to bone resorption, followed by a slow decline due to altered bone formation5. Conventional steroid replacement therapy administered in two or three daily doses is characterized by a peak-to-trough release profile with inevitable supraphysiological cortisol values, notably in the evening hours, affecting bone formation and resorption.
The steroid effects on BMD and fractures in patients with AI have been investigated, providing conflicting results. The first studies conducted on patients with AI did not report changes in BMD in patients on long-duration treatment with steroids13,14. Other studies suggested a gender difference in BMD values, unfortunately providing conflicting results15,16,17. Braatvetdt et al. did not report differences between patients with AI and healthy controls, even though they showed a negative correlation among BMD, steroid dose and duration of disease, in line with our results18.
Several recent studies have investigated the relationship between steroid dose and BMD in adrenal insufficiency. Koetz et al. did not show changes in BMD in 81 patients with PAI and 41 with congenital adrenal hyperplasia (CAH) treated with low doses of steroids (mean equivalent hydrocortisone dose of 21.9 mg/day)19. Lovas et al. reported a decrease in BMD in patients with PAI treated with equivalent HC dose of 30 mg/day or more compared to healthy controls20. Schulz et al. conducted a prospective study on 57 subjects with PAI and 33 with CAH evaluating three different schemes of HC treatment, patients who maintained unchanged dose of HC (mean dose 25 mg/day), patients who increased the HC dose (from a mean of 18.7 to 25.2 mg/day) and patient who decreased the HC dose (from a mean of 30.8 to 21.4 mg/day). The authors showed that after 2 years, those patients who reduced the mean HC dose had an improvement in BMD, while patients with increased dose of HC had a decrease in BMD21. Zelissen et al. showed a linear decrease in BMD with the increasing of HC dose in men17. By contrast, another study did not report any correlation between reduced spinal BMD and weight-adjusted HC dose in 28 patients with PAI22. Further, Danilowicz et al. and Chikada et al. reported no changes in BMD with low HC doses of 10–15 mg in patients with SAI23,24. In addition, patients treated with prednisolone showed lower BMD values compared to the other conventional steroid therapies25. Overall, these studies showed that high doses of HC (more than 25 mg/day) were associated with reduced BMD, while low doses of HC (less than 25 mg/day) did not affect BMD.
With regard to the correlation between fractures and steroids, a large study on 3129 patients with PAI conducted from 1964 to 2006 reported 221 hip fractures, notably in the first year of observation, independently from age, sex and coexistence of other autoimmune disorders26. Camozzi et al. showed a 3–4 fold risk increase of vertebral fractures in 87 patients with PAI compared to healthy controls27. Authors did not observe any correlation with BMD, while reported that long duration of disease and supra-physiological doses of steroids were likely associated with fractures. Recently, a metanalysis on 7 studies and a systematic review on 17 studies on fracture rate in patients with CAH, SAI and PAI showed an increased overall risk of fractures compared to healthy controls with a slight and not significant correlation between steroid equivalent dose and osteoporotic fracture rate28. However, this metanalysis had several limitations due to the heterogeneity of the studies included.
DR-HC has been shown to ensure a more physiological release of HC due to its pharmacokinetics, with favourable effects on glucose metabolism, body weight, low-grade inflammation and immune response, and reducing the adverse metabolic effects and the related mortality risk frequently observed in patients chronically treated with conventional steroids29,30,31. The switch from conventional steroids to DR-HC is associated with resynchronization of clock genes and restoration of expression of many genes involved in inflammation, immune response, adipogenesis, and oxidative stress32,33. Only one study investigated the effects of DR-HC on BMD, showing a significant improvement in BMD values at lumbar spine and femoral neck after 24 months of treatment. However, this study had a retrospective design and was conducted in a small sample of patients with SAI8.
The limitations of the current study are the following: first, the lack of randomization in assignment of patients to group A or group B; second, the small sample of patients enrolled due to the rarity of the disease; third, most of the patients enrolled in the study had combined autoimmune deficiencies which could play an adjunctive negative role on bone health, even though the other autoimmune disorders (hypothyroidism, celiac disease and type 1 diabetes mellitus) were all well controlled; forth, higher frequency of osteoporosis in patients of group B than group A could be a bias of the study. However, we excluded patients with premature ovarian failure to avoid any interference from gonadal hormones on bone. Lastly, we did not evaluate genetic polymorphisms in P-glycoproteins, 11-B-hydroxylase and glucocorticoid receptors, which could have an influence on BMD. The strength of the study was the comparison of the effects of two different schemes of steroid replacement treatment for PAI on bone health and metabolism, the conventional one subdivided into two or three daily doses, and the novel one administered once daily. To our knowledge, this has never been done before.
In conclusion, our study shows that in patients with PAI, 5-years conventional steroid replacement therapy is associated with a significant decrease in lumbar BMD T and Z scores and increase in vertebral fractures rate. In these patients, the decrease in lumbar BMD is associated with long duration of disease and high daily steroid dose per body surface, while the increased rate of vertebral fractures is associated with the daily steroid dose per body surface. By contrast, patients treated with DR-HC treatment show an improvement of femoral neck T and Z scores and lumbar T score. Our findings suggest that DR-HC having a physiological glucocorticoid profile release do not affect bone physiology.
However, further larger randomized studies are required to confirm our data.
References
Chotiyarnwong, P. & McCloskey, E. V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 16, 437–447 (2020).
Husebye, E. S., Pearce, S. H., Krone, N. P. & Kämpe, O. Adrenal insufficiency. Lancet 397, 613–629 (2021).
Falhammar, H. Skeletal fragility induced by overtreatment of adrenal insufficiency. Endocrine 59, 239–241 (2018).
Briot, K. & Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 1, e000014 (2015).
Buckley, L. & Humphrey, M. B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 379, 2547–2556 (2018).
Kiko, N. & Kalhan, A. Comparison of various glucocorticoid replacement regimens used in chronic adrenal insufficiency: A systematic review. Dubai Diabetes Endocrinol. J. 26, 50–68 (2020).
Van Staa, T. P. et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Reum. 48, 3224–3229 (2003).
Frara, S. et al. Bone safety of dual-release hydrocortisone in patients with hypopituitarism. Endocrine 60, 528–531 (2018).
Bornstein, S. R. et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 364–389 (2016).
Schousboe, J. T., Shepherd, J. A., Bilezikian, J. P. & Baim, S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J. Clin. Densitom. 16, 455–466 (2013).
Bentivegna, G., Osella, G., Pia, A. & Terzolo, M. Effects on bone health of glucocorticoid replacement therapy in primary and secondary adrenal insufficiency: A review. Curr. Opin. Endocrine Metab. Res. 3, 31–37 (2018).
Steinbuch, M., Youket, T. E. & Cohen, S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos. Int. 15, 323–328 (2004).
Arlt, W., Rosenthal, C., Hahner, S. & Allolio, B. Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin. Endocrinol. 64, 384–389 (2006).
Jódar, E., Valdepeñas, M. P., Martinez, G., Jara, A. & Hawkins, F. Long term follow-up of bone mineral density in Addison’s disease. Clin. Endocrinol. 58, 617–620 (2003).
Devogelaer, J. P., Crabbé, J. & Nagant de Deuxchaisnes, C. Bone mineral density in Addison’s disease: evidence for an effect of adrenal androgens on bone mass. Br. Med. J. 294, 798–800 (1994).
Florkowski, C. M., Holmes, S. J., Elliot, J. R., Donald, R. A. & Espiner, E. A. Bone mineral density is reduced in female but not male subjects with Addison’s disease. N. Z. Med. J. 107, 52–53 (1994).
Zelissen, P. M., Croughs, R. J., Van Rijk, P. P. & Raymakers, J. A. Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann. Intern. Med. 120, 207–210 (1994).
Braatvedt, G. D., Joyce, M., Evans, M., Clearwater, J. & Reid, I. R. Bone mineral density in patients with treated Addison’s disease. Osteoporos. Int. 10, 435–440 (1999).
Koetz, K. R., Ventz, M., Diederich, S. & Quinkler, M. Bone mineral density is not significantly reduced in adult patients on low dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metabol. 97, 85–92 (2012).
Løvås, K. et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. Eur. J. Endocrinol. 160, 993–1002 (2009).
Schulz, J. et al. Reduction in daily HC dosage improves bone health in primary adrenal insufficiency. Eur. J. Endocrinol. 174, 531–538 (2016).
Leelarathna, L. et al. Co-morbidities, management and clinical outcome of auto-immune Addison’s disease. Endocrine 38, 113–117 (2010).
Danilowicz, K., Bruno, O. D., Manavela, M., Gomez, R. M. & Barkan, A. Correction of cortisol overreplacement ameliorates morbidities in patients with hypopituitarism: a pilot study. Pituitary 11, 279–285 (2008).
Chikada, N., Imaki, T., Hotta, M., Sato, K. & Takano, K. An assessment of bone mineral density in patients with Addison’s disease and isolated ACTH deficiency treated with glucocorticoid. Endocr. J. 51, 355–360 (2004).
Chandy, D. D. & Bhatia, E. Bone mineral density in patients with addison disease on replacement therapy with prednisolone. Endocr. Pract. 22, 434–439 (2016).
Björnsdottir, S. et al. Risk of hip fracture in Addison’s disease: a population-based cohort study. J. Intern. Med. 270, 187–195 (2011).
Camozzi, V. et al. Vertebral fractures assessed with dual-energy X-ray absorptiometry in patients with Addison’s disease on glucocorticoid and mineralocorticoid replacement therapy. Endocrine 59, 319–329 (2017).
Li, L., Bensing, S. & Falhammar, H. Rate of fracture in patients with glucocorticoid replacement therapy: a systematic review and meta-analysis. Endocrine 74, 29–37 (2021).
Quinkler, M., Miodini, Nilsen, R., Zopf, K., Ventz, M., Øksnes, M. Modified-release hydrocortisone decreases BMI and HbA1c in patients with primary and secondary adrenal insufficiency. Eur. J. Endocrinol. 172, 619–626 (2015).
Guarnotta, V., Ciresi, A., Pillitteri, G. & Giordano, C. Improved insulin sensitivity and secretion in prediabetic patients with adrenal insufficiency on dual-release hydrocortisone treatment: A 36-month retrospective analysis. Clin. Endocrinol. 88, 665–672 (2018).
Guarnotta, V. et al. Dual-release hydrocortisone vs conventional glucocorticoids in adrenal insufficiency. Endocr. Connect. 8, 853–862 (2019).
Isidori, A. M. et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): A single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 6, 173–185 (2018).
Guarnotta, V., Amodei, R. & Giordano, C. Metabolic comorbidities of adrenal insufficiency: Focus on steroid replacement therapy and chronopharmacology. Curr. Opin. Pharmacol. 60, 123–132 (2021).
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
V.G., C.D.S. and C.G. had full control of study design, data analysis and interpretation, and preparation of the article. All authors were involved in planning the analysis and drafting the article. The final draft article was approved by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guarnotta, V., Di Stefano, C. & Giordano, C. Long-term outcomes of conventional and novel steroid replacement therapy on bone health in primary adrenal insufficiency. Sci Rep 12, 13280 (2022). https://doi.org/10.1038/s41598-022-13506-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13506-5
- Springer Nature Limited